Abstract
Extracellular acidification occurs not only in pathological conditions such as inflammation and brain ischemia, but also in normal physiological conditions such as synaptic transmission. Acid-sensing ion channels (ASICs) can detect a broad range of physiological pH changes during pathological and synaptic cellular activities. ASICs are voltage-independent, proton-gated cation channels widely expressed throughout the central and peripheral nervous system. Activation of ASICs is involved in pain perception, synaptic plasticity, learning and memory, fear, ischemic neuronal injury, seizure termination, neuronal degeneration, and mechanosensation. Therefore, ASICs emerge as potential therapeutic targets for manipulating pain and neurological diseases. The activity of these channels can be regulated by many factors such as lactate, Zn2+, and Phe-Met-Arg-Phe amide (FMRFamide)-like neuropeptides by interacting with the channel’s large extracellular loop. ASICs are also modulated by G protein-coupled receptors such as CB1 cannabinoid receptors and 5-HT2. This review focuses on the physiological roles of ASICs and the molecular mechanisms by which these channels are regulated. [BMB Reports 2013; 46(6): 295-304]
Keywords: Acidosis, Acid-sensing ion channels, G protein-coupled receptors, Modulation, Pain, pH
INTRODUCTION
Tissue acidosis is a common feature in pain-generating pathological conditions such as inflammation, ischemic stroke, infections, and cancer. Tissue injury leads to the release of inflammatory mediators, including substance P, bradykinin, histamine, 5-hydroxytryptamin (5-HT or serotonin), glutamate, ATP, interleukin-1, nerve growth factor (NGF), and proton (1). Application of an acidic solution on human skin induces pain (2,3). It is well known that the extracellular pH levels drop to 5.4 in acute inflammation (4). Severe ischemia also induces the reduction of pH to 6.3 or even lower (5,6). In the central nervous system (CNS), the pH of the synaptic cleft can fall during neurotransmission, as the synaptic vesicles are acidic (7,8). Recently, local pH changes during normal brain activity were detected in mouse and human brains, although the extent of pH fluctuations still needs to be clarified (9).
Acid-sensing ion channels (ASICs) play a critical role in the perception of a wide range of pH changes in conditions related to tissue acidosis. ASICs are voltage-insensitive, proton-gated cation channels which are activated by extracellular pH fall. These channels are expressed throughout the central and peripheral nervous system. ASICs belong to the ENaC (Epithelial Na+ Channel)/DEG (Degenerin)/ASIC (Acid-sensing ion channel) superfamily of ion channels. The members of ENaC/DEG/ASIC (EDA) superfamily share the same topology, consisting of two hydrophobic transmembrane domains, a large cysteine-rich extracellular loop, and short intracellular N- and C- termini (Fig. 1A).
Fig. 1. ASIC subunits and activation of ASICs by extracellular acidification. (A) Each subunit has two hydrophobic transmembrane domains, a large cysteine-rich extracellular loop, and short intracellular N- and C- termini. (B) Three subunits assemble to form a functional homo- or hetero- trimeric channel. (C) ASIC currents evoked by extracellular pH fall in tsA cells. ASIC1a, ASIC2a, and ASIC3 were activated by application of pH 6.0, 4.5, and 5.0 solution, respectively. The membrane potential was clamped to −70 mV. The dashed line indicates zero current level.
