Abstract
Nephrin, a structural molecule, is also a signaling molecule after phosphorylation. Inhibition of nephrin phosphorylation is correlated with podocyte injury. The PINCH-1-ILK-α-parvin (PIP) complex plays a crucial role in cell adhesion and cytoskeleton formation. We hypothesized that nephrin phosphorylation influenced cytoskeleton and cell adhesion in podocytes by regulating the PIP complex. The nephrin phosphorylation, PIP complex formation, and F-actin in Wistar rats intraperitoneally injected with puromycin aminonucleoside were gradually decreased but increased with time, coinciding with the recovery from glomerular/podocyte injury and proteinuria. In cultured podocytes, PIP complex knockdown resulted in cytoskeleton reorganization and decreased cell adhesion and spreading. Nephrin and its phosphorylation were unaffected after PIP complex knockdown. Furthermore, inhibition of nephrin phosphorylation suppressed PIP complex expression, disorganized podocyte cytoskeleton, and decreased cell adhesion and spreading. These findings indicate that alterations in nephrin phosphorylation disorganize podocyte cytoskeleton and decrease cell adhesion through a PIP complex-dependent mechanism. [BMB Reports 2013; 46(4): 230-235]
Keywords: Cell adhesion, Nephrin phosphorylation, PINCH-1-ILK-α-parvin complex, Podocyte
INTRODUCTION
The slit diaphragm (SD), the intercellular junction between podocyte foot processes, plays a critical role in cell survival, polarity, cytoskeletal organization, and glomerular filtration function (1,2). SD dysfunction results in injury of glomerular filtration barrier, thereby leading to proteinuria and progressive renal injury. Nephrin, an important structural component of the podocyte, maintains the integrity of SD. Aside from being a structural molecule, nephrin is also a crucial signaling molecule that, after phosphorylation, mediates signal transduction in podocytes and thereby participates in its biological function (3,4). The expression of phosphorylated nephrin can be suppressed by Src family kinase inhibitors (PP1 or PP2) and overexpressed by protamine sulfate or the Src family kinase Fyn (5-7). Dysregulation of nephrin phosphorylation results in proteinuria (8), but the molecular mechanisms in this event are not fully delineated.
Podocyte foot processes anchor on the glomerular basement membrane (GBM) with adhesion molecules to maintain the morphology and mobility of the podocyte. The PIP complex, as a hetero-oligomer consisting of PINCH-1, integrin-linked kinase (ILK), and a-parvin, is one of the adhesion molecules between podocytes and GBM (9). Known as a fundamental component of focal adhesion, the PIP complex is supposed to regulate the adhesion between cells and the extracellular matrix and take part in the location of the cytoskeleton (10-12). In addition, it functions as a signaling platform for integrins by interacting with the actin cytoskeleton and other signaling pathways (13). These processes interrelate and determine cell behaviors, such as adhesion, spreading, architecture, morphology, and survival. The detailed signaling mechanisms by which the PIP complex is modulated remain unclear.
Recent reports have demonstrated that ILK, a member of the PIP complex, maybe have a function in bridging and integrating the integrin and SD signals by interacting with cytoplasmic domains of β-integrins and nephrin (14). Furthermore, the pathological characteristics of podocytes with abnormal nephrin expression and aberrant nephrin phosphorylation are similar to those that result from PIP complex abnormalities. Based on these available evidence, we hypothesize that abnormal nephrin phosphorylation is involved in the signal transduction of the PIP complex. In this study, we determined the influence of nephrin phosphorylation on podocyte adhesion and investigated its possible relationship with PIP complex formation.
RESULTS
Glomerular expression of nephrin, phosphorylated nephrin, PIP complex and F-actin in puromycin aminonucleoside nephrosis (PAN)
Kidneys from PAN were used to study the possible role of nephrin, phosphorylated nephrin, PIP complex in glomerular/ podocyte injury. As shown in Fig. 1, the expression levels of nephrin and phosphorylated nephrin were decreased significantly on day 1, 4 and 7 after the injection of puromycin aminonucleoside (PA) compared with that of control rats on day 0 (Fig. 1A and B). Furthermore, nephrin expression was started to recover on day 14 and was almost similar to that of the control rats on day 28 (Fig. 1A and B). However, phosphorylated nephrin was still low on day 14 and recovered on day 28 (Fig. 1B). The PIP complex was decreased initially on day 4 after the injection, reached its lowest level on day 7, and then restored to control levels on day 28 (Fig. 1C). F-actin was reduced on day 1 after the injection of PA and then started to recover on day 4. The protein expression was approximate to the normal level on day 7 (Fig. 1D).
