Abstract
Cecropin A and papiliocin are novel 37-residue cecropin-like antimicrobial peptides isolated from insect. We have confirmed that papiliocin possess high bacterial cell selectivity and has an α-helical structure from Lys3 to Lys21 and from Ala25 to Val35, linked by a hinge region. In this study, we demonstrated that both peptides showed high antimicrobial activities against multi-drug resistant Gram negative bacteria as well as fungi. Interactions between these cecropin-like peptides and phospholipid membrane were studied using CD, dye leakage experiments, and NMR experiments, showing that both peptides have strong permeabilizing activities against bacterial cell membranes and fungal membranes as well as Trp2 and Phe5 at the N-terminal helix play an important role in attracting cecropin-like peptides to the negatively charged bacterial cell membrane. Cecropin-like peptides can be potent peptide antibiotics against multi-drug resistant Gram negative bacteria and fungi. [BMB Reports 2013; 46(5): 282-287]
Keywords: Antimicrobial peptide, Cecropin A, NMR spectroscopy, Papiliocin, Structure
INTRODUCTION
All living organisms including mammals, insects, amphibians, and plants produce antimicrobial peptides (AMPs) which are important components of the innate immune systems (1). Insects are extremely resistant to bacterial infections (2,3). AMPs are important in the innate immune systems of insects for their defense. A majority of such defensive peptides in insects are produced in either their fat body or haemocytes and then released into their haemolymph (2,3). To date, more than 200 AMPs have been identified in insects. Most of these peptides are membrane-active peptides which rapidly kill invading pathogens by causing membrane permeabilization (4). Since the isolation of cecropin A from the moth Hyalophora cecropia (5), more than 200 AMPs have been characterized from various insect species.
Among the insect-derived AMPs, the first α-helical form discovered was cecropin, which was found to contribute an indispensable proportion of the innate immunity of insects (6). Cecropin-like peptides constitute a large family of cationic α-helical AMPs that are active against bacteria and fungi (7). The mechanism by which these peptides exercise their bactericidal effect is not yet fully understood but is thought to involve the formation of pores, leading to depolarization of the bacterial membrane. Cecropin-like peptides are composed of 35-39 amino acids and were isolated from Bombyx mori (8), Drosophila melanogaster (9), Musca domestica (10), Acalolepta luxuriosa (11), Helicoverpa armigera (12), and Papilio xuthus (13).
We have determined the tertiary structure of papiliocin, a 37-residue cecropin-like peptide (RWKIFKKIEKVGRNVRDGIIK AGPAVAVVGQAATVVK-NH2) isolated from the larvae of the swallowtail butterfly, Papilio xuthus and also proved that papiliocin exhibit high anti-inflammatory activity which is comparable to that of LL-37 (14). Similar to other cecropins, papiliocin consist of an N-terminal amphipathic α-helix linked to a more hydrophobic C-terminal α-helix by a hinge region.
In this study, we compared the structures and antimicrobial activities of cecropin–like peptides, cecropin A and papiliocin. Even though papiliocin exhibits a 78.4% sequence homology with cecropin A from the giant silk moth, Hyalophora cecropia (KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK-NH2) (15), papiliocin demonstrated a little higher antibacterial activity compared to cecropin A. We investigated, for the first time, their antimicrobial activities against multi-drug resistant bacteria and various fungi to verify their potency as potent antibiotics. Furthermore, we investigated their ability to permeate model phospholipid membranes which mimic the bacterial membrane as well as the fungal membrane and investigated its modes of action using nuclear magnetic resonance (NMR) spectroscopy and Fluorescence spectroscopy.
