Abstract
Sulforaphane [1-isothiocyanato-4-(methylsulfinyl)-butane] is an isothiocyanate found in some cruciferous vegetables, especially broccoli. Sulforaphane has been shown to display anti-cancer properties against various cancer cell lines. Matrix metalloproteinase-9 (MMP-9), which degrades the extracellular matrix (ECM), plays an important role in cancer cell invasion. In this study, we investigated the effect of sulforaphane on 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced MMP-9 expression and cell invasion in MCF-7 cells. TPA-induced MMP-9 expression and cell invasion were decreased by sulforaphane treatment. TPA substantially increased NF-κB and AP-1 DNA binding activity. Pre-treatment with sulforaphane inhibited TPA-stimulated NF-κB binding activity, but not AP-1 binding activity. In addition, we found that sulforaphane suppressed NF-κB activation, by inhibiting phosphorylation of IκB in TPA-treated MCF-7 cells. In this study, we demonstrated that the inhibition of TPA-induced MMP-9 expression and cell invasion by sulforaphane was mediated by the suppression of the NF-κB pathway in MCF-7 cells. [BMB Reports 2013; 46(4): 201-206]
Keywords: Invasion, MCF-7, MMP-9, NF-κB, Sulforaphane
INTRODUCTION
Sulforaphane [1-isothiocyanato-4-(methylsulfinyl)-butane] is one of the most abundant isothiocyanates in some cruciferous vegetables, especially broccoli (1). Sulforaphane has been shown to display anti-cancer properties against various cancer cell lines (2-4). In recent studies, NF-κB was reported to be a molecular target for sulforaphane mediated cancer chemoprevention through anti-inflammatory mechanisms (5-9). Sulforaphane has also been reported to inhibit the cell-mediated immune response in B16F-10 melanoma-induced metastasis-bearing C57BL/6 mice (10,11).
Breast cancer is one of the leading causes of malignancy-related death in women (12). Most cases of breast cancer death are caused by distant metastasis from the primary tumor site. Despite successful treatment of the primary malignancy, relapse and subsequent metastatic spread can still occur at other areas of the body, through the bloodstream or lymphatic channels. This leads to local, regional or distant metastasis, including bone, lung, liver, kidney, thyroid and brain (13). This process requires degradation of the extracellular matrix (ECM) and basement membranes, which provides biochemical and mechanical barriers to cell movement in cancer cells (14). Many studies have assessed matrix metalloproteinase (MMP) expression correlated with invasive ability or metastatic potential of tumor (15), and the many factors that influence the transcription of MMP genes include cytokines, growth factors and phorbol ester (16). Among the MMPs, MMP-9 is a key enzyme for degrading type IV collagen, which is localized in the basement membrane (17). Elevated MMP-9 levels are functionally linked with elevated metastasis in many tumors (18,19), and MMP-9-deficient mice exhibit suppression of metastasis (20).
Cytokine and phorbol-ester treatments have been shown to MMP-9 expression, via activation of transcription factors such as NF-κB and activator protein-1 (AP-1) (15-17). Also, the mitogen-activated protein kinase (MAPK) signaling pathway is important for NF-κB activation and AP-1 activation (15,18). Consequently, MMP-9 expression and/or its upstream regulatory pathways would be critical targets in treating malignant tumors, including breast cancer. Therefore, we hypothesized that sulforaphane may have anticancer properties, by inhibiting MMP-9 expression and cell invasion in breast cancer cells.
Our results demonstrated that sulforaphane suppresses TPA-induced MMP-9 expression through inhibition of the NF-κB, which occurs by blocking IκB phosphorylation, and the suppression of MMP-9 expression correlated well with its ability to inhibit cell invasion.
RESULTS
Effect of sulforaphane on MCF-7 cell viability
In order to investigate the cytotoxicity of sulforaphane on MCF-7 cells, the cells were seeded into plate at a density of 1×105 cells/plate. MCF-7 cellular toxicity was analyzed using the MTT assay. Treatment of MCF-7 cells with (0.5-5 μM) sulforaphane for 24 h did not cause any significant change in cell viability (Fig. 1B). But, the pro-survival effect of TPA did not show significant at treatment with sulforaphane (1 μM and 5 μM) (Fig. 1C), indicating that sulforaphane does not affect cell proliferation. Therefore, we performed experiments at the optimal non-toxic sulforaphane concentration (1 μM and 5 μM).
