Abstract
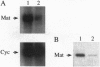
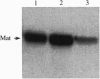
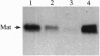
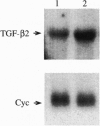
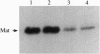
Unlike most normal adult tissues, cyclic growth and tissue remodeling occur within the uterine endometrium throughout the reproductive years. The matrix metalloproteinases (MMPs), a family of structurally related enzymes that degrade specific components of the extracellular matrix are thought to be the physiologically relevant mediators of extracellular matrix composition and turnover. Our laboratory has identified MMPs of the stromelysin family in the cycling human endometrium, implicating these enzymes in mediating the extensive remodeling that occurs in this tissue. While the stromelysins are expressed in vivo during proliferation-associated remodeling and menstruation-associated endometrial breakdown, none of the stromelysins are expressed during the progesterone-dominated secretory phase of the cycle. Our in vitro studies of isolated cell types have confirmed progesterone suppression of stromal MMPs, but a stromal-derived paracrine factor was found necessary for suppression of the epithelial-specific MMP matrilysin. In this report, we demonstrate that transforming growth factor beta (TGF-beta) is produced by endometrial stroma in response to progesterone and can suppress expression of epithelial matrilysin independent of progesterone. Additionally, we find that an antibody directed against the mammalian isoforms of TGF-beta abolishes progesterone suppression of matrilysin in stromal-epithelial cocultures, implicating TGF-beta as the principal mediator of matrilysin suppression in the human endometrium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman D. J., Schneider S. L., Thompson D. A., Cheng H. L., Tomasi T. B. A transforming growth factor beta 2 (TGF-beta 2)-like immunosuppressive factor in amniotic fluid and localization of TGF-beta 2 mRNA in the pregnant uterus. J Exp Med. 1990 Nov 1;172(5):1391–1401. doi: 10.1084/jem.172.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. L., Gorstein F., Osteen K. G. Stromal-epithelial cell communication, growth factors, and tissue regulation. Lab Invest. 1990 May;62(5):519–521. [PubMed] [Google Scholar]
- Bascom C. C., Sipes N. J., Coffey R. J., Moses H. L. Regulation of epithelial cell proliferation by transforming growth factors. J Cell Biochem. 1989 Jan;39(1):25–32. doi: 10.1002/jcb.240390104. [DOI] [PubMed] [Google Scholar]
- Busiek D. F., Ross F. P., McDonnell S., Murphy G., Matrisian L. M., Welgus H. G. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992 May 5;267(13):9087–9092. [PubMed] [Google Scholar]
- Butler T. A., Zhu C., Mueller R. A., Fuller G. C., Lemaire W. J., Woessner J. F., Jr Inhibition of ovulation in the perfused rat ovary by the synthetic collagenase inhibitor SC 44463. Biol Reprod. 1991 Jun;44(6):1183–1188. doi: 10.1095/biolreprod44.6.1183. [DOI] [PubMed] [Google Scholar]
- Chegini N., Zhao Y., Williams R. S., Flanders K. C. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994 Jul;135(1):439–449. doi: 10.1210/endo.135.1.8013382. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Bigsby R. M., Cooke P. S., Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985 Sep;17(3):137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- Curry T. E., Jr, Mann J. S., Huang M. H., Keeble S. C. Gelatinase and proteoglycanase activity during the periovulatory period in the rat. Biol Reprod. 1992 Feb;46(2):256–264. doi: 10.1095/biolreprod46.2.256. [DOI] [PubMed] [Google Scholar]
- Damsky C., Sutherland A., Fisher S. Extracellular matrix 5: adhesive interactions in early mammalian embryogenesis, implantation, and placentation. FASEB J. 1993 Nov;7(14):1320–1329. doi: 10.1096/fasebj.7.14.8224605. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Dawood M. Y. Corpus luteal insufficiency. Curr Opin Obstet Gynecol. 1994 Apr;6(2):121–127. [PubMed] [Google Scholar]
- Gambrell R. D., Jr Update on hormone replacement therapy. Am Fam Physician. 1992 Nov;46(5 Suppl):87S–96S. [PubMed] [Google Scholar]
- Giudice L. C. Growth factors and growth modulators in human uterine endometrium: their potential relevance to reproductive medicine. Fertil Steril. 1994 Jan;61(1):1–17. doi: 10.1016/s0015-0282(16)56447-4. [DOI] [PubMed] [Google Scholar]
- Gold L. I., Saxena B., Mittal K. R., Marmor M., Goswami S., Nactigal L., Korc M., Demopoulos R. I. Increased expression of transforming growth factor beta isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: evidence for paracrine and autocrine action. Cancer Res. 1994 May 1;54(9):2347–2358. [PubMed] [Google Scholar]
- Graham C. H., Lala P. K. Mechanism of control of trophoblast invasion in situ. J Cell Physiol. 1991 Aug;148(2):228–234. doi: 10.1002/jcp.1041480207. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Reife R. A., Kang A. H., Stuart J. M. The role of stromelysin in the cartilage destruction that accompanies inflammatory arthritis. Arthritis Rheum. 1990 Mar;33(3):388–397. doi: 10.1002/art.1780330312. [DOI] [PubMed] [Google Scholar]
- Healy D. L., Hodgen G. D. The endocrinology of human endometrium. Obstet Gynecol Surv. 1983 Aug;38(8):509–530. doi: 10.1097/00006254-198308000-00025. [DOI] [PubMed] [Google Scholar]
