Abstract
Many bioactive molecules like recombinant human bone morphogenetic protein 2 (rhBMP-2) have been developed for mineralized bone grafts, for which proper scaffolds are necessary to successfully apply the bioactive molecules. In this study, we tested the osteogenic efficacy of rhBMP-2 produced in-house in combination with gelatin sponge as the scaffold carrier in a rabbit radial defect model. The efficacy of the rhBMP-2 was determined by alkaline phosphatase activity assay of C2C12 cells. Two groups of ten rabbits each were treated with rhBMP-2/gelatin sponge, or gelatin sponge only. At 4 weeks, rhBMP-2/gelatin sponge grafts showed more bone regeneration than gelatin sponge grafts, as determined by X-ray radiography, micro-computed tomography, and histological analyses. At 8 weeks, rhBMP-2/gelatin sponge grafts exerted much stronger osteogenic effects. The study demonstrates the improved osteogenic efficacy of the rhBMP-2/gelatin sponge grafts in a rabbit radial bone defect model acting as a bone-inductive material. [BMB Reports 2013; 46(6): 328-333]
Keywords: BMP-2, Bone defect, Bone graft, Gelatin sponge, Osteogenic activity
INTRODUCTION
The successful reconstruction of bone defects is a major medical challenge. Although autologous bone is the gold standard for the reconstruction of bone defects, donor site morbidity and a limited amount of bone are obstacles to its use (1). Therefore, the development of a suitable bone substitute is important, and many candidate materials have been studied (1,2).
Mineralized allografts are strong candidates as bone graft materials, and many related products have been introduced (2). The advantages of mineralized materials are low immunogenicity and good space maintenance, but their disadvantages are slow replacement by regenerative bone, and rapid degradation under acidic conditions (3). Demineralized bone matrix was developed as a substitute for mineralized bone grafts (4), but it also degrades rapidly, and as a result, the space-maintaining ability of demineralized bone matrix is poor. Therefore, a suitable scaffold is needed for demineralized bone-matrix grafts (5).
Recombinant human bone morphogenetic protein-2 (rh-BMP-2) has strong osteoinduction ability, through the activation of the Smad signaling pathway and the induction of Runx2 expression (6-8). rhBMP-2 can be produced in different ways (9,10), and the activity of rhBMP-2 derived from Escherichia coli has been reported to be similar to that derived from Chinese hamster ovary cells (10). However, its production cost hinders clinical applications. Recently, Joint Protein Central (jPC, Incheon, Korea) developed bioactive artificial BMP ligands cost-effectively using RASCH (Random Assembly of Segmental Chimera and Heteromer) (11). We have used this E. coli-derived rhBMP-2 to perform osteogenic assays in vivo, using gelatin scaffold material. The scaffold used for rhBMP-2 is important. For example, when rhBMP-2 is used with a proper carrier, it significantly increases bone regeneration, but when solubilized, it is ineffective. Thus, the time-dependent release of rhBMP-2 in bony defects is important for bone regeneration. Many organic and inorganic materials have been tested as an rhBMP-2 carrier. Among these, collagen has been most widely tested, and has been successfully used as an rhBMP-2 carrier in many types of bone defects, including peri-implant bone defects (12,13). Despite its advantages, however, collagen is expensive, and gelatin sponge offers a more cost-effective alternative. Gelatin sponge is commonly used to promote blood coagulation (14), absorbs hydrophilic material easily in the solution state, and takes approximately 4 weeks to degrade in vivo.
In this study, we demonstrate that gelatin sponge could be used as a scaffold for rhBMP-2, and in combination with rhBMP-2, it increases bone regeneration in a rabbit radial bone defect model.