RESULTS
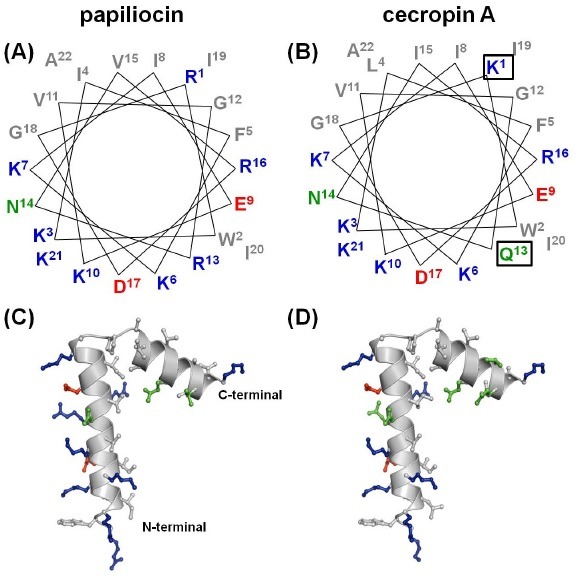
Papiliocin and cecropin A were synthesized as described. Papiliocin has a net charge of +8 and hydrophobicity of −1.48, whereas cecropin A has a net charge of +7 and hydrophobicity of −1.37, calculated according to the combined consensus scale (CCS) (16). The NMR structure of cecropin A in 15% (v/v) hexafluoroisopropyl (HFIP) alcohol has been determined by Holak et al., showing that cecropin A has two helical regions extending from residues 5 to 21 and from residues 24 to 37 (17). We have determined the three-dimensional structure of papiliocin in 300 mM DPC micelles by NMR spectroscopy, showing that papiliocin has an α-helical structure from Lys3 to Lys21 and from Ala25 to Val35, linked by a hinge region (14). Therefore, the structure of papiliocin in DPC micelles has a longer N-terminal helix and shorter C-terminal helix compared to the structure of cecropin A determined in HFIP organic solvent. However, the coordinate of cecropin A is not available since it was not deposited in the Protein Data Bank (PDB) in 1988 (17). Therefore, for comparison, the model structure of cecropin A was built by substitution regarding the sequence of cecropin A onto the structure of papiliocin in DPC micelles. Fig. 1 presents the helical wheel diagrams showing the N-terminal amphipathic helix and the overall structures of papiliocin and cecropin A. Arg1 in papiliocin is substituted with Lys1 in cecropin A and Lys13 is substituted with Gln13 in cecropin A as marked with the box in Fig. 1. Although the sequence identity between the two peptides is very high at 78.4% and the overall structures of both peptides are very similar, the N-terminal amphipathic helix of papiliocin is more positively charged than that of cecropin A, whereas the C-terminal helix of papiliocin is somewhat more hydrophobic than that of cecropin A.
Fig. 1. Helical-wheel diagram and ribbon diagram of papiliocin and cecropin A. Helical-wheel diagram of N-terminal helix region from (A) Arg1 to Ala22 of papiliocin and (B) Lys1 to Ala22 of cecropin A. Ribbon diagram of the lowest structures of (C) papiliocin and (D) cecropin A. Residues are color-coded as follows: gray, hydrophobic; blue, positive: red, negative; green, neutral.
Antimicrobial activity
We investigated the antifungal activities of papiliocin and cecropin A against Candida albicans, Malassezia obtuse, and Malassezia sloofiae and compared with the activities of melittin, which is known to have profound antibacterial activity as well as antifungal activities against all bacterial strains and fungi. The C. albicans is the major fungal infections in humans that can cause infections ranging from superficial mucosal infections to hematogenously disseminated candidiasis and finally lead to nosocomial bloodstream infections (18). The Malassezia species are related to several human skin diseases including seborrhoeic eczema, atopic eczema, dandruff and pityriasis versicolor, as well as systemic diseases in immunodeficient humans (19). As listed in Table 1, antifungal activities of papiliocin against C. ablicans, M. obtuse and M. sloofiae were very similar compared to those of cecropin A.
Table 1.