Fig. 1. Expression of nephrin, phosphorylated nephrin, PIP complex and F-actin in rats with PAN. (A) Immunofluorescence detection of glomerular nephrin expression in different groups. Original magnification ×400. (B) Western blot for phosphorylated nephrin and nephrin in the glomeruli of rats of different groups. *P < 0.05 compared with the group of day 0. (C) Co-immunoprecipitation of the PIP complex in the glomeruli of rats of different groups. ILK and PINCH-1 were immunoprecipitated by an α-parvin antibody. *P < 0.05 compared with the group of day 0. (D) Western blot for F-actin expression in the glomeruli of rats of different groups. *P < 0.05 compared with the group of day 0.
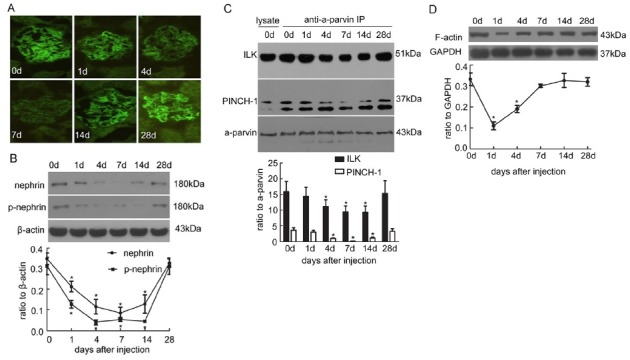
Effect of the PIP complex on cytoskeleton and podocyte adhesion in vitro
To test the effect of PIP complex formation on the cytoskeleton in podocyte injury, cultured murine podocytes exposed to PA (50 mg/l) were transfected with PINCH-1 siRNA. Transfection of podocytes with PINCH-1 siRNA reduced significantly the PIP complex compared with normal podocytes and cells transfected with scrambled siRNA (Fig. 2A). Reduction of PIP complex did not alter the expression levels of nephrin and phosphorylated nephrin (Fig. 2B). After inhibition of PIP complex, the cytoskeleton was arranged disorderly and displayed disorganization (Fig. 2C). Furthermore, PIP complex knockdown decreased cell adhesion and spreading (Fig. 2D and E).
Fig. 2. Knockdown of the PIP complex resulted in the reorganization of the cytoskeleton, and down-regulated podocyte adhesion and spreading. (A) ILK and PINCH-1 were immunoprecipitated by an α-parvin antibody in cultured normal podocytes and podocytes stimulated by PA with/without PINCH-1 siRNA or scrambled siRNA transfection. The immunoprecipitates were analyzed by Western blot with antibodies of ILK, PINCH-1 and α-parvin. *P < 0.05 compared with the normal group; #P < 0.05 compared with the groups stimulated by PA with/without scrambled siRNA. (B) Nephrin and phosphorylated nephrin were detected by Western blot in different groups. *P < 0.05 compared with the normal group; #P < 0.05 compared with the groups stimulated by PA with/without scrambled siRNA. (C) Stress fiber of the cytoskeleton was detected by immunofluorescence with FITC-phalloidin. Original magnification ×400. (a) Normal podocytes; (b) podocytes transfected with PINCH-1 siRNA; (c) podocytes transfected with scrambled siRNA; (d) podocytes stimulated by PA; (e) podocytes stimulated by PA with PINCH-1 siRNA transfection; (f) podocytes stimulated by PA with scrambled siRNA transfection. (D) Cell adhesion was measured with spectrophotometry. *P < 0.05 compared with the normal group; #P < 0.05 compared with the PA or scrambled siRNA group. (E) Cell spreading was detected and counted under inverted microscope. Original magnification ×400. *P < 0.05 compared with the normal group; #P < 0.05 compared with the PA or scrambled siRNA group.