Fig. 1. Structure of sulforaphne and effect of sulforaphane on cell viability of MCF-7 viability. Chemical structure of sulforaphane (A). To examine the cytotoxicity of sulforaphane, cells were cultured in 96-well plates up to a confluency of 70%, and various concentrations of sulforaphane were added to cells for 24 h (B). MTT assay was used to detect the viability of the cells. Cells were pretreated with 1 μM and 5 μM sulforaphanes for 1 h, before incubation with 100 nM TPA for 24 h (C). The optical density of the control was regarded as 100%. Data points are the mean ± SEM of three independent experiments.
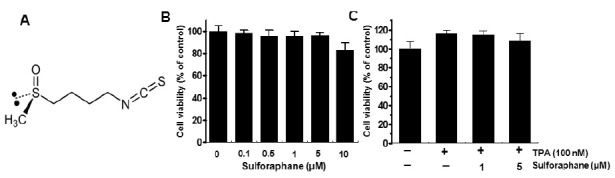
Effect of sulforaphane on TPA-induced MMP-9 expression in MCF-7 cells
To investigate the effect of sulforaphane on TPA-induced MMP-9 expression, we performed western blot analysis, real-time PCR, and zymography in MCF-7 cells. Western blot analysis/real-time PCR/zymography revealed that sulforaphane blocked the up-regulation of TPA-induced MMP-9 protein/mRNA expression and secretion (Fig. 2A-C). These results indicate that sulforaphane is a potent inhibitor of TPA-induced MMP-9 expression and secretion in MCF-7 cells.
Fig. 2. Sulforaphane inhibits TPA-induced MMP-9 expression in MCF-7 cells. MCF-7 cells were treated with the indicated sulforaphane concentrations in the presence of TPA for 24 h. Cell lysates were analyzed by western blot analysis with anti-MMP-9. The blot was reprobed with anti-β-actin to confirm equal loading (A). MMP-9 mRNA levels were analyzed by real-time PCR, and GAPDH was used as an internal control (B). Conditioned medium was prepared, and used for gelatin zymography (C). Each value represents the mean ± SEM of three independent experiments. *P < 0.01 vs. TPA.
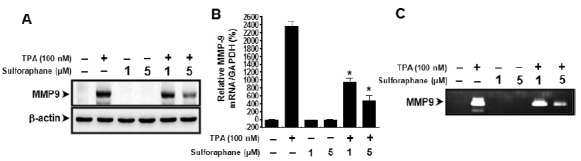
Effect of sulforaphane on TPA-induced NF-κB and AP-1 activation
To determine the mechanism of sulforaphane-mediated inhibition of MMP-9 expression, the effects of sulforaphane on TPA-induced activation of NF-κB and AP-1 were evaluated using EMSA and western blot analysis. As shown in Fig. 3A, TPA substantially increased NF-κB and AP-1 binding activity. Pre-treatment with sulforaphane inhibited TPA-stimulated NF-κB binding activity, but not AP-1 binding activity. Sulforaphane itself had no effect on NF-κB and AP-1 binding activity. In addition, we determined the levels of p65, p50 and p-c-Jun in the nuclear fraction. TPA treatment resulted in increase of p65, p50 and p-c-Jun. However, sulforaphane blocked the TPA-induced translocation of NF-κB to the nucleus (Fig. 3B). These results suggest that sulforaphane specifically blocked NF-κB activation in MCF-7 cells. The IκB Kinase (IKK) enzyme complexs are part of the upstream NF-κB signal transduction cascade (19). IKK phosphorylation results in the dissociation of IκB from NF-κB, and thereby activates NF-κB. Therefore, we examined the effect of TPA and sulforaphane treatment on the p-IKKαβ, p-IκBα levels in the cytoplamic fraction. TPA-stimulated MCF-7 cells showed increased levels of p-IKKαβ and p-IκBα level. The p-IκBα level was significantly suppressed by treatment with sulforaphane, but not p-IKKαβ (Fig. 3C).