MICs of papiliocin and cecropin A for multidrug-resistant bacterial strains determined in the presence and absence of NaCl, CaCl2, and MgCl2 and antifungal activities the peptides against Candida and Malassezia strains
| MIC (μM) | |||||
|---|---|---|---|---|---|
|
| |||||
| Papiliocin | Cecropin A | Melittin | |||
|
| |||||
| Bacterial strains | S. typhimurium 8003 (R) | None | 0.50 | 0.50 | 8.0 |
| 150 mM NaCl | 0.50 | 0.50 | 16 | ||
| 1 mM CaCl2 | 1.0 | 1.0 | 32 | ||
| 1 mM MgCl2 | 1.0 | 1.0 | 32 | ||
| S. typhimurium 8007 (R) | None | 0.50 | 0.50 | 8.0 | |
| 150 mM NaCl | 1.0 | 1.0 | 16 | ||
| 1 mM CaCl2 | 1.0 | 1.0 | 32 | ||
| 1 mM MgCl2 | 1.0 | 1.0 | 32 | ||
| S. typhimurium 8009 (R) | None | 0.50 | 0.50 | 8.0 | |
| 150 mM NaCl | 0.50 | 0.50 | 16 | ||
| 1 mM CaCl2 | 0.50 | 0.50 | 32 | ||
| 1 mM MgCl2 | 0.50 | 0.50 | 32 | ||
| E. coli 1229 (R) | None | 1.0 | 1.0 | 4.0 | |
| 150 mM NaCl | 4.0 | 8.0 | 16 | ||
| 1 mM CaCl2 | 2.0 | 4.0 | 32 | ||
| 1 mM MgCl2 | 4.0 | 8.0 | 16 | ||
| E. coli 1238 (R) | None | 0.50 | 0.50 | 4.0 | |
| 150 mM NaCl | 0.50 | 0.50 | 8.0 | ||
| 1 mM CaCl2 | 1.0 | 1.0 | 16 | ||
| 1 mM MgCl2 | 1.0 | 1.0 | 8.0 | ||
| S.aureus 3089 (R) | None | 32 | 64 | 4.0 | |
| S.aureus 3090 (R) | None | 16 | 64 | 4.0 | |
| S.aureus 3108 (R) | None | 32 | 64 | 2.0 | |
| S.aureus 3114 (R) | None | 16 | 64 | 4.0 | |
| S.aureus 3126 (R) | None | 16 | 64 | 4.0 | |
| Fungal strains | C. albicans | None | 4.0 | 8.0 | 2.0 |
| M. obitus | None | 8.0 | 8.0 | 4.0 | |
| M. sloofiae | None | 8.0 | 8.0 | 1.0 | |
The antimicrobial activities of papiliocin and cecropin A were examined against multi-drug resistant bacterial strains and compared with the activities of melittin. Papiliocin and cecropin A were more potent than melittin against all multi-drug resistant Gram-negative bacteria tested, but were much less potent than melittin against Gram-positive bacteria. The antimicrobial activities of papiliocin were a little higher than those of cecropin A against multi-drug resistant Gram-negative bacteria, and much higher than those of cecropin A against multi-drug resistant Gram-positive bacteria.
It has been known that cecropin like peptides demonstrate high antimicrobial activities against gram negative bacteria due to the hydrophobic C-terminal helix and papiliocin shows similar MIC against Gram negative bacteria compared with cecropin A. However, papiliocin has a net charge of +6 in N-terminal helix, whereas cecropin A has a net charge of +5 in the N-terminal helix. Therefore, the N-terminal helix of papiliocin has a higher cationicity than that of cecropin A. The difference in net charge at the N-terminal helix may result in the difference in antimicrobial activity against gram positive bacteria. This might be the reason for somewhat higher antibacterial activities of papiliocin against Gram-positive bacteria compared to cecropin A.
These differences may result from differences in the net positive charge at the N-terminal helix and hydrophobicity of the two peptides.