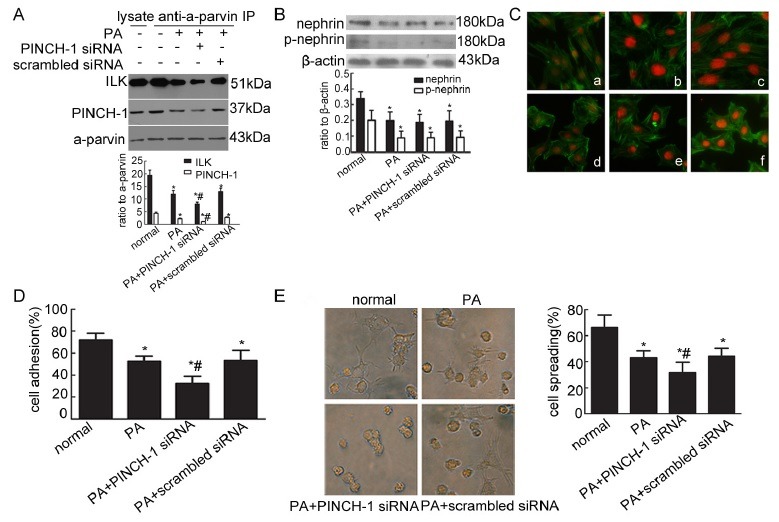
Effect of phosphorylated nephrin on cytoskeleton and podocyte adhesion in vitro
Cultured murine podocytes exposed to PA (50 mg/l) were transfected with nephrin siRNA for 48 h to further verify the effect of phosphorylated nephrin on the cytoskeleton, podocyte spreading and adhesion in podocyte injury. Podocytes transfected with nephrin siRNA reduced significantly nephrin and phosphorylated nephrin compared with the normal podocytes and cells permeated with scrambled siRNA (Fig. 3A). The inhibition of nephrin phosphorylation after nephrin knockdown suppressed the expression of PIP complex (Fig. 3B) and led to cytoskeleton disorder and rearrangement (Fig. 3C). Moreover, cell adhesion and spreading were decreased significantly as nephrin phosporylation decreased (Fig. 3D and E). Then, cultured murine podocytes were stimulated by the Src family kinase inhibitor (PP2; 10 μM) for 1 h to further test the effect of phosphorylated nephrin on the cytoskeleton, podocyte spreading and adhesion in the physiological state. Phosphorylated nephrin was inhibited in podocytes stimulated by the Src family kinase inhibitor (PP2) compared with that of normal podocytes (Fig. 4A). The inhibition of phosphorylated nephrin resulted in the decrease of PIP complex (Fig. 4B) and reorganization of podocyte cytoskeleton (Fig. 4C). Furhermore, the inhibition of phosphorylated nephrin decreased podocyte adhesion (Fig. 4D) and spreading (Fig. 4E).
Fig. 3. Diminishment of phosphorylated nephrin resulted in cytoskeleton reorganization, decreased podocyte adhesion and spreading in podocyte injury. (A) Nephrin and phosphorylated nephrin were detected by Western blot in different groups. *P < 0.05 compared with the normal group; #P < 0.05 compared with the PA or scrambled siRNA group. (B) ILK and PINCH-1 were immunoprecipitated by an α-parvin antibody in different groups. The immunoprecipitates were analyzed by Western blot with antibodies against ILK and PINCH-1. *P < 0.05 compared with the control group; #P < 0.05 compared with the PA or scrambled siRNA group. (C) Stress fiber of the cytoskeleton was detected by immunofluorescence with FITC-phalloidin. Original magnification ×400. (a) Normal podocytes; (b) podocytes transfected with nephrin siRNA; (c) podocytes transfected with scrambled siRNA; (d) podocytes stimulated by PA; (e) podocytes stimulated by PA with nephrin siRNA transfection; (f) podocytes stimulated by PA with scrambled siRNA transfection. (D) Cell adhesion was measured with a spectrophotometer. *P < 0.05 compared with the normal group; #P < 0.05 compared with the PA or scrambled siRNA group. (E) Cell spreading was detected and counted under an inverted microscope. Original magnification ×400. *P < 0.05 compared with the normal group; #P < 0.05 compared with the PA or scrambled siRNA group.