RESULTS
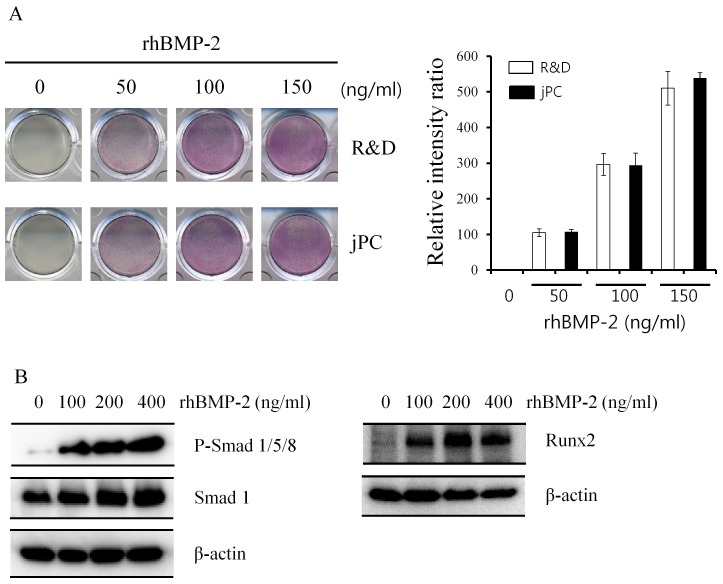
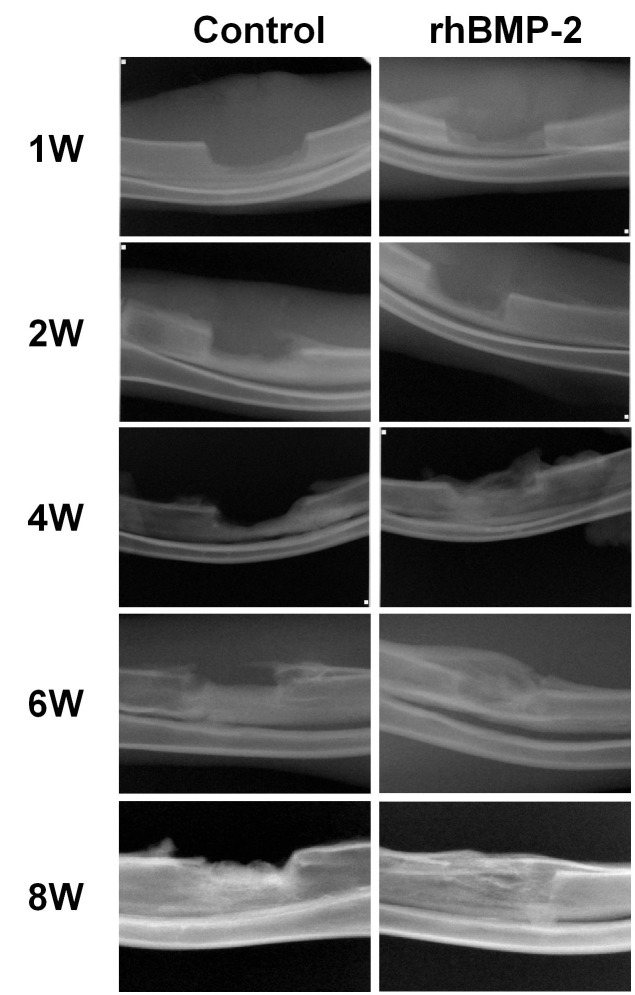
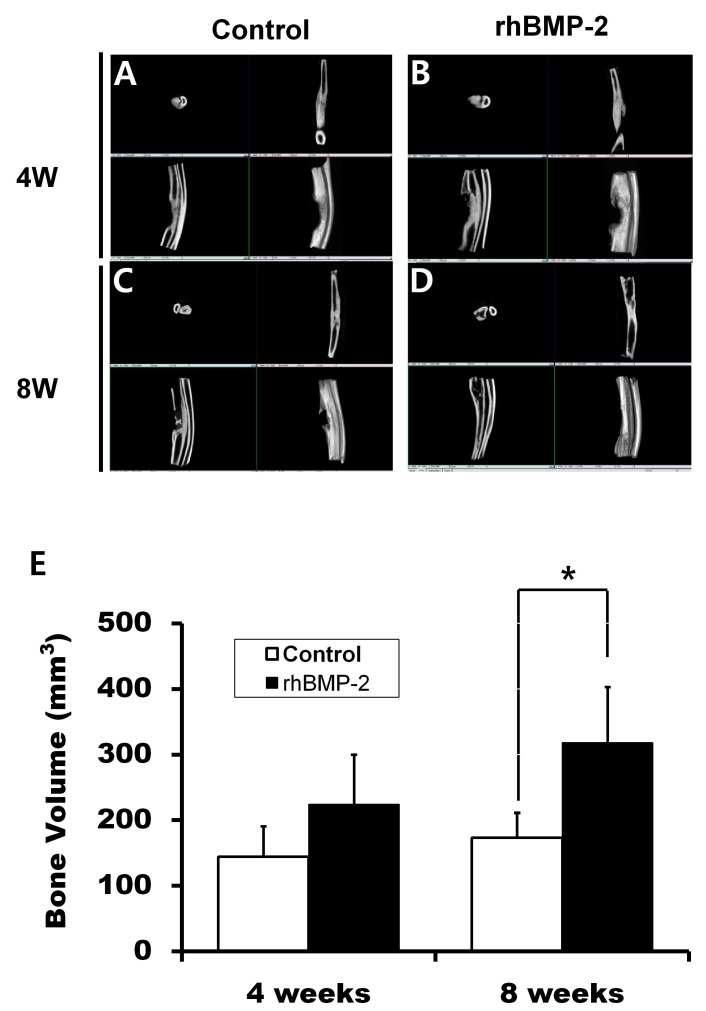
First, the effects of rhBMP-2 and RD-rhBMP-2 were examined by ALP staining. As shown in Fig. 1A, they were found to stimulate ALP activity to similar extents. To further test rhBMP-2 activity, we measured the activation of the BMP signalling pathway with regard to Smad phosphorylation and Runx2 expression (8). rhBMP-2 strongly increased Smad1,5,8 phosphorylation and Runx2 expression, indicating intact osteogenic activity of E. coli-derived rhBMP-2 (Fig. 1B). Hence, all other experiments were performed using rhBMP-2. In the serial radiography results, the rhBMP-2/gelatin sponge experimental group showed greater increases in radiopacity than the gelatin-sponge-treated control group after surgery (Fig. 2). μ-CT analysis supported the findings of radiographs taken at 4 and 8 weeks (Fig. 3A-D). The bone volume in the control group at 4 weeks was 144.49 ± 46.25 mm3, whereas in the experimental group, it was 224.35 ± 75.48 mm3. However, these values were not significantly different (Fig. 3E, P > 0.05). On the other hand, at 8 weeks, bone volumes in the control and experiment groups were 173.27 ± 38.19 mm3 and 318.27 ± 84.89 mm3, respectively, and these values were significantly different (Fig. 3E, P = 0.008).