In order to investigate the therapeutic potency of these peptides, salt resistance tests regarding papiliocin and cecropin A for three multi-drug resistant Gram-negative bacteria were determined in the presence of 150 mM NaCl, 1 mM CaCl2 and 1 mM MgCl2, which are the concentrations of these salts in human body fluids and compared to those of melittin. As summarized in Table 1, papiliocin exhibited strong antibacterial activity against all Gram-negative bacteria in 150 mM NaCl. The minimal inhibitory concentrations (MICs) of papiliocin and cecropin A against all Gram-negative bacteria increased approximately 2 to 4-fold in the presence of 1 mM CaCl2 and 1 mM MgCl2, whereas those for melittin increased substantially. The reported concentrations of calcium and magnesium in human body fluids are in the order of 1 mM (20). At this concentration of CaCl2 and MgCl2, papiliocin showed good antimicrobial activity, exhibiting an MIC of 1-2 μM against all Gram-negative bacteria. Therefore, both cecropin-like peptides are salt resistant and can be described as calcium- and magnesium-resistant against Gram-negative bacteria in a physiological environment.
Cytotoxicity against mammalian cells
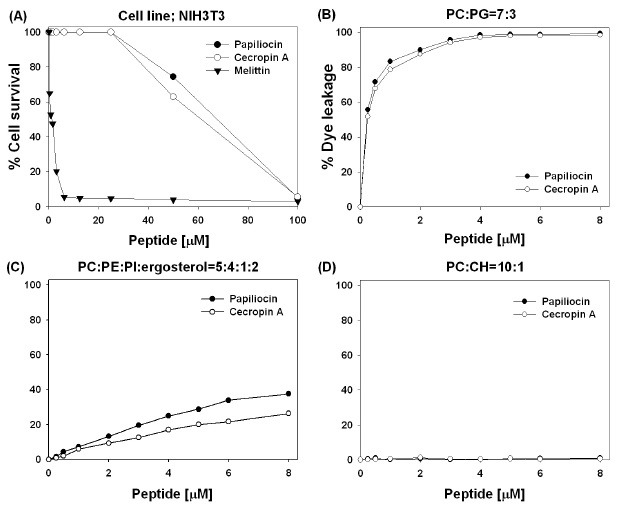
We examined the cytotoxicity of papiliocin and cecropin A against NIH 3T3 cells, assessing the effects on cell growth by measuring the mitochondrial reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to a colored product by live cells. As shown in Fig. 2A, melittin exhibited remarkable cytotoxicity at its MIC, whereas papiliocin as well as cecropin A was not toxic at all until 25 μM which is much higher than their MIC. Therefore, papiliocin and cecropin A were highly selective for bacterial cells, whereas melittin was not.
Fig. 2. (A) Dose-response curves for the cytotoxicity of papiliocin (●), cecropin A (○), and melittin (▼) toward NIH 3T3 cells. Doseesponse curves of calcein leakage from (B) EYPC/EYPG (7:3, w/w), (C) EYPC/EYPE/EYPI/ergosterol (5:4:1:2, w/w) and (D) EYPC/CH LUVs (10:1, w/w) induced by papiliocin and cecropin A. The lipid concentration used in the leakage experiments was 64 μM.
Peptide-induced permeabilization of lipid vesicles
In order to investigate the mechanism of action for papiliocin and cecropin A, we measured the membrane-permeabilizing ability of papiliocin by monitoring the release of the fluorescent marker, calcein, from large unilamellar vesicles (LUVs) with different composition. We employed zwitterionic LUVs composed of 10:1 (w/w) egg yolk L-α-phosphatidylcholine (EYPC)/cholesterol (CH), negatively charged LUVs composed of 7:3 (w/w) EYPC/egg yolk L-α-phosphatidylglycerol (EYPG), and negatively charged LUVs composed of 5:4:1:2 (w/w) EYPC/egg yolk L-α-phosphatidylethanolamine (EYPE)/egg yolk L-α-phosphatidylinositol (EYPI)/ergosterol. The percentage of calcein leakage 2 min after exposure to the peptide was used as a measure of membrane permeability. The dose-response curves of peptide-induced calcein release presented in Fig. 2 show that papiliocin as well as cecropin A permeated negatively charged vesicles, which mimic bacterial cell component membranes, much more effectively than zwitterionic vesicles, which mimic the major components of the outer leaflet of human erythrocytes. In EYPC/EYPE/EYPI/ergosterol (5:4:1:2, w/w) which mimic the fungal membrane, papiliocin permeated more effectively than cecropin A. Therefore, dye leakage data as well as cytotoxicity data imply that these cecropin-like peptides are cell selective.