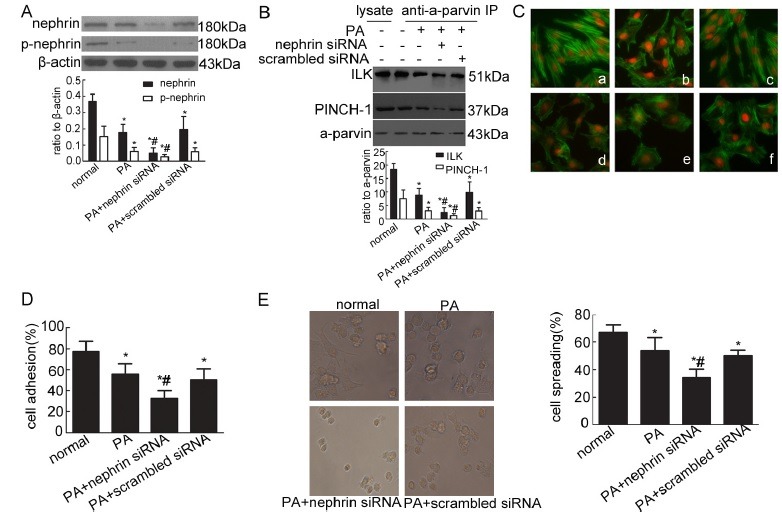
Fig. 4. Effect of phosphorylated nephrin on podocyte cytoskeleton, adhesion and spreading in the physiological state. (A) Phosphorylated nephrin was detected by Western blot with antibody against phosphorylated nephrin (pY1217) in cultured normal podocytes and podocytes stimulated by the Src family kinase inhibitor (PP2). *P < 0.05 compared with the normal group. (B) ILK and PINCH-1 were immunoprecipitated by an α-parvin antibody in cultured normal podocytes and podocytes stimulated by PP2. The immunoprecipitates were analyzed by Western blot with antibodies against ILK, PINCH-1 and α-parvin. *P < 0.05 compared with the normal group. (C) Stress fiber of the cytoskeleton was detected by immunofluorescence with FITC-phalloidin. Original magnification ×400. (D) Cell adhesion was measured with a spectrophotometer. *P < 0.05 compared with the normal group. (E) Cell spreading was detected and counted under an inverted microscope. Original magnification ×400. *P < 0.05 compared with the normal group.
DISCUSSION
A common denominator of glomerular diseases, such as minimal change nephropathy, membranous nephropathy, and focal segmental glomerulosclerosis, is the presence of proteinuria and podocyte foot processes fusion or effacement, the latter being a characteristic feature of podocyte injury. In the present study, our data indicated that the rat model injected with PA, a classic model of podocyte injury, was similar to human minimal change nephritic syndrome with regard to its clinical and pathological representation (Supplemental Fig. 1 and Supplemental Fig. 2). The establishment of the model contributed to the subsequent research in the pathogenesis of proteinuria.
The abnormalities in nephrin expression and phosphorylation of nephrin are involved in the development of proteinuria, foot processes effacement, and loss of podocytes. Aside from being a structural protein, nephrin transducts signals from the SD into the interior of podocyte, thereby initiating a signaling cascade that leads to actin polymerization through the phosphorylation of nephrin (15,16). In addition, the tyrosine phosphorylation of nephrin is significant to the maintenance of normal podocyte morphology and function through the modulation of skeleton proteins (e.g., F-actin) (17). The phosphorylation of nephrin was significantly reduced, and podocyte apoptosis was promoted after Ang II stimulation (18). In the present study, nephrin phosphorylation was significantly decreased, the cytoskeleton was rearranged, and cell adhesion and spreading were reduced distinctly after puromycin aminonucleoside stimulation. These results demonstrate that nephrin phosphorylation is cardinal to maintain podocyte structure, function and survival. We also found that inhibition of nephrin phosphorylation resulted in cytoskeleton reorganization, decreased cell spreading and adhesion. These findings further indicate that nephrin phosphorylation plays a crucial role in the maintenance of podocyte cytoskeleton stability and the adhesion between podocytes and GBM.
Proper podocyte adhesion to extracellular matrix, morphology, and survival is pivotal to normal renal function. However, the molecular mechanisms that mediate podocyte behavior, such as adhesion, architecture and survival, are still unknown. The PIP complex, a cytoplasmic component of the cell-extracellular matrix adhesion, functions both as an adaptor between integrins and the actin cytoskeleton, as well as a hub that regulates several signaling pathways. The PIP complex transduces diverse signals from ECM to intracellular effectors through its direct interaction with factors that function as upstream regulators of several signaling pathways. The PIP complex plays a crucial role in the adhesion, morphology, and survival of podocytes, which is mediated by the phosphorylation of α-parvin (19). TGF-β regulates the expression of the PIP complex in glomerular cells, thereby promoting glomerular mesangial matrix deposition and enhancing podocyte apoptosis through modulating p38 activation (20). We found that the reduction of PINCH-1 (using siRNA) induced the diminishment of the PIP complex; this disruption of the PIP complex resulted in cytoskeleton reorganization and decreased podocyte adhesion and spreading. These findings indicate that the PIP complex has an important role in podocyte behavior including cytoskeleton stability and adhesion to GBM.