Fig. 1. Evaluation of rhBMP-2 efficacy. C2C12 cells were cultured and treated with rhBMP-2 obtained from two different sources (R & D Systems, R&&D, and joint Protein Central, jPC). (A) Osteogenic efficacies were similar, as determined by ALP staining. The intensity of each well was quantified by an image analysis program. Bars indicate the relative intensity ratio of experimental groups after normalization with the control group (right panel, n = 3). (B) rhBMP-2 increased Smad1,5,8 phosphorylation and Runx2 expression. Cells were cultured and treated with various concentrations of rhBMP-2 described in the Materials and Methods section. Total proteins were extracted and determined by phosphorylation of Smad1/5/8 (P-smad 1/5/8, left panel) and Runx2 expression (right panel) by western blot analyses. Smad1 and β-actin were loading controls.
Fig. 2. Serial radiography. The experimental group showed increasing radiopacity as compared with the control group with the passage of time. Control: Gelatin sponge only. rhBMP 2: Gelatin sponge plus rhBMP2. W: Weeks.
Fig. 3. Micro-computed tomographic view. (A) Gelatin sponge graft at 4 weeks postoperatively. (B) The gelatin sponge/rhBMP-2 graft at 4 weeks postoperatively. (C) Gelatin sponge graft at 8 weeks. (D) Gelatin sponge/rhBMP-2 graft at 8 weeks. W: Weeks. (E) Analysis of bone volumes in defect regions (BV). At 4 weeks, mean BV in the control and experimental groups were 144.49 ± 46.25 mm3 and 224.35 ± 75.48 mm3, respectively, and at 8 weeks, mean BVs were 173.27 ± 38.19 mm3 and 318.27 ± 84.89 mm3, respectively (*P = 0.008) (n = 5 for each group).