Fig. 3. Sulforaphane blocks TPA-induced NF-κB activation in MCF-7 cells. Cells were treated with sulforaphane in the presence of TPA. Following 3 h incubation, nuclear extracts were prepared as described in the Materials and Methods. NF-κB/AP-1 DNA binding was analyzed by EMSA, as described in Materials and Methods (A). Nuclear/cytoplasm extracts were subjected to western blot analysis (B, C). Cell extracts were prepared from MCF-7 cells with TPA for 15 min in the absence or presence of sulforaphane, and subjected to western blotting (D).
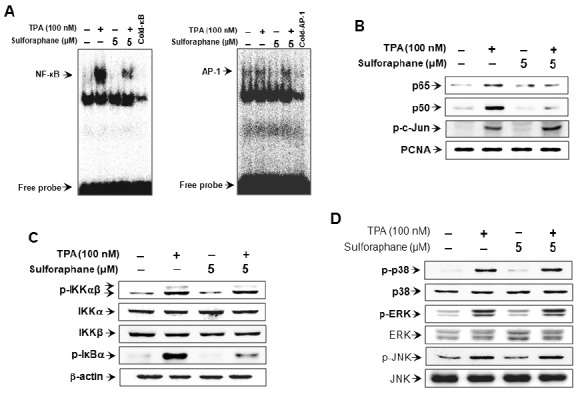
Effect of sulforaphane on TPA-induced MAPKs signaling pathway
MAPKs (ERK, p38 and JNK) have been shown to be upstream modulators of NF-κB and AP-1, which results in the activation of MMP-9 expression (20,21). Thus, we investigated the effect of sulforaphane on TPA-induced activation of MAPKs. Sulforaphane showed no effect on the MAPKs (Fig. 3D). These results suggest that the MAPK pathway is not involved in the regulation of TPA-induced MMP-9 expression by sulforaphane.
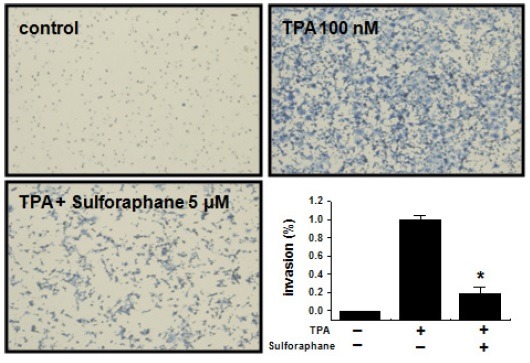
Effect of sulforaphane on TPA-induced MCF-7 cell invasion in vitro
It has been reported that the up-regulation of MMP-9 expression contributes to the invasion of cancer cells (22,23). An in vitro invasion assay was used to investigate the effects of sulforaphane on the invasive potency of breast carcinoma MCF-7 cells. TPA treatment increased MCF-7 cell invasion when compared with untreated control cells, as determined by a Matrigel invasion assay. However, sulforaphane inhibited TPA-induced MCF-7 cell invasion by 80% (Fig. 4).
Fig. 4. Effect of sulforaphane on TPA-induced Matrigel invasion in MCF-7 cells. Cells were seeded onto the upper chamber, and TPA and sulforaphane placed in the well. Each value represents the mean ± SEM of three independent experiments. *P < 0.01 vs. TPA.
DISCUSSION
In this study, we demonstrated that sulforaphane inhibited TPA-induced MMP-9 expression and cell invasion in MCF-7 cells. Furthermore, sulforaphane strongly blocked TPA-mediated activation of NF-κB, but not AP-1, in MCF-7 cells. These findings suggest that the inhibition of TPA-induced MMP-9 expression and cell invasion by sulforaphane is mediated by the suppression of the NF-κB pathway in MCF-7 cells.
Recent studies have clearly demonstrated that the action of sulforaphane involves multiple targets. Early research focused on Phase 2 enzyme induction by sulforaphane, as well as inhibition of enzymes involved in carcinogen activation, but recent studies have identified other activities of sulforaphane, including chemoprotection and anti-inflammation (6,7,11,24,25). Previous studies have demonstrated that NF-κB is a molecular target in sulforaphane treated cells (5-7,18,19). These results indicate that sulforaphane can affect proliferation signals and apoptotic signals, via modulation of NF-κB activity.