Although the function of the PIP complex was demonstrated, the possible mechanisms by which the PIP ternary complex is regulated and recruited to ECM adhesion sites still need further exploration. Therefore, we further investigated the mutual effects of the PIP complex and nephrin phosphorylation (Fig. 2, 3 and Supplemental Fig. 3). Our data showed the reduction of nephrin phosphorylation resulted in the disruption of the PIP complex. This result demonstrates that nephrin phosphorylation regulates the formation and disruption of the PIP complex, thereby maintaining the cytoskeleton of podocyte and cell adhesion and spreading. In addition, we found that the disruption of the PIP complex did not influence the protein expression of nephrin and its phosphorylation. However, Dai et al. (14) discovered that ILK deficiency caused an aberrant distribution of nephrin in podocytes. Therefore, the PIP complex may also modulate SD signaling as nephrin phosphorylation by regulating the distribution of nephrin and not the protein expression.
In conclusion, our present study demonstrated that nephrin phosphorylation regulated GBM signaling through the PIP complex, which had a key role in cytoskeleton reorganization and adhesion to GBM in podocyte injury. Dual-direction regulation between glomerular SD signaling and GBM signaling cannot be excluded. The nephrin phosphorylation-PIP complex signaling pathway may provide a new effective therapeutic target for podocyte injury in human minimal change nephrotic syndrome.
MATERIALS AND METHODS
Animals
A total of 36 male SPF Wistar rats (140 g to 160 g body weight; 4 to 6 weeks of age) were purchased from the Hubei Research Center of Experimental Animals (Disease Prevention and Control Centers of Hubei Province, China). Rats were randomized into two groups (n=18 per group). The model animals were subjected to a single intraperitoneal injection of puromycin aminonucleoside (PA, 15 mg/100 g). Saline-injected animals served as controls. Twenty-four-hour urine was collected in metabolic cages, and urinary protein concentration was measured on day 0, 1, 4, 7, 14, and 28 after the injection. The animals were sacrificed on day 0, 1, 4, 7, 14, and 28. Kidneys were infused with the phosphatase inhibitor vanadate before retrieval and then stored at −80℃ for biochemical and renal pathological analysis.
Cell culture and treatment
A conditionally immortalized murine podocyte cell line was obtained from Dr. Peter Mundel (Mount Sinai School of Medicine, New York). Podocytes were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% heat-inactivated fetal calf serum (Gibco, USA), 100 U/ml penicillin G, and 100 μg/ml streptomycin in an incubator with 5% CO2. During proliferation, the medium was mixed with 10 U/ml recombinant mouse interferon-γ (Sigma, USA), and the cells were maintained at 33℃. Afterward, the podocytes were cultured at 37℃ to induce differentiation without interferon-γ for 10 d to 14 d. Differentiated podycytes were used between passages 19 and 29 for all experiments. Cultured murine podocytes were treated with puromycin aminonucleoside (PA) at various times (0, 6, 12, 24, and 48 h) and concentrations (0, 25, 50, 75, and 100 mg/l). Cultured murine podocytes were stimulated by the Src family kinase inhibitor (PP2; 10 μM) for 1 h (6).