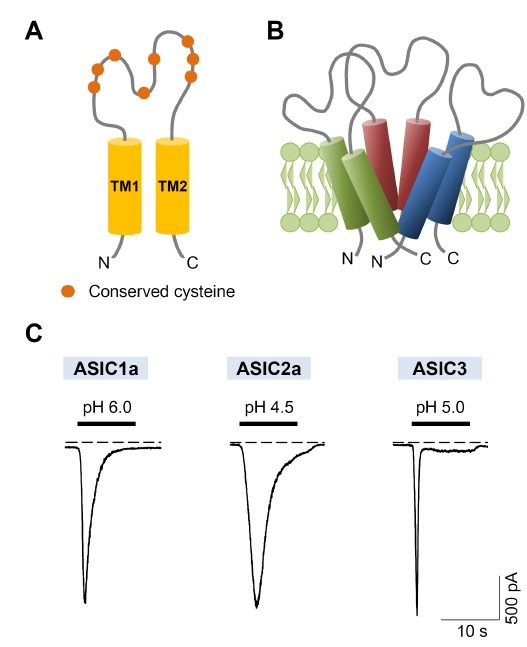
Four genes, including ACCN2, ACCN1, ACCN3, and ACCN4, encode at least six different ASIC subunits, ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 (Table 1). ASIC1a and ASIC1b are protein products of alternative splicing from the ACCN2 gene, and ASIC2a and ASIC2b are products of the alternative spliced ACCN1 gene. The CNS primarily expresses ASIC1a, ASIC2a, and ASIC2b, while all subunits are expressed in the peripheral nervous system (PNS) (6).
Table 1.
Properties, inhibitors, and regulators of ASICs
| Gene | Protein | Alternative name | pH0.5 activation | Non-proton ligand | Inhibitor | Regulator | Distribution | Physiology |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ACCN2 | ASIC1a | ASICα, BNaC2α | 6.2-6.8 (6, 11, 17, 100-104) | MitTx (14) | Amiloride (102), Mambalgin (28), PcTx1 (16, 24), A-317567 (27), NSAIDs (26), Pb2+ (30), Ni2+ (31), Ca2+ (33), Zn2+ (34) | GMQ (43), Dynorphin A (39), Big dynorphin (39), FMRFamide-like neuropeptides (41, 42), Lactate (36), Arachidonic acid (37), Spermine (38), Nitric oxide (40) | PNS/CNS | In the CNS Synaptic plasticity (47) Learning and memory (47) Fear (44) Central sensitization (104) Visual transduction (105) In the PNS Primary muscle hyperalgesia (106) |
| ASIC1b | ASICβ, BNaC2β | 5.1-6.2 (6, 11, 17, 100, 101) | MitTx (14) | Amiloride (102, Mambalgin (28), Pb2+ (30) | GMQ (43), Nitric oxide (40) | PNS | ||
| ACCN1 | ASIC2a | MDEG1, BNaC1α, BNC1a | 3.8-5.0 (6, 11, 100, 103, 104) | MitTx (14) | Amiloride (102), A-317567 (27), Cd2+(31), Ca2+ (33) | Zn2+ (35), Nitric oxide (40) | PNS/CNS | In the CNS Visual transduction (107) Perception of sour taste (108) In the PNS Mechanosensation (65) Arterial baroreceptor reflex (109) |
| ASIC2b | MDEG2, BNaC1β | N/A | PNS/CNS | |||||
| ACCN3 | ASIC3 | DRASIC, TNaC | 6.2-6.7 (6, 11, 100, 101, 103) | GMQ (13), MitTx (14), Amiloride (15) | Amiloride (102), APETx2 (25), A-317567 (27), NSAIDs (26), Gd3+ (29), Pb2+ (30), Cd2+(31), Hypertonicity (62) | FMRFamide-like neuropeptides (41, 42), Lactate (36), Arachidonic acid (37), Nitric oxide (40) | PNS | In the PNS Acidic and primary inflammatory pain (62) Cardiac pain (101) Secondary mechanical hyperalgesia (60) |
| ACCN4 | ASIC4 | SPASIC | N/A | PNS | ||||
In 2007, Eric Gouaux et al. first showed the tridimensional structure of a chicken ASIC1 channel (10). They found that three subunits are required to form a functional channel (Fig. 1B). All ASIC subunits assemble to form homo- or hetero- trimeric channels, except for ASIC2b and ASIC4. ASIC2b and ASIC4 may only contribute to forming heteromeric channels with other ASIC subunits, and modulate the expression or properties of the channels (11,12). Although the canonical ligand for ASICs is the proton, the large extracellular loop of these channels allowed the possibility of the existence of other non-proton ligands (6,7). Some examples include 2-guanidine-4-methylquinazoline (GMQ) (13) and MitTx from the venom of the Texas coral snake (14) (Table 1). Moreover, amiloride, a non-specific blocker of ASICs, paradoxically triggered the sustained currents of ASIC3 at normal pH through binding to the non-proton ligand sensing domain of channels (15). Therefore, one possibility is that homomeric ASIC2b and homomeric ASIC4 have their own specific ligands (6).