Globally, breast cancer is the main cause of death from cancer in women. Metastasis is the primary cause of breast cancer mortality. Tumor metastasis is a multistep process in a complex process that includes cell proliferation, ECM degradation, cell migration, and tumor growth at metastatic sites (15,26). Morphologically, tumor invasion is associated with a distorted edge of the primary tumor, where individual or cohorts of tumor cells actively invade the tissue surrounding ECM tissue (27).
MMP-9 has been regarded as major critical molecule in processing tumor invasion and metastasis. MMP-9 activation has been shown to be associated with tumor progression and invasion, including mammary tumors (28). In previous reports, inflammatory cytokines, growth factors, or phorbol esters were shown to stimulate MMP-9 by activating different intracellular-signaling pathways in breast cancer cells (29-31). The PKCs can be activated by phorbol esters in vitro, and TPA acts as a potential inducer of tumor invasion and migration in various tumor cells. Up-regulation and activation of PKCs are highly correlated with an increased invasiveness in breast carcinomas (32-34). The inhibitory effects on expression are important for the development of a therapeutic experimental model of tumor metastasis.
The three major MAPKs families, JNK, ERK, and p38 kinase, are expressed, and the active phosphorylated forms of these proteins have been detected in MCF-7 cells (12). The results of the present study suggest that sulforaphane does not inhibit the phosphorylation of MAPKs in TPA-mediated signaling pathways. These findings suggest that sulforaphane is not involved in the TPA-stimulated MAPKs pathway.
NF-κB is a transcription factor that regulates MMP-9 expression binding sites on its promoter (35,36). NF-κB comprises a family of inducible transcription factors that regulate host inflammatory and immune responses. Diverse signal transduction cascades mediate NF-κB pathway stimulation (37). NF-κB is an inducible dimeric transcription factor that belongs to the Rel/NF-κB family of transcription factors, and consists of two major polypeptides, p65 and p50 (38). NF-κB is initially located in the cytoplasm in an inactive form complexed with IκB, an inhibitory factor of NF-κB. NF-κB elements are centrally involved in MMP-9 gene induction by TPA (15,16,39). Our results show that sulforaphane inhibited MMP-9 expression by suppression of NF-κB in breast carcinoma cells.
In this study, we identified the molecular mechanisms of the MAPKs, NF-κB and AP-1 signal pathways in breast cancer cells responsible for the sulforaphane inhibitory effect. Our results have demonstrated that sulforaphane is a potent inhibitor of TPA-induced MMP-9 expression, and strongly blocks the NF-κB signaling pathway in breast carcinoma cells. This is the first study demonstrating that sulforaphane suppresses TPA-stimulated cancer cell invasion by inhibiting MMP-9 expression. Thus, sulforaphane may be a potential candidate in the development of novel therapeutics to preventing breast tumor invasion and metastasis in vivo.
MATERIALS AND METHODS
Cells and chemicals
MCF-7 cells were purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in high glucose-containing Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics, at 37℃ in a 5% CO2 incubator. Sulforaphane was obtained from Sigma (St. Louis, MO. USA). 12-O-tetradecanoylphorbol-13-acetate (TPA) and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and anti-β-actin and toluidine were obtained from Sigma (St. Louis, MO, USA). Primary p-c-Jun, p-IκBα, p-IKKαβ, p38, p-p38, JNK, p-JNK, ERK, and p-ERK antibodies were from Cell Signaling Technology (Beverly, MA, USA). MMP-9, p50, p65, IKKα, IKKβ, IκBα, PCNA, and Horseradish peroxidase (HRP)-conjugated IgG were from Santa Cruz Biotechnology (Santa Cruz, CA. USA). [α-32P]dCTP was purchased from Amersham (Buckinghamshire, UK). DMEM, FBS and phosphate-buffered saline (PBS) were obtained from Gibco-BRL (Gaithersburg, ME, USA).