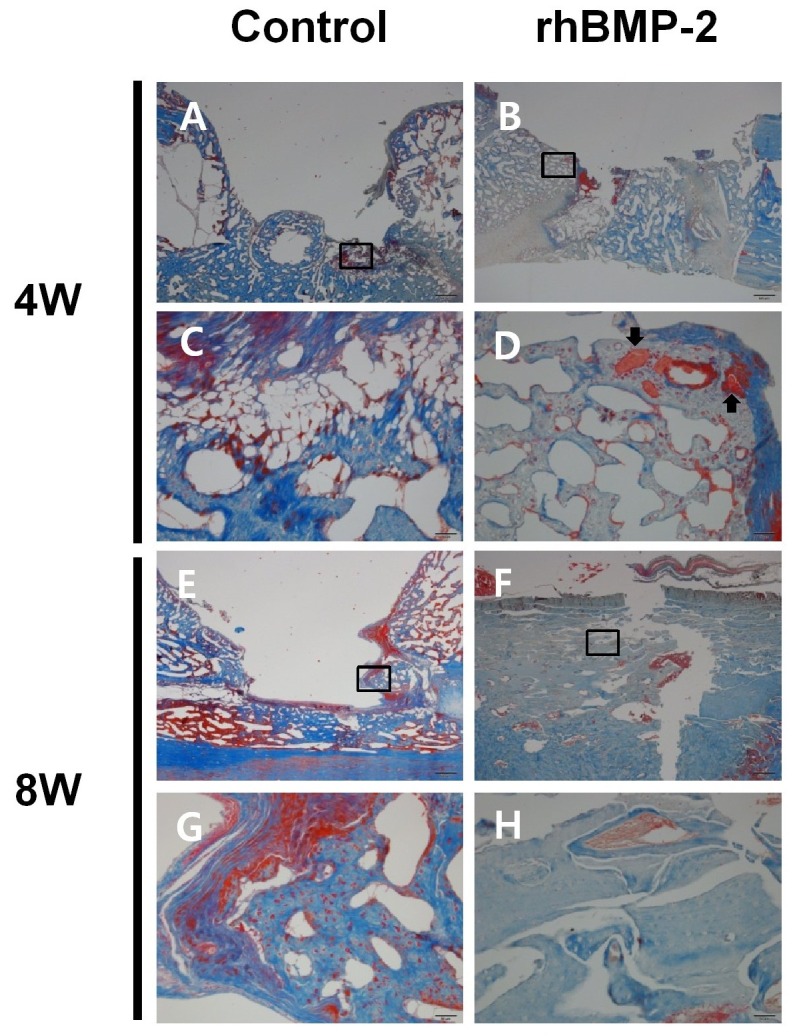
In histological examinations, the control group showed large bone defects at 4 weeks after surgery (Fig. 4A and C), whereas bony bridges were observed in the experimental group (Fig. 4B and D), in which residual grafts were encompassed by regenerated bone (Fig. 4D). At 8 weeks after surgery, the control group showed bony bridges over the ulnar bone (Fig. 4E), and incompletely regenerated bone defects. However, at 8 weeks, the experimental group showed completely regenerated bone defects (Fig. 4F). Furthermore, whereas the control group showed immature bone at the edges of the surgical defect (Fig. 4G), the experimental group showed mature lamellar bone (Fig. 4H).
Fig. 4. Postoperative histological examinations. Histological analysis at 4 weeks postoperatively (A-D). The control group showed large bone defects (A, bar = 0.5 mm), but bony bridges were observed in the rhBMP-2 group (B, bar = 0.5 mm). Boxed areas in A and B are shown as magnified images in C and D, respectively (bar = 50 μm). In the experimental group, residual grafts (arrows) were encompassed by regenerative bone (D). Histological examination at 8 weeks postoperatively (E-G). The control group showed bony bridges over ulnar bone (E, bar = 0.5 mm), and the rhBMP-2 group showed complete bone regeneration in defects (F, bar = 0.5 mm). The boxed areas in E and F are shown as magnified images in G and H, respectively (bar = 50 μm). The control group showed immature bone at the edges of surgical defect (G), whereas the experimental group showed mature lamellar bone (H).