BIOPHYSICAL PROPERTIES OF ASICs
All ASIC subunits have different pH sensitivities that enable them to detect a wide range of physiological pH. ASIC1a and ASIC3 are sensitive to slight extracellular acidosis, whereas ASIC2a requires more acidic pH value for activation. The pH values for half maximal activation (pH0.5 activation) of each subunit are described in Table 1. A slight discrepancy is apparent in the pH0.5 activation values depending on different studies. This discrepancy may be due to the different species and heterologous expression systems used by the various researchers. In addition, each subunit shows different biophysical properties in kinetics and ion selectivity. ASICs are mainly permeable to Na+ ion. However, ASIC1a homomeric channels, human ASIC1b homomeric channels, and ASIC1a/2b heteromeric channels are also permeable to Ca2+ ion (6,16,17). Activation of ASIC1a increased intracellular Ca2+ concentration in hippocampal, cortical, and dorsal root ganglion (DRG) neurons (18-20). Although Ca2+ permeability of ASICs is very low, the increase of intracellular Ca2+ through ASICs can induce neuronal injury during brain ischemia accompanied by prolonged acidosis (19,21,22).
A rapid drop in the extracellular pH from 7.4 to 6.0 for 10 seconds evoked transient ASIC1a inward currents that inactivated within seconds (Fig. 1C). ASIC2a homomers were activated by more acidic pH values, and displayed slow activating and slow inactivating currents. ASIC3 homomers have biphasic currents, a rapidly activating transient current followed by a sustained current that does not fully inactivate during prolonged acidosis. The increase in cation conductance that allows fluxes of K+, Cs+, and Na+ ions contributes to the sustained currents of ASIC3. This plays a role in the perception of non-adapting pain. ASIC1a homomeric channels have the unique property of tachyphylaxis. Tachyphylaxis means that the current amplitude is gradually reduced to successive acid stimuli, even though the time interval between repetitive acid stimulations is sufficient for recovery from desensitization (23). Tachyphylaxis occurs due to proton permeation through ASIC1a channels, and it is attenuated by the extracellular Ca2+ ion (23).
A number of pharmacological tools can be used for investigating the functions and properties of ASICs (Table 1). PcTx1, a polypeptide from the venom of spider toxin, specifically inhibits homomeric ASIC1a currents (16,24). ASIC3 channels and ASIC3-containing heteromeric channels are inhibited by APETx2, a sea anemone toxin (25). Non-steroidal anti-inflammatory (NSAIDs) drugs, such as ibuprofen and flurbiprofen, can inhibit the activities of ASIC1a and ASIC3 (26). Above these molecules, A-317567 (27) and mambalgin (28) can be used as inhibitors of ASICs. The activity of ASICs can be modulated by both divalent and trivalent metal ions, including Gd3+ (29), Pb2+ (30), Ni2+ (31), Cd2+ (31), Cu2+ (32), Ca2+ (33), and Zn2+ ions (34). Zinc (Zn2+) inhibits homomeric ASIC1a channels at nanomolar concentrations, whereas this metal ion can also potentiate homomeric ASIC2a, heteromeric ASIC1a/2a, and heteromeric ASIC2a/3 channels at micromolar concentrations (35).
The activity of ASICs can be regulated by a variety of molecules, such as lactate (36), arachidonic acid (37), spermine (38), dynorphins (39), nitric oxide (40), and Phe-Met-Arg-Phe amide (FMRFamide) (41,42) (Table 1). GMQ, a non-proton ligand for ASIC3 can also regulate the function of ASIC1a and ASIC1b, although it does not trigger ASIC1a and ASIC1b currents at normal pH. It has been reported that GMQ shifts the pH dependence of the ASIC1a and ASIC1b activation to lower pH values (43). The regulators of ASICs will be reviewed in a later section.