- Kauma S., Matt D., Strom S., Eierman D., Turner T. Interleukin-1 beta, human leukocyte antigen HLA-DR alpha, and transforming growth factor-beta expression in endometrium, placenta, and placental membranes. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1430–1437. doi: 10.1016/0002-9378(90)90601-3. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Jeang K. T., Glick A. B., Sporn M. B., Roberts A. B. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989 Apr 25;264(12):7041–7045. [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Limacher J. M., Hutin P., Wendling C., LeMeur M., Basset P., Rio M. C. The breast cancer-associated stromelysin-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol. 1992 Nov;119(4):997–1002. doi: 10.1083/jcb.119.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librach C. L., Werb Z., Fitzgerald M. L., Chiu K., Corwin N. M., Esteves R. A., Grobelny D., Galardy R., Damsky C. H., Fisher S. J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991 Apr;113(2):437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990 Apr;1(2):99–106. [PubMed] [Google Scholar]
- Marbaix E., Donnez J., Courtoy P. J., Eeckhout Y. Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11789–11793. doi: 10.1073/pnas.89.24.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshburn P. B., Arici A. M., Casey M. L. Expression of transforming growth factor-beta 1 messenger ribonucleic acid and the modulation of deoxyribonucleic acid synthesis by transforming growth factor-beta 1 in human endometrial cells. Am J Obstet Gynecol. 1994 Apr;170(4):1152–1158. doi: 10.1016/s0002-9378(94)70112-1. [DOI] [PubMed] [Google Scholar]
- Martelli M., Campana A., Bischof P. Secretion of matrix metalloproteinases by human endometrial cells in vitro. J Reprod Fertil. 1993 May;98(1):67–76. doi: 10.1530/jrf.0.0980067. [DOI] [PubMed] [Google Scholar]
- Marti H. P., Lee L., Kashgarian M., Lovett D. H. Transforming growth factor-beta 1 stimulates glomerular mesangial cell synthesis of the 72-kd type IV collagenase. Am J Pathol. 1994 Jan;144(1):82–94. [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993 Dec;53(4):288–295. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- McClellan M., West N. B., Brenner R. M. Immunocytochemical localization of estrogen receptors in the macaque endometrium during the luteal-follicular transition. Endocrinology. 1986 Dec;119(6):2467–2475. doi: 10.1210/endo-119-6-2467. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Navre M., Coffey R. J., Jr, Matrisian L. M. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991;4(6):527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- Noble N. A., Harper J. R., Border W. A. In vivo interactions of TGF-beta and extracellular matrix. Prog Growth Factor Res. 1992;4(4):369–382. doi: 10.1016/0955-2235(92)90017-c. [DOI] [PubMed] [Google Scholar]
- Osteen K. G., Hill G. A., Hargrove J. T., Gorstein F. Development of a method to isolate and culture highly purified populations of stromal and epithelial cells from human endometrial biopsy specimens. Fertil Steril. 1989 Dec;52(6):965–972. doi: 10.1016/s0015-0282(16)53160-4. [DOI] [PubMed] [Google Scholar]
- Osteen K. G., Rodgers W. H., Gaire M., Hargrove J. T., Gorstein F., Matrisian L. M. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10129–10133. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Kim S. J., Noma T., Glick A. B., Lafyatis R., Lechleider R., Jakowlew S. B., Geiser A., O'Reilly M. A., Danielpour D. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp. 1991;157:7–28. doi: 10.1002/9780470514061.ch2. [DOI] [PubMed] [Google Scholar]
- Rodgers W. H., Matrisian L. M., Giudice L. C., Dsupin B., Cannon P., Svitek C., Gorstein F., Osteen K. G. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994 Sep;94(3):946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W. H., Osteen K. G., Matrisian L. M., Navre M., Giudice L. C., Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am J Obstet Gynecol. 1993 Jan;168(1 Pt 1):253–260. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- Schatz F., Papp C., Toth-Pal E., Lockwood C. J. Ovarian steroid-modulated stromelysin-1 expression in human endometrial stromal and decidual cells. J Clin Endocrinol Metab. 1994 Jun;78(6):1467–1472. doi: 10.1210/jcem.78.6.8200951. [DOI] [PubMed] [Google Scholar]
- Schwarz L. C., Wright J. A., Gingras M. C., Kondaiah P., Danielpour D., Pimentel M., Sporn M. B., Greenberg A. H. Aberrant TGF-beta production and regulation in metastatic malignancy. Growth Factors. 1990;3(2):115–127. doi: 10.3109/08977199009108274. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta. Multiple actions and potential clinical applications. JAMA. 1989 Aug 18;262(7):938–941. doi: 10.1001/jama.262.7.938. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. Human endometrium: an active site of cytokine production and action. Endocr Rev. 1991 Aug;12(3):272–290. doi: 10.1210/edrv-12-3-272. [DOI] [PubMed] [Google Scholar]
- Talhouk R. S., Bissell M. J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992 Sep;118(5):1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Wakefield L. M., Sporn M. B. Suppression of carcinogenesis: a role for TGF-beta and related molecules in prevention of cancer. Immunol Ser. 1990;51:217–243. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]