DISCUSSION
In this study, we tested the osteogenic efficacy of rhBMP-2 obtained in-house (joint Protein Central), in a gelatin sponge carrier in a rabbit radial defect model. It was found that gelatin sponge plus rhBMP-2 achieved significantly more bone regeneration than gelatin sponge only. Therefore, we suggest that rhBMP-2-impregnated gelatin sponge be considered as a potential osteogenic bone-inductive material.
The rhBMP2 is a strong osteogenic protein (6,12,13), and its local administration has been tested in many bone defect types (12,13). This study has also shown that E. coli-derived rhBMP-2 has a comparable osteogenic efficacy. For maximum bone formation, the therapeutic concentration of rhBMP-2 must be achieved at the bone regeneration stage (15), and thus, timely delivery is critical for bone regeneration. Many materials have been examined as potential rhBMP-2 carriers. Tissue-originated and synthetic polymers have been used as rhBMP-2 scaffolds (16). Type I collagen is typically sourced from bone (17), and collagen-based biomaterials can be produced in sheet, sponge, or block forms. Collagen can bind rhBMP-2, and releases rhBMP-2 slowly at sites of application (18). However, because most collagen is produced from animal materials, it can transmit disease and cause immune reactions. Hyaluronic acid (18), chitosan (19), fibrin (20), and alginate (21) have also been used as rhBMP-2 carriers.
Mineralized materials, such as hydroxyapatite and tricalcium phosphate, have also been used as scaffolds for rhBMP-2 (6,22), but their biodegradability is poor (23). Although regenerated bone can grow into pores, these materials are not replaced completely by viable bone. Furthermore, mineralized materials are vulnerable to acidic conditions, and are thus attacked under inflammatory conditions and provide attachment sites for microorganisms (3).
Gelatin sponge is a widely used material for haemostasis. For example, the local application of gelatin sponge decreases postoperative swelling and ecchymosis after third molar extraction (24). Gelatin sponge is also used as a scaffold for stem cells and for the controlled release of growth factor (25). Interestingly, mesenchymal stem cells produce more chondrogenic markers in gelatin than in alginate or chitosan (26). Recently, a gelatin/tricalcium phosphate graft was used as a BMP-2 carrier, but the addition of tricalcium phosphate failed to confer mechanical benefits to regenerated bone as compared with gelatin/rhBMP-2 (27). In this study, the use of gelatin/rhBMP-2 resulted in apparent bone regeneration, whereas the use of only gelatin sponge did not.
Collectively, this study shows that rhBMP-2-impregnated gelatin sponge implantation encouraged bone regeneration in a rabbit bone defect model. Because of its availability and known safety, we recommend the combination of rhBMP-2/gelatin as a carrier for clinical applications.
MATERIALS AND METHODS
Materials and cell cultures
We used rhBMP-2 developed at Joint Protein Central (jPC, Incheon, Korea) (rhBMP-2) using a conventional bacterial expression method (11). As a reference, recombinant human BMP-2 was purchased from R & D Systems (Minneapolis, MN, USA) (RD-rhBMP-2). Chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). The efficacy of RD-rhBMP-2 and rhBMP-2 were compared by measuring alkaline phosphatase (ALP) activity in an osteoblast culture system. C2C12 cells were cultured at 2 × 104 cells per well in a 24-well plate until confluence. Cells were treated with 50, 100, and 150 ng/ml concentrations of rhBMP-2 and RD-rhBMP-2 for 48 hours each, and ALP activities were determined by staining, as previously described (28). The intensity of each well was measured using an image analysis program (BIOQUANT Image Analysis Corporation, Nashville, TN, USA).