PHYSIOLOGICAL ROLES OF ASICs
In the CNS
Synaptic plasticity: ASIC1a and ASIC2a are widely expressed throughout the brain areas, such as the hippocampus, cingulate cortex, striatum, amygdala, cerebellar cortex, and olfactory bulb (44,45). ASIC2a is predominantly localized in dendrites, dendritic spines, and synaptosomes (46). Localization of ASIC1a and ASIC2a in the excitatory synaptic area suggested that ASICs may contribute to synaptic plasticity (44,47). Mice lacking the ASIC1a gene displayed impaired long-term potentiation (LTP) and spatial memory (47). It was proposed that the activation of postsynaptic ASIC1a induces membrane depolarization which facilitates N-methyl-D-aspartate (NMDA) receptor activation and contributes to hippocampal LTP. However, the functional relevance of ASIC1a in synaptic transmission remains unclear (47,48). Wemmie et al. recently detected local pH changes during normal brain activity in mouse and human brains (9). This study directly supports the potential activation of ASICs during brain activity, although attempts to measure the ASIC-mediated currents during synaptic transmission in hippocampal neurons have not been successful (6,45). However, another recent report suggests that ASIC1a is not necessary for normal hippocampal LTP and spatial memory (48). Normal LTP was observed in ASIC1a-null mice, and disrupting the ASIC1a gene did not impair hippocampal spatial memory (48). Therefore, the role of ASIC1a in learning and memory remains controversial.
Brain ischemia: Severe ischemic stroke results in dramatic extracellular pH decrease to 6.0 (5,6). Deprivation of oxygen due to the reduction of blood flow increases anaerobic metabolism, which leads to the accumulation of lactic acid (36,49,50). Buildup of lactic acid, accompanying proton release from ATP hydrolysis, induces a dramatic decrease in pH value (49). Tissue acidosis from ischemic stroke has been known to induce neuronal injury in the brain, although the underlying mechanism is not clear. Ca2+ toxicity is also essential for ischemic neuronal injury (19,51). Excessive Ca2+ overload in the cell induces neuronal injury by activating cytotoxic cascades (19). Glutamate receptors were considered to be responsible for Ca2+ overload in the ischemic brain (51). However, while clinical trials to protect the brain from Ca2+ toxicity by using glutamate antagonists have failed (52), recent findings suggest that the activation of ASIC1a can induce neuronal injury during brain ischemia through the increase of [Ca2+]i (19,21,22). The infarct volume after transient middle cerebral artery occlusion (MCAO)-induced ischemia was surprisingly reduced by intracerebroventricular injection of ASIC1a blockers, amiloride and PcTx1 in rodents (19). Moreover, disrupting the ASIC1a gene protected the brain from ischemic neuronal injury (19). Therefore, the activation of Ca2+-permeable ASIC1a can induce neuronal injury during brain ischemia, and ASIC1a is a novel neuroprotective therapeutic target for brain ischemia (18,53).
Seizure: ASIC1a has a critical role in seizure termination. It is well known that seizure and intense neuronal excitation induce the decrease of pH levels in the brain (54), and acidosis terminates epileptic activity (55,56). Disrupting the ASIC1a gene enhanced the severity of the seizure, whereas overexpression of the ASIC1a gene displayed the opposite effect in mice (57). Acidosis-induced ASIC1a activation facilitates the function of inhibitory interneurons, which contributes to seizure termination by increasing the inhibitory tone (57). Therefore, ASIC1a activators could be potent therapeutic drugs for seizure.