Circular dichroism (CD) measurements
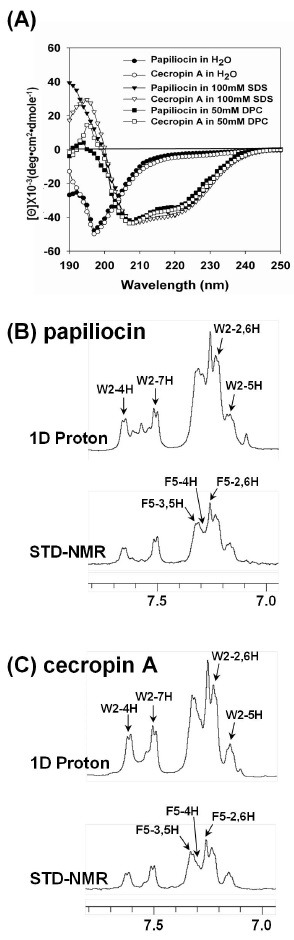
To investigate the secondary structures of papiliocin and cecropin A in membrane-like environments, we analyzed the CD spectra of the peptide dissolved under a variety of membrane-mimicking conditions. As shown in Fig. 3A, the peptides demonstrated unordered structures in aqueous solution, but exhibited conformational changes in dodecylphosphocholine (DPC) micelles and sodium dodecyl sulfate (SDS) micelles. In all membrane-mimetic environments, papiliocin and cecropin A exhibited characteristic double negative maxima at 205 nm and 220 nm, suggesting that they adopt a significant degree of α helical structure under these conditions. We also analyzed the possibility of oligomerization of papiliocin as well as cecropin. At a concentration from 50 to 400 uM, two characteristic negative ellipticities at 208 and 222 nm were observed, indicating the presence of an α-helical secondary structure. If there is an increased degree of oligomerization, the CD spectrum should give rise to a stronger α-helical structure since the molar residue ellipticity (degㆍcm2/dmol) at 222 nm is a negatively increased intensity as concentration of the peptide was increased from 50 μM to 400 μM. CD spectra of papiliocin and cecropin at concentrations from 50 μM to 400 μM showed very similar patterns of CD spectra and very similar molar ellipticity at all wavelengths, implying that papiliocin and cecropin A do not aggregate from 50 μM to 400 μM.
Fig. 3. CD spectra and STD-NMR spectra. (A) CD spectra of peptides (50 μM, pH 4.1) in H2O, 100 mM SDS micelles, 50 mM DPC micelles (B) 1H NMR spectra of papiliocin and STD-NMR results in aromatic region, showing the interaction between papiliocin and EYPC/EYPG SUVs and (C) 1H NMR spectra of cecropin A and STD-NMR results in aromatic region, showing the interaction between cecropin A and EYPC/EYPG SUVs. Spectral differences primarily constituted resonances belonging to peptide protons bound to SUVs.
NMR studies of peptides bound to SUVs
In order to investigate the mode of actions for papiliocin and cecropin A, interaction with 0.1 mM EYPC/EYPG small unilamellar vesicles (SUVs) which mimic the bacterial cell membrane, we performed saturation transfer difference (STD)-NMR experiments to identify residues close to the SUVs. Fig. 3B illustrates the one-dimensional spectrum of free papiliocin and the STD spectrum of papiliocin bound to SUVs in D2O and Fig. 3C shows those of cecropin A. The intermolecular saturation transfer between the peptide and the SUVs resulted in a difference regarding the intensity of resonances between SUV-bound peptides and free peptides. Enhancements are observed for the resonance of protons in close contact with the peptide exhibiting the phospholipid membrane. This allows direct observation of areas of the peptide that comprise the epitope. The STD effect was observed for the aromatic ring protons of Trp2 and Phe5 in cecropin A as well as papiliocin, indicating their close contacts with SUVs. The individual signal protons of Trp2 and Phe5 in papiliocin as well as cecropin A were analyzed from the intensity values in the reference 1H 1D spectra and STD spectra. For these aromatic protons in cecropin A as well as papiliocin, intensity ratios ranged from 20 to 30%. For 7H of Trp2 in papiliocin, the intensity ratio was 30%. These data confirmed that Trp2 and Phe5 in the N-terminal helix play important roles in interacting with the bacterial cell membrane.