Western blot analysis
C2C12 cells were cultured at 2 × 105 cells per well in a 6-well plate and treated with rhBMP-2 for 20 minutes and 24 hours to determine the activation of BMP signaling and Runx2 expression, respectively. Whole cells were lysed in ProteoJETTM Mammalian Cell Lysis Reagent (Thermo Scientific, Rockford, IL, USA) containing protease inhibitor cocktail (Sigma-Aldrich). Western blot analysis was performed using anti-Phospho Smad1,5,8 (Cell Signaling Technology, Inc. Danvers, MA, USA), anti-Smad1 (Bioworld Technology, Inc. Minneapolis, MN, USA), anti-Runx2 (Abcam, Cambridge, UK), and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies.
Animals
Twenty four-month-old New Zealand white rabbits with an average weight of 2.7 kg (range 2.5-3.0 kg) were used in this study. This experiment was approved by the Institutional Animal Care and Use Committee of Gangneung-Wonju National University (GWNU 2011-19).
Operative procedure
General anesthesia was induced by an intramuscular injection of 0.4 ml of ketamine (100 mg/ml) (Ketara; Yuhan, Seoul) plus 0.3 ml of xylazine (10 mg/kg body weight; Rompun; Bayer Korea, Seoul). The right forelimb area of each rabbit was shaved and disinfected with povidine-iodine, and then the operation site was infiltrated with 0.5 ml of 2% lidocaine containing a 1:100,000 ratio of epinephrine. A 3 cm longitudinal, superomedial incision was then made in the periosteum. Soft tissues around the distal part of the diaphysis of the radius were dissected, and the right radius was exposed. Under copious sterile saline irrigation, segmental ostectomy was performed on the right diaphysis of the radius so as to create a 10 mm segmental defect. In the experiment group, rhBMP-2-soaked cubic gelatin sponges (side 10 mm) (Cutanplast; Mascia Brunelli Spa, Viale Monza, Italy) were placed into the defects of ten rabbits, and in the control group, untreated gelatin sponges were inserted into the defects. Internal fixation between proximal and distal segments was not performed, because the radius remained connected to the ulna, which provided sufficient stabilization. The soft tissues were closed in layers using 3-0 silk. Postoperatively, rabbits were administrated 1 mg/kg of antibiotics (Gentamicin; Kookje Inc., Seoul) and 0.5 ml/kg of analgesics (Pyrin; Green Cross Veterinary Products, Seoul) intramuscularly three times daily for three days. Rabbits were individually caged, and received food and water ad lib. Five rabbits in the experimental group and five rabbits in the control group were humanely sacrificed 4 weeks after surgery, and the others were sacrificed 8 weeks after surgery. After sacrifice, specimens of the defect areas and adjacent connected ulna were removed with a saw, fixed in 10% formalin, and subjected to micro–computed tomography and histological evaluation.
Standard radiography evaluation
Under general anesthesia postoperative standard radiographs were taken at 1, 2, 4, 6, and 8 weeks after surgery.
Micro-computed tomography (μ-CT) analysis
The prepared specimens were examined by μ-CT using an InveonTM unit (Siemens Healthcare USA, Inc., Pennsylvania, USA). After calibrating the optimal exposure conditions, radius specimens were scanned at a section thickness of 0.05 mm. Images were reconstructed using Inveon Research Workplace software (Siemens Healthcare USA, Inc., Pennsylvania, USA) to create 3-D images of newly formed bone and gross profiles of specimens. The regions of interest were established according to the initial sizes and shapes of the defects. The bone volumes of regenerated bone were calculated from the images acquired.
Histological evaluation
Histological analysis of in vivo bone formation was performed after μ-CT. Specimens were decalcified using 5% nitric acid for 2 weeks, dehydrated in an ethanol series (concentration 75% to 100%), and embedded in paraffin blocks, which were then sectioned at 4 μm and stained with Masson’s trichrome. Selected sections were examined under a microscope, and photomicrographs were taken using a digital camera (DP-73; Olympus, Tokyo).
Statistical analysis
The significances of bone volume differences between the experiment and control groups were determined using the independent samples t-test using SPSS software (SPSS Inc., Chicago, IL). Statistical significance was accepted for P values < 0.05.