Determination of cell viability
The effect of sulforaphane on MCF-7 cell viability was determined using an MTT assay. After incubation, the cells were treated with MTT (0.5 mg/ml) solution for 30 min. The dark blue formazan crystals were solubilized with DMSO (100 μl/well), and detected at 570 nm using a microplate reader (Model 3550, BIO-RAD, Richmond, CA, USA).
Western blot analysis
After treatment, cells were collected and washed with PBS. The protein concentration in the lysate was determined using the Bradford method (40). The samples (20 μg) of total cell lysates or cytosolic fractions/nuclear fractions were separated by 10% SDS-PAGE. The PVDF membranes were blocked with 5% bovine serum albumin or 5% skim milk, and then incubated overnight with 1 μg/ml primary antibodies. HRP-conjugated IgG was used as a secondary antibody. Protein expression levels were determined by signal analysis using an image analyzer (Fuji-Film, Japan).
Gelatin zymography assay
The enzyme activity of MMP-9 was determined by gelatin zymography. Conditioned media were collected after 24 h stimulation, mixed with non-reducing sample buffer, and electrophoresed using a polyacrylamide gel containing 0.1% (w/v) gelatin. The gel was washed at room temperature for 30 min with 2.5% Triton X-100 solution, and subsequently incubated at 37℃ for 16 h in 5 mM CaCl2, 0.02% Brij, and 50 mM Tris–HCl (pH7.5). The gel was stained for 30 min with 0.25% (w/v) Coomassie brilliant blue in 40% (v/v) methanol/7% (v/v) acetic acid, and photographed on an image analyzer (Fuji-Film, Japan). Proteolysis was assessed based on the white zone in a dark blue field.
Quantitative real-time PCR assay
Total RNA was isolated with a FastPureTM RNA Kit (TaKaRa, Shiga, Japan). RNA concentration and purity were determined by absorbance at 260/280 nm. 1 μg total RNA was converted to cDNA using a PrimeScriptTM RT reagent Kit (TaKaRa, Shiga, Japan). MMP-9 and GAPDH mRNA expression were determined by real-time PCR using the ABI PRISM 7900 sequence detection system and SYBRⓇ Green (Applied Biosystems, Foster City, CA, USA). The primers were: MMP-9 (NM004994) sense, CCTGGAG ACCTGAGAACCAATCT; antisense, CCACCCGAG TGTA ACCATAGC and GAPDH (NM002046) sense, ATGGA AATCCCATCACCATCTT; antisense, CGCCCCACTTG ATTTT GG. To control for variation in mRNA concentration, all results were normalized to the GAPDH, a housekeeping gene. Relative quantitation was performed using the comparative ΔΔCt method, according to the manufacturer’s instructions.
Preparation of nuclear extract
Cytoplasmic and nuclear extracts were prepared using the NE-PERⓇ Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL), according to the manufacturer’s instructions.
Electrophoretic mobility shift assay (EMSA)
Labeled [α-32P]dCTP oligonucleotides (10,000 cpm), 10 μg of nuclear extracts, and binding buffer (10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly )undefined(dI•dC), 1 mM DTT) were then incubated for 30 min at room temperature in a final volume of 20 μl. The reaction mixtures were analyzed by electrophoresis on 4% polyacrylamide gels in 0.5X Tris-borate buffer. The gels were dried and examined by autoradiography. The oligonucleotide sequences of NF-κB and AP-1 probe were those from human MMP-9 promoter; NF-κB, 5’-CCGGTTAACAGAGGGGGCTTTCCGAG-3’ and AP-1, 5’-C GCTTGATGAGTCAGCCGGAA-3’. The oligomers were synthesized, and used as a probe in the gel retardation assay. Specific binding was controlled by competition, with a 50-fold excess of cold κB and AP-1 oligonucleotide.
Invasion assay
Cells (2 × 105) were added to an inner cup of a 24-well chamber (BD Biosciences) that had been coated with 20 μl matrigel (BD Biosciences, Franklin Lakes, NJ) 1:6 dilution in serum-free medium). TPA and sulforaphane were placed in the bottom well. Cells on the upper side of the chamber were removed using cotton swabs, and cells that had migrated were fixed and stained with toluidine blue solution. Invading cells were counted in five random areas of the membrane, using a light microscope. Analyzed data are, in each case, the mean ± SE from three individual experiments performed in triplicate.