Multiple sclerosis: ASIC1a also contributes to axonal degeneration during multiple sclerosis. Multiple sclerosis, which is an autoimmune inflammatory disease accompanying tissue acidosis in inflammatory lesions, leads to axonal degeneration in the CNS (58). Excessive accumulation of Ca2+ ion through ASIC1a activation during multiple sclerosis contributes to induce axonal degeneration. Disrupting the ASIC1a gene attenuates axonal degeneration during multiple sclerosis. Amiloride, a non-specific blocker of ASICs, has also displayed neuroprotective effects against axonal degeneration (58). These results suggest that ASIC1a could be an effective neuroprotective target for the treatment of neuronal degeneration.
In the PNS
Nociception: Noxious stimuli is mainly carried by the thin nerve fibers, such as thin myelinated Aδ-fibers and unmyelinated C-fibers, and the neurons participating in nociception are mostly small (59). Almost all ASIC subunits are abundantly expressed in the small and medium nociceptive sensory neurons, which are responsible for pain perception. Among the ASIC subunits, ASIC3, which is the most essential pH sensor for pain, is specifically localized in nociceptive fibers innervating the skeletal and cardiac muscles, joints, and bone (1,11,60). In these tissues, anaerobic metabolism during severe exercise or tissue injury induces the accumulation of lactate and protons, resulting in the activation of nociceptors. In addition, inflammation and tissue injury increase the expression levels of ASIC1a, ASIC2b, and ASIC3 mRNA in DRG neurons (26). Activation of ASICs, which is important for sensitizing cutaneous nociceptors, is upregulated by inflammatory mediators such as bradykinin, arachidonic acid (37,61), and nitric oxide (40). ASIC3-like currents were activated by tissue acidification in rat cutaneous sensory neurons, and acidosis-induced pain was suppressed by APETx2, a specific blocker of ASIC3, or knockdown of ASIC3 with siRNA (62).
Mechanosensation: ASICs have also been found in large mechanosensitive neurons (mechanoreceptors), which are responsible for the perception of mechanical stimuli or proprioception. It was initially proposed that ASICs have a role in mechanotransduction, since their phylogenetic homologues in the nematode Caenorhabditis elegans (C. elegans) (the mechanosensory abnormality 4- or 10- (MEC-4/MEC-10) proteins, which are expressed in touch receptor neurons in C. elegans), is important for touch sensation (63). ASIC subunits are localized in specialized nerve endings of skin and muscle spindles, and cutaneous mechanosensory structures such as Meissner corpuscles, Merkel cell neurite complexes, and Pacinian corpuscles (11,64). Therefore, ASICs have been considered to participate in neurosensory mechanotransduction, although the role of ASICs in mechanosensation is still controversial. The role of ASICs in mechanotransduction has been investigated in behavioral studies by using mice with the targeted gene deleted. Disrupting the ASIC2 gene significantly reduced the firing rates of Aβ-fibers in response to low-threshold mechanical stimuli. Thus, ASIC2 was proposed to be involved in the perception of light touch (65). However, disrupting the ASIC2 gene had no effect on the current amplitude or kinetics in response to mechanical stimuli in large DRG neurons (66). These conflicting results could be generated from the compensatory effects of multiple mechanosensitive ion channels (e.g. TRP channels) or receptors in ASIC knockout mice (64). The role of ASICs in echanosensation remains elusive.
REGULATION OF ASICs
Lactate: During brain ischemia, the concentration of lactate has been reported to increase up to 15 mM from the resting level of below 1 mM (36). It is well known that the buildup of lactic acid accompanying acidosis contributes to neuronal injury. Lactate significantly potentiated the amplitude of ASIC currents in rat sensory neurons innervating the heart (36). Potentiation of ASICs by lactate was also observed in excised outside-out membrane patches, indicating that the effect of lactate is not mediated by receptor activation or signaling cascade (36). One hypothesis suggested that lactate may potentiate ASIC currents by chelating extracellular divalent ions such as Ca2+ and Mg2+ ions, which in turn modulate the activities of membrane receptors and ion channels (49). The effect of lactate on ASICs was mimicked by reducing the concentration of Ca2+ and Mg2+ ions in the extracellular solution, even without treating lactate. Moreover, potentiation of ASIC currents by lactate was diminished by increasing the concentration of divalent ions (36). These results suggest that lactate potentiates the activity of ASICs by chelating extracellular divalent ions.