Acknowledgments
This study was supported by a Hankok Scholarship, the Next-Generation BioGreen21 Program (Center for Nutraceutical & Pharmaceutical Materials, Grant no. PJ009051) of the Rural Development Administration, the National Research Foundation of Korea grant funded by the MEST (Grant no. 2010-0026741), IFEZ (jCB), WCU program (R32-10215, R32-10064), and by Kyungpook National University Research Fund, 2012.
References
- 1.Jang E. S., Park J. W., Kweon H., Lee K. G., Kang S. W., Baek D. H., Choi J. Y., Kim S. G. Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. (2010);109:831–836. doi: 10.1016/j.tripleo.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Kweon H., Lee K. G., Chae C. H., Balazsi C., Min S. K., Kim J. Y., Choi J. Y., Kim S. G. Development of nano-hydroxyapatite graft with silk fibroin scaffold as a new bone substitute. J. Oral Maxillofac. Surg. (2011);69:1578–1586. doi: 10.1016/j.joms.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Lee E. H., Ryu S. M., Kim J. Y., Cho B. O., Lee Y. C., Park Y. J., Kim S. G. Effects of installation depth on survival of an hydroxyapatite-coated Bicon implant for single-tooth restoration. J. Oral Maxillofac. Surg. (2010);68:1345–1352. doi: 10.1016/j.joms.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Takano-Yamamoto T., Kawakami M., Sakuda M. Effect of a pulsing electromagnetic field on demineralized bone-matrix-induced bone formation in a bony defect in the premaxilla of rats. J. Dent. Res. (1992);71:1920–1925. doi: 10.1177/00220345920710121301. [DOI] [PubMed] [Google Scholar]
- 5.Ozturk A., Yetkin H., Memis L., Cila E., Bolukbasi S., Gemalmaz C. Demineralized bone matrix and hydroxyapatite/tri-calcium phosphate mixture for bone healing in rats. Int. Orthop. (2006);30:147–152. doi: 10.1007/s00264-006-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino M., Egi T., Terai H., Namikawa T., Takaoka K. Repair of long intercalated rib defects using porous beta-tricalcium phosphate cylinders containing recombinant human bone morphogenetic protein-2 in dogs. Biomaterials. (2006);27:4934–4940. doi: 10.1016/j.biomaterials.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Kim M., Choe S. BMPs and their clinical potentials. BMB Rep. (2011);44:619–634. doi: 10.5483/BMBRep.2011.44.10.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K. S., Kim H. J., Li Q. L., Chi X. Z., Ueta C., Komori T., Wozney J. M., Kim E. G., Choi J. Y., Ryoo H. M., Bae S. C. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. (2000);20:8783–8792. doi: 10.1128/MCB.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruoka Y., Oida S., Iimura T., Takeda K., Asahina I., Enomoto S., Sasaki S. Production of functional human bone morphogenetic protein-2 using a baculovirus/Sf-9 insect cell system. Biochem. Mol. Biol. Int. (1995);35:957–963. [PubMed] [Google Scholar]
- 10.Bessho K., Konishi Y., Kaihara S., Fujimura K., Okubo Y., Iizuka T. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br. J. Oral Maxillofac. Surg. (2000);38:645–649. doi: 10.1054/bjom.2000.0533. [DOI] [PubMed] [Google Scholar]
- 11.Allendorph G. P., Read J. D., Kawakami Y., Kelber J. A., Isaacs M. J., Choe S. Designer TGFbeta superfamily ligands with diversified functionality. PLoS One. (2011);6:e26402. doi: 10.1371/journal.pone.0026402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa Y., Sato S., Takayama T., Murai M., Suzuki N., Ito K. Short-term effects of rhBMP-2-enhanced bone augmentation beyond the skeletal envelope within a titanium cap in rabbit calvarium. J. Periodontol. (2008);79:348–354. doi: 10.1902/jop.2008.070305. [DOI] [PubMed] [Google Scholar]
- 13.Tatakis D. N., Koh A., Jin L., Wozney J. M., Rohrer M. D., Wikesjo U. M. Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: a dose-response study. J. Periodontal. Res. (2002);37:93–100. doi: 10.1034/j.1600-0765.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 14.Evans B. E. Local hemostatic agents. N. Y. J. Dent. (1977);47:109–114. [PubMed] [Google Scholar]