Statistical analysis
Statistical data analysis was performed using ANOVA. Differences with a P < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by the research fund of Chonbuk National University in 2009, a National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (No-2011-0004671), and by a grant from the Ministry of Science & Technology (MoST)/Korea Science & Engineering Foundation (KOSEF) (M10528010003-05N2801-00310), Republic of Korea.
References
- 1.Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. (1992);89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gamet-Payrastre L., Li P., Lumeau S., Cassar G., Dupont M. A., Chevolleau S., Gasc N., Tulliez J., Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. (2000);60:1426–1433. [PubMed] [Google Scholar]
- 3.Herman-Antosiewicz A., Johnson D. E., Singh S. V. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. (2006);66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 4.Parnaud G., Li P., Cassar G., Rouimi P., Tulliez J., Combaret L., Gamet-Payrastre L. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr. Cancer. (2004);48:198–206. doi: 10.1207/s15327914nc4802_10. [DOI] [PubMed] [Google Scholar]
- 5.Heiss E., Herhaus C., Klimo K., Bartsch H., Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. (2001);276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 6.Woo K. J., Kwon T. K. Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int. Immunopharmacol. (2007);7:1776–1783. doi: 10.1016/j.intimp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Xu C., Shen G., Chen C., Gelinas C., Kong A. N. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through Ikappa-Balpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. (2005);24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 8.Song M. Y., Kim E. K., Moon W. S., Park J. W., Kim H. J., So H. S., Park R., Kwon K. B., Park B. H. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol. Appl. Pharmacol. (2009);235:57–67. doi: 10.1016/j.taap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Wang X. F., Wu D. M., Li B. X., Lu Y. J., Yang B. F. Synergistic inhibitory effect of sulforaphane and 5-fluorouracil in high and low metastasis cell lines of salivary gland adenoid cystic carcinoma. Phytother. Res. (2009);23:303–307. doi: 10.1002/ptr.2618. [DOI] [PubMed] [Google Scholar]
- 10.Thejass P., Kuttan G. Modulation of cell-mediated immune response in B16F-10 melanoma-induced metastatic tumor-bearing C57BL/6 mice by sulforaphane. Immunopharmacol. Immunotoxicol. (2007);29:173–186. doi: 10.1080/08923970701511728. [DOI] [PubMed] [Google Scholar]
- 11.Chen X. L., Dodd G., Kunsch C. Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm. Res. (2009);58:513–521. doi: 10.1007/s00011-009-0017-7. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A., Murray T., Ward E., Samuels A., Tiwari R. C., Ghafoor A., Feuer E. J., Thun M. J. Cancer statistics, 2005. CA. Cancer J. Clin. (2005);55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Friedel G., Pastorino U., Ginsberg R. J., Goldstraw P., Johnston M., Pass H., Putnam J. B., Toomes H. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur. J. Cardiothorac. Surg. (2002);22:335–344. doi: 10.1016/S1010-7940(02)00331-7. [DOI] [PubMed] [Google Scholar]
- 14.Woessner J. F., Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. (1991);5:2145–2154. [PubMed] [Google Scholar]
- 15.Chung T. W., Moon S. K., Chang Y. C., Ko J. H., Lee Y. C., Cho G., Kim S. H., Kim J. G., Kim C. H. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. (2004);18:1670–1681. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 16.Hong S., Park K. K., Magae J., Ando K., Lee T. S., Kwon T. K., Kwak J. Y., Kim C. H., Chang Y. C. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J. Biol. Chem. (2005);280:25202–25209. doi: 10.1074/jbc.M413985200. [DOI] [PubMed] [Google Scholar]