DISCUSSION
In this study, we demonstrated that papiliocin and cecropin A are potent therapeutic agents against multi-drug resistant Gram-negative bacteria. To study the mechanism of cecropin-like peptide antibacterial actions as well as antifungal action, we investigated interactions between peptide and bacterial cell membranes as well as fungal cells and their mode of actions. Papiliocin showed a little higher antibacterial activity against multi-drug resistant Gram-negative bacterial strains compared to cecropin A while papiliocin demonstrated much higher activities against Gram-positive multi-drug resistant bacterial strains. Both peptides have bacterial cell selectivities with no cytotoxicity against NIH 3T3 cells at 25 uM (Fig. 2A). All antimicrobial activities implied that higher cationicity of papiliocin resulted in a little improved antibacterial activities compared to those of cecropin A and demonstrated that cecropin-like peptides are very selective against Gram-negative bacteria.
Salt-resistance experiments indicated that papiliocin as well as cecropin A retained its antibacterial activity against all Gram-negative bacteria at high salt concentrations and under serum conditions. Therefore, they are highly salt resistant in a physiological environment. Since high ionic concentrations generally prevail in the body fluids of patients suffering from inflammatory disease, cecropin-like peptides may be potent antibiotic candidates for use in patients with cystic fibrosis or inflammatory disease.
As shown in Fig. 1, the C-terminal region from residue 25 to 35 is very hydrophobic, and the hydrophobic sector is much larger compared to the hydrophilic sector. In contrast, the N-terminal helix from residue 3 to 21 has a larger hydrophilic sector than hydrophobic sector. This is a common feature found in all cecropin family members. Since Arg1 in papiliocin is substituted with Lys1 in cecropin A and Lys13 is substituted with Gln13 in cecropin A, the N-terminal amphipathic α helix of papiliocin is more positively charged than that of cecropin A, resulting in the slightly higher antibacterial activities of papiliocin compared to cecropin A. This amphipathicity of cecropin-like peptides is important because the hydrophilic sector faces toward the negatively charged head groups of the lipid membrane, and the hydrophobic side-chains, including the aromatic rings of Trp2 and Phe5, have close contact with the acyl chains of the hydrophobic lipid. Cecropin-like peptides have helix-hinge-helix structures in membrane-mimicking environments (20). Furthermore, this typical structural characteristic is likely important for the high antimicrobial and anti-inflammatory activities of cecropin-like peptides.
To address the antimicrobial mechanism of actions for cecropin-like peptides, we compared their abilities to damage LUVs of varying phospholipid composition mammalian- and microbial-mimetic membranes. Dye leakage results showed that papiliocin and cecropin A at their MIC bind strongly and permeate more efficiently into phospholipid membranes which mimic bacterial membranes and fungal membrane than into zwitterionic phospholipid membranes, which are the major components of the outer leaflet of human erythrocytes (21). These results suggest that the bactericidal action of cecropin-like peptides is attributable to perturbation of the bacterial cell membrane.
STD effects of both peptides in EYPC/EYPG SUVs imply that Trp2 and Phe5 in the N-terminal helix play important roles in interacting with the bacterial cell membrane as well as with the fungal membrane. All of the results in this study implied that a strong interaction between cecropin-like peptides and the phospholipid membrane may lead to a plausible disruption of phospholipid membrane structures and this may be the reason for the susceptibility of Gram-negative bacteria to cecropin-like peptides. In conclusion, cecropin-like peptides can be potent peptide antibiotics suitable for treating Gram-negative bacterial infections.
MATERIALS AND METHODS
Peptide synthesis
Peptides were prepared by solid-phase synthesis using fluorenylmethoxycarbonyl (Fmoc) chemistry. The peptide was purified as described previously (14).