Western immunoblotting
Isolated glomeruli or cells were lysed in a cell disruption buffer (Beyotime, China) with protease/phosphatase inhibitors (1% phenylmethanesulfonylfluoride and 1% cocktail) and then centrifuged at 12,000 rpm for 20 min at 4℃. The protein samples were mixed with loading buffer and then heated at 95℃ to 100℃ for 5 min. The protein concentration of the samples was measured using a bicinchoninic acid protein assay kit (Thermo Scientific Pierce, USA). Equal samples were separated on 8% to 12% sodium dodecyl sulfate-polyacrylamide gels and then transferred to nitrocellulose membrane (GE Healthcare, UK) by semidry blotting. Guinea pig anti-nephrin (1:500; Progen Biotechnik, Germany), mouse anti-nephrin phospho (pY1217) (1:10,000; Epitomics, USA), mouse anti-β-actin (1:1,000; Santa Cruz, USA), rabbit anti-PINCH-1 (1:1,000; Cell Signaling Technology, USA), rabbit anti-ILK (1:1,000; Cell Signaling Technology, USA), and rabbit anti-α-parvin (1:1,000; Cell Signaling Technology, USA) were used as primary antibodies. Horseradish peroxidase-conjugated secondary antibodies (1:1,000; Cell Signaling Technology, USA) were used at 1:5,000. Blots were visualized by the enhanced chemiluminescence reaction (Santa Cruz, USA) and developed on the film.
Immunofluorescence assay
Frozen kidney sections (6 μm) were incubated with bovine serum (5%) for 30 min at room temperature. Guinea pig anti-nephrin (1:50, Progen Biotechnik, Germany) was used as primary antibody for overnight incubation at 4℃. Fluorescein isothiocyanate (FITC)-conjugated IgG antibody was used as secondary antibody at 37℃ for 90 min.
Co-immunoprecipitation
Isolated glomeruli or cells were lysed in immunoprecipitation buffer and centrifuged at 12,000 rpm for 20 min. The supernatant was mixed with rabbit IgG and protein A/G agarose for 2 h at 4℃ and then centrifuged at 2,500 rpm for 5 min. The supernatants were incubated with rabbit α-parvin monoclonal antibodies overnight at 4℃. The samples were mixed with protein A/G agarose for 3 h and then centrifuged at 2,500 rpm for 5 min. The precipitates were collected and washed five times with phosphate-buffered saline (PBS). The complex was mixed with 5× Lane Marker Sample Buffer to make a 1× final solution and then heated at 100℃ for 5 min. The samples were separated by SDS-PAGE and analyzed by immunoblotting with PINCH-1, ILK, and α-parvin antibodies.
Transfection of PINCH-1 and nephrin siRNA
PINCH-1 siRNA and nephrin siRNA transfection was performed according to the Santa Cruz transfection reagent handbook (Santa Cruz, USA). Briefly, 2 × 105 cells were seeded in six-well plates and transfected with 10 nM PINCH-1 or nephrin siRNA (Santa Cruz, USA) with or without a negative control (scrambled siRNA provided by the manufacturer). The cells were incubated with the transfection complexes at 37℃ for 48 h.
Cell adhesion and spreading assays
Cell adhesion and spreading were analyzed as described (19,21). Cells (2 × 105) were seeded in six-well plates coated with fibronectin (FN; 10 μg/ml). Free-floating cells were washed off after being incubated for 5 h, and cells sticking to the bottom were fixed with 4% paraformaldehyde, stained by 0.1% crystal violet, and then lysed with 10% acetic acid. The control cells were fixed without being washed. Absorbance was obtained using a spectrophotometer at 570 nm optical density. The percentage of podocyte adhesion is presented as the absorbance of experimental groups (including the normal group) divided by the absorbance of the control cells (without being washed). Cells (2 × 105) were seeded in six-well plates coated with FN (10 μg/ml) after being digested by pancreatin. The podocyte morphology was observed after 5 h under an inverted microscope. Unspread cells were round, whereas spread cells were of extended processes. The percentage of podocyte spreading is presented as the number of spread cells divided by the number of total cells.
Staining of cytoskeleton segments
The cells were washed with precooled (4℃) PBS and then fixed in 4% paraformaldehyde containing 0.1% Triton X-100 for 30 min at 4℃. FITC-phalloidin (5 μg/ml) was used to label the stress fiber overnight at 4℃ or 2 h at 37℃. After the cells were washed thrice with PBS, the nucleus was restained with propidium iodide (2 μg/ml) for 15 min at room temperature without light. Subsequently, the sections were imaged by fluorescence microscopy.
Statistical analysis
Data were presented as mean ± SD. Statistically significant differences in mean values were tested by analysis of variance. Differences at P < 0.05 were considered statistically significant. The data were analyzed using SPSS 17.0.
Acknowledgments
This study is supported by grants from the National Science Foundation of China (30900688 to C.C., 81100478 to W.L., and 81270762 to G. D.).