- 17.Woo M. S., Jung S. H., Kim S. Y., Hyun J. W., Ko K. H., Kim W. K., Kim H. S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. (2005);335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 18.Karin M., Liu Z., Zandi E. AP-1 function and regulation. Curr. Opin. Cell Biol. (1997);9:240–246. doi: 10.1016/S0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 19.Crawford H. C., Matrisian L. M. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. (1996);49:20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- 20.Chambers A. F., Matrisian L. M. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. (1997);89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 21.Stetler-Stevenson W. G., Hewitt R., Corcoran M. Matrix metallo-proteinases and tumor invasion: from correlation and causality to the clinic. Semin. Cancer Biol. (1996);7:147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 22.Clarke J. D., Dashwood R. H., Ho E. Multi- targeted prevention of cancer by sulforaphane. Cancer Lett. (2008);269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y. C., Hsieh C. W., Weng Y. C., Chuang S. H., Hsieh C. Y., Wung B. S. Sulforaphane inhibition of monocyte adhesion via the suppression of ICAM-1 and NF-kappaB is dependent upon glutathione depletion in endothelial cells. Vascul. Pharmacol. (2008);48:54–61. doi: 10.1016/j.vph.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Choi S., Lew K. L., Xiao H., Herman-Antosiewicz A., Xiao D., Brown C. K., Singh S. V. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. (2007);28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- 25.Jeong W. S., Kim I. W., Hu R., Kong A. N. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm. Res. (2004);21:661–670. doi: 10.1023/B:PHAM.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 26.Lee S. O., Jeong Y. J., Kim M., Kim C. H., Lee I. S. Suppression of PMA-induced tumor cell invasion by capillarisin via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem. Biophys. Res. Commun. (2008);366:1019–1024. doi: 10.1016/j.bbrc.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 27.Deryugina E. I., Quigley J. P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. (2006);25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 28.Scorilas A., Karameris A., Arnogiannaki N., Ardavanis A., Bassilopoulos P., Trangas T., Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br. J. Cancer. (2001);84:1488–1496. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho H. J., Kang J. H., Kwak J. Y., Lee T. S., Lee I. S., Park N. G., Nakajima H., Magae J., Chang Y. C. Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ ERKand Ap1-dependent mechanisms. Carcinogenesis. (2007);28:1104–1110. doi: 10.1093/carcin/bgl217. [DOI] [PubMed] [Google Scholar]
- 30.Kajanne R., Miettinen P., Mehlem A., Leivonen S. K., Birrer M., Foschi M., Kahari V. M., Leppa S. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J. Cell Physiol. (2007);212:489–497. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A. K., Qin X., Wedhas N., Arnush M., Linkhart T. A., Chadwick R. B., Kumar A. Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J. Biol. Chem. (2007);282:35113–35124. doi: 10.1074/jbc.M705329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton A. C. Protein kinase C: structure, function, and regulation. J. Biol. Chem. (1995);270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 33.Datta R., Yoshinaga K., Kaneki M., Pandey P., Kufe D. Phorbol ester-induced generation of reactive oxygen species is protein kinase cbeta-dependent and required for SAPK activation. J. Biol. Chem. (2000);275:41000–41003. doi: 10.1074/jbc.M009322200. [DOI] [PubMed] [Google Scholar]
- 34.Schlingemann J., Hess J., Wrobel G., Breitenbach U., Gebhardt C., Steinlein P., Kramer H., Furstenberger G., Hahn M., Angel P., Lichter P. Profile of gene expression induced by the tumour promotor TPA in murine epithelial cells. Int. J. Cancer. (2003);104:699–708. doi: 10.1002/ijc.11008. [DOI] [PubMed] [Google Scholar]
- 35.Eberhardt W., Huwiler A., Beck K. F., Walpen S., Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J. Immunol. (2000);165:5788–5797. doi: 10.4049/jimmunol.165.10.5788. [DOI] [PubMed] [Google Scholar]
- 36.Kim H. S., Kim H. J., Park K. G., Kim Y. N., Kwon T. K., Park J. Y., Lee K. U., Kim J. G., Lee I. K. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp. Mol. Med. (2007);39:106–113. doi: 10.1038/emm.2007.12. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y., Gaynor R. B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest. (2001);107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanos D., Maniatis T. NF-kappa B: a lesson in family values. Cell. (1995);80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 39.Shin Y., Yoon S. H., Choe E. Y., Cho S. H., Woo C. H., Rho J. Y., Kim J. H. PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp. Mol. Med. (2007);39:97–105. doi: 10.1038/emm.2007.11. [DOI] [PubMed] [Google Scholar]
- 40.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. (1976);72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]