Antifungal activity
M. slooffiae (KCTC 17431) and M. obtuse (KCTC 7847) were purchased from Korean Collection for Type Cultures (Daejeon, Korea). Fungal strains were cultured during 3 days at 37℃ on Sabouraud dextrose broth (Sigma) containing 1% olive oil to evaluate the antifungal activity of peptides. The MICs were measured in 1% bactopeptone with 2 × 105 cells/ml of fungal cells. The lowest concentration of peptides that completely inhibited growth was defined as the MIC.
Antibacterial activity
The clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA; CCARM 3089, 3090, 3108, 3114 and 3126), multi-drug resistant Salmonella typhimurium (MDRST; CCARM 8003, 8007 and 8009), and multi-drug resistant Escherichia coli (MDREC; CCARM 1229 and 1238) were supplied by the Culture Collection of Antibiotic-Resistant Microbes (CCARM) at Seoul Women's University in Korea. MICs and salt-resistance tests were determined as reported previously (14).
Cytotoxicity against NIH 3T3 cell
The NIH-3T3 fibroblast cell line was obtained from the Korea Research Institute of Chemical Technology (KRICT) (Daejeon, Korea). Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and antibiotic solution (100 units/ml penicillin and 100 μg/ml streptomycin) at 37℃ in a humidified 5% CO2 atmosphere. The cytotoxicity of peptides against mammalian cells was determined using a MTT assay as previously reported (22), with minor modifications as described earlier (14).
Peptide-induced permeabilization of lipid vesicles
Calcein-entrapped LUVs composed of EYPC/EYPG (7:3, w/w), EYPC/EYPE/EYPI/ergosterol (5:4:1:2, w/w) or EYPC/CH (10:1, w/w) were prepared by vortexing dried lipids in a dye buffer solution (70 mM calcein, 10 mM Tris [pH 7.4], 150 mM NaCl, and 0.1 mM EDTA). Peptide-induced permeabilization of lipid vesicles was determined as reported previously (14).
CD analysis
CD spectroscopy was used to investigate the secondary structure adopted by peptides in the following membrane-mimetic environments: 50 mM DPC micelles and 100 mM SDS micelles. The peptide concentration was 50 μM for all CD experiments. CD experiments were performed as reported previously (14).
STD NMR experiments
Peptides were added at a concentration of 0.5 mM to 0.1 mM EYPC/EYPG SUVs in D2O at a pH of 5.9. STD-NMR experiments were recorded on a Bruker 500 MHz spectrometer at a temperature of 298 K. The STD-NMR spectra were obtained with 512 scans and selective saturation of SUV resonances at −1.0 ppm (30 ppm for reference spectra). A cascade of 40 selective Gaussian-shaped pulses of 50-ms duration and a 100-ms delay between each pulse were used in all STD-NMR experiments with a total saturation time of 2 s. Subtraction of the two spectra (on resonance - off resonance) leads to the difference spectrum, which contains signals arising from the saturation transfer. Therefore, spectral differences primarily constituted resonances belonging to peptide protons bound to SUV.
Acknowledgments
This paper was supported by Konkuk University in 2012.