- 15.Wikesjo U. M., Qahash M., Polimeni G., Susin C., Shanaman R. H., Rohrer M. D., Wozney J. M., Hall J. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J. Clin. Periodontol. (2008);35:1001–1010. doi: 10.1111/j.1600-051X.2008.01321.x. [DOI] [PubMed] [Google Scholar]
- 16.Li R. H., Wozney J. M. Delivering on the promise of bone morphogenetic proteins. Trends. Biotechnol. (2001);19:255–265. doi: 10.1016/S0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 17.Phipps M. C., Clem W. C., Grunda J. M., Clines G. A., Bellis S. L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cellinfiltration. Biomaterials. (2012);33:524–534. doi: 10.1016/j.biomaterials.2011.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhakta G., Rai B., Lim Z. X., Hui J. H., Stein G. S., van Wijnen A. J., Nurcombe V., Prestwich G. D., Cool S. M. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. (2012);33:6113–6122. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S., Kang Y., Krueger C. A., Sen M., Holcomb J. B., Chen D., Wenke J. C., Yang Y. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with gelatin microspheres enhances early osteoblastic differentiation. Acta. Biomater. (2012);8:1768–1777. doi: 10.1016/j.actbio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo K. H., Yeo do H., Ahn J. M., Kim B. S., Kim C. S., Im G. I. Lumbar posterolateral fusion using heparin-conjugated fibrin for sustained delivery of bone morphogenic protein-2 in a rabbit model. Artif. Organs. (2012);36:629–634. doi: 10.1111/j.1525-1594.2012.01444.x. [DOI] [PubMed] [Google Scholar]
- 21.Jeon O., Powell C., Solorio L. D., Krebs M. D., Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Control Release. (2011);154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J. H., Yu C. H., Yang J. J., Baek H. R., Lee K. M., Koo T. Y., Chang B. S., Lee C. K. Comparative study of fusion rate induced by differentdosages of Escherichia coli-derived recombinant human bone morphogenetic protein-2 using hydroxyapatite carrier. Spine J. (2012);12:239–248. doi: 10.1016/j.spinee.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Ding X., Takahata M., Akazawa T., Iwasaki N., Abe Y., Komatsu M., Murata M., Ito M., Abumi K., Minami A. Improved bioabsorbability of synthetic hydroxyapatite through partial dissolution-precipitation of its surface. J. Mater. Sci. Mater. Med. (2011);22:1247–1255. doi: 10.1007/s10856-011-4291-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim J. C., Choi S. S., Wang S. J., Kim S. G. Minor complications after mandibular third molar surgery: type, incidence, and possible prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. (2006);102:e4–11. doi: 10.1016/j.tripleo.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 25.Omata K., Matsuno T., Asano K., Hashimoto Y., Tabata Y., Satoh T. Enhanced bone regeneration by gelatin-beta-tricalcium phosphate composites enabling controlled release of bFGF. J. Tissue Eng. Regen. Med.; (2012). (in press). [DOI] [PubMed] [Google Scholar]
- 26.Bertolo A., Mehr M., Aebli N., Baur M., Ferguson S. J., Stoyanov J. V. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur. Spine. J. (2012);21(Suppl 6):S826–838. doi: 10.1007/s00586-011-1975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita N., Matsushita T., Ishida K., Sasaki K., Kubo S., Matsumoto T., Kurosaka M., Tabata Y., Kuroda R. An analysis of bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein 2 from a biodegradable sponge composed of gelatin and beta-tricalcium phosphate. J. Tissue Eng. Regen. Med. (2012);6:291–298. doi: 10.1002/term.432. [DOI] [PubMed] [Google Scholar]
- 28.Kim H. N., Min W. K., Jeong J. H., Kim S. G., Kim J. R., Kim S. Y., Choi J. Y., Park B. C. Combination of Runx2 and BMP2 increases conversion of human ligamentum flavum cells into osteoblastic cells. BMB Rep. (2011);44:446–451. doi: 10.5483/BMBRep.2011.44.7.446. [DOI] [PubMed] [Google Scholar]