References
- 1.Faul C., Asanuma K., Yanagida-Asanuma E., Kim K., Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. (2007);17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Schermer B., Benzing T. Lipid-protein interactions along the slit diaphragm of podocytes. J. Am. Soc. Nephrol. (2009);20:473–478. doi: 10.1681/ASN.2008070694. [DOI] [PubMed] [Google Scholar]
- 3.Miner J. H. Life without nephrin: it's for the birds. J. Am. Soc. Nephrol. (2012);23:369–371. doi: 10.1681/ASN.2012010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin X. S., Tsukaguchi H., Shono A., Yamamoto A., Kurihara H., Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J. Am. Soc. Nephrol. (2009);20:2534–2545. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiser J., Pixley F. J., Hug A., Kriz W., Smoyer W. E., Stanley E. R., Mundel P. Regulation of mouse podocyte process dynamics by protein tyrosine phosphatases rapid communication. Kidney Int. (2000);57:2035–2042. doi: 10.1046/j.1523-1755.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 6.Lahdenperä J., Kilpeläinen P., Liu X. L., Pikkarainen T., Reponen P., Ruotsalainen V., Tryggvason K. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int. (2003);64:404–413. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 7.New L. A., Keyvani Chahi A., Jones N. Direct regulation of nephrin tyrosine phosphorylation by nck adaptor proteins. J. Biol. Chem. (2013);288:1500–1510. doi: 10.1074/jbc.M112.439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jim B., Ghanta M., Qipo A., Fan Y., Chuang P. Y., Cohen H. W., Abadi M., Thomas D. B., He J. C. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS One. (2012);7:e36041. doi: 10.1371/journal.pone.0036041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim. Biophys. Acta. (2004);1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. (2005);15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Braun A., Bordoy R., Stanchi F., Moser M., Kostka G. G., Ehler E., Brandau O., Fassler R. PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp. Cell Res. (2003);284:239–250. doi: 10.1016/S0014-4827(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 12.Attwell S., Mills J., Troussard A., Wu C., Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol. Biol. Cell. (2003);14:4813–4825. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legate K. R., Montanez E., Kudlacek O., Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. (2006);7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 14.Dai C., Stolz D. B., Bastacky S. I., St-Arnaud R., Wu C., Dedhar S., Liu Y. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J. Am. Soc. Nephrol. (2006);17:2164–2175. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- 15.Verma R., Kovari I., Soofi A., Nihalani D., Patrie K., Holzman L. B. Nephrin ectodomain engagement results in src kinase activation, nephrin phosphorylation, nck recruitment, and actin polymerization. J. Clin. Invest. (2006);116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones N., Blasutig I. M., Eremina V., Ruston J. M., Bladt F., Li H., Huang H., Larose L., Li S. S., Takano T., Quaggin S. E., Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. (2006);440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 17.Uchida K., Suzuki K., Iwamoto M., Kawachi H., Ohno M., Horita S., Nitta K. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. (2008);73:926–932. doi: 10.1038/ki.2008.19. [DOI] [PubMed] [Google Scholar]
- 18.Ren Z., Liang W., Chen C., Yang H., Singhal P. C., Ding G. Angiotensin II induces nephrin dephosphorylation and podocyte injury: Role of caveolin-1. Cellular Signalling. (2012);24:443–450. doi: 10.1016/j.cellsig.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y., Guo L., Blattner S. M., Mundel P., Kretzler M., Wu C. Formation and phosphorylation of the PINCH-1–integrin linked kinase-α-parvin complex are important for regulation of renal glomerular podocyte adhesion, architecture, and survival. J. Am. Soc. Nephrol. (2005);16:1966–1976. doi: 10.1681/ASN.2004121112. [DOI] [PubMed] [Google Scholar]
- 20.Jung K. Y., Chen K., Kretzler M., Wu C. TGF-b1 Regulates the PINCH-1-Integrin-Linked Kinase-α-Parvin Complex in Glomerular Cells. J. Am. Soc. Nephrol. (2007);18:66–73. doi: 10.1681/ASN.2006050421. [DOI] [PubMed] [Google Scholar]
- 21.Humphries M. J. Cell adhesion assays. Methods Mol. Biol. (2009);522:203–210. doi: 10.1007/978-1-59745-413-1_14. [DOI] [PubMed] [Google Scholar]