References
- 1.Hancock R. E., Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. (1998);16:82–88. doi: 10.1016/S0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 2.Otvos L. Jr. Antibacterial peptides isolated from insects. J. Pept. Sci. (2000);6:497–511. doi: 10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Dimarcq J. L., Bulet P., Hetru C., Hoffmann J. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers. (1998);47:465–477. doi: 10.1002/(SICI)1097-0282(1998)47:6<465::AID-BIP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Yeaman M. R., Yount N. Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. (2003);55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Broqden K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev. Microbiol. (2005);3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 6.Bulet P., Hetru C., Dimarcq J. L., Hoffmann D. Antimicrobial peptides in insects: structure and function. Dev. Comp. Immunol. (1999);23:329–344. doi: 10.1016/S0145-305X(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 7.Boman H. G., Hultmark D. Cell-free immunity in insects. Cell-free immunity in insects. Annu. Rev. Microbiol. (1987);41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- 8.Morishima I., Suginaka S., Ueno T., Hirano H. Isolation and structure of cecropins, inducible antibacterial peptides, from the silkworm, Bombyx mori. Comp. Biochem. Physiol. (1990);95:551–554. doi: 10.1016/0305-0491(90)90019-p. [DOI] [PubMed] [Google Scholar]
- 9.Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. (1990);9:217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y., Wang J. X., Zhao X. F., Du X. J., Xue J. F. Molecular cloning and characterization of cecropin from the housefly (Musca domestica) and its expression in Escherichia coli. Dev. Comp. Immunol. (2006);30:249–257. doi: 10.1016/j.dci.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Saito A., Ueda K., Imamura M., Atsumi S., Tabunoki H., Miura N., Watanabe A., Kitami M., Sato R. Purification and cDNA cloning of a cecropin from the longicorn beetle, Acalolepta luxuriosa. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. (2005);142:317–323. doi: 10.1016/j.cbpb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Li Z., Du C., Chen W., Pang Y. Characterization and expression of a cecropin-like gene from Helicoverpa armigera. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. (2007);148:417–425. doi: 10.1016/j.cbpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Kim S. R., Hong M. Y., Park S. W., Choi K. H., Yun E. Y., Goo T. W., Kang S. W., Suh H. J., Kim I., Hwang J. S. Characterization and cDNA cloning of a cecropin-like antimicrobial peptide, papiliocin, from the swallowtail butterfly, Papilio xuthus. Mol. Cells. (2010);29:419–423. doi: 10.1007/s10059-010-0050-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim J. K., Lee E., Shin S., Jeong K. W., Lee J. Y., Bae S. Y., Kim S. H., Lee J., Kim S. R., Lee D. G., Hwang J. S., Kim Y. Structure and function of papiliocin with antimicrobial and anti-inflammatory activities isolated from the Swallowtail Butterfly, Papilio xuthus. J. Biol. Chem. (2011);285:3883–3895. doi: 10.1074/jbc.M111.269225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boman H. G., Faye I., Hofsten P., Kockum K., Lee J. Y., Xanthopoulos K. G., Bennich H., Engström A., Merrifield R. B., Andreu D. On the primary structures of lysozyme, cecropins and attacins from Hyalophora cecropia. Dev. Comp. Immunol. (1985);9:551–558. doi: 10.1016/0145-305X(85)90018-7. [DOI] [PubMed] [Google Scholar]
- 16.Pliška V., Schmidt M., Fauchère J. L. Partition coefficients of amino acids and hydrophobic parameters π of their side-chains as measured by thin-layer chromatography. J. Chromatography. (1981);A216:79–92. doi: 10.1016/S0021-9673(00)82337-7. [DOI] [Google Scholar]
- 17.Holak T. A., Engström A., Kraulis P. J., Lindeberg G., Bennich H., Jones T. A., Gronenborn A. M., Clore G. M. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. (1998);27:7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- 18.Kauffman C. A. Fungal infections. Proc. Am. Thorac. Soc. (2006);3:35–40. doi: 10.1513/pats.200510-110JH. [DOI] [PubMed] [Google Scholar]
- 19.Ashbee R. H., Scheynius A. Malassezia. In Pathogenic Yeasts, Ashbee, R. H. and Bignell, E. (eds.) Springer Verlag Heidelberg; Germany: (2010). pp. 209–230. [Google Scholar]
- 20.Cole A. M., Darouiche R. O., Legarda D., Connell N., Diamond G. Characterization of a fish antimicrobial peptide: Gene expression, subcellular localization and spectrum of activity. Antimicrob. Agents Chemother. (2000);44:2039–2045. doi: 10.1128/AAC.44.8.2039-2045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gennis R. B. In Biomembranes: Molecular Structure and Function. Springer-Verlag New York Inc; New York, USA: (1989). pp. 155–156. [Google Scholar]
- 22.Scudiero D. A., Shoemaker R. H., Paull K. D., Monks A., Tierney S., Nofziger T. H., Currens M. J., Seniff D., Boyd M. R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. (1988);48:4827–4833. [PubMed] [Google Scholar]