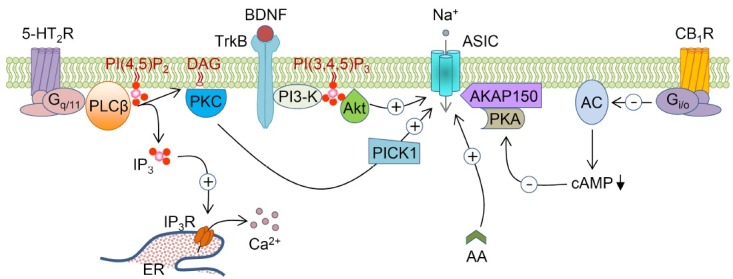
Arachidonic acid: Arachidonic acid (AA), a polyunsaturated fatty acid with a 20-carbon chain and four double bonds, is involved in cellular signaling activities as a lipid second messenger (37,61). AA is liberated from membrane phospholipids by the activation of phospholipase A2 (PLA2). The increase of intracellular Ca2+ concentration during brain ischemia leads to the activation of PLA2, resulting in increased production of AA. AA is also one of the proinflammatory factors playing a critical role in pathological conditions such as inflammation and neurological disorders including ischemic neuronal injury (67). Moreover, AA has been known to regulate the functions of various types of potassium channels, L-type and N-type Ca2+ channels (68), and transient receptor potential (TRP) channels (69). The activity of ASICs is also regulated by AA (37,61) (Fig. 2). The effects of AA can be mediated either by the direct action of AA or by the AA metabolites (70). However, inhibition of AA metabolism has no significant effects on AA-mediated potentiation of ASIC currents (37). Moreover, the regulation of ASICs by AA was not attributed to cell swelling or membrane stretch, both of which were induced by the bath application of hypotonic solution (37). These results suggest that AA increases the activity of ASICs by a direct action, and not by AA metabolism or membrane stretch.
Fig. 2. ASICs are regulated by signal transduction pathways. 5-HT2 receptors activate PLCβ through the heterotrimeric Gq/11 proteins. PLCβ hydrolyzes membrane PI(4,5)P2 to two second messengers, IP3 and DAG. IP3 releases Ca2+ from the internal Ca2+ stores in ER. DAG activates PKC, which enhances the activity of ASICs by interaction with PICK1. CB1 receptors inhibit ASICs via suppression of AC/cAMP pathway. AC is inhibited by the heterotrimeric Gi/o proteins, and inhibition of AC leads to reduction of the cAMP levels, which in turn inhibits binding of PKA to AKAP150. TrkB activates PI3-K, and enhances the membrane expression of ASICs through Akt proteins. Arachidonic acid directly potentiates the amplitude of ASIC currents. Abbreviations: ASICs: Acid-sensing ion channels, 5-HT2R: 5-HT2 receptor, CB1R: Cannabinoid-1 receptor, PLCβ: phospholipase C β, PI(4,5)P2: phosphatidylinositol 4,5-bisphosphate, PI(3,4,5)P3: phosphatidylinositol 3,4,5-trisphosphate, IP3: inositol 1,4,5-trisphosphate, IP3R: inositol 1,4,5-trisphosphate receptor, DAG: diacylglycerol, PKC: protein kinase C, PICK1: protein interacting with C-kinase, ER: endoplasmic reticulum, AC: adenylyl cyclase, cAMP: cyclic AMP, PKA: protein kinase A, AKAP150: A-kinase anchoring protein 150, TrkB: tropomyosin-related kinase B, BDNF: brain-derived neurotrophic factor, PI3-K: phosphatidylinositol 3-kinase, Akt: protein kinase B, AA: arachidonic acid.
FMRFamide-like neuropeptides: FMRFamide and structurally related peptides, which function as neurotransmitters and neuromodulators, are abundant in invertebrates, including C. elegans (71) and Drosophila melanogaster (72). Several FMRFamide-like neuropeptides such as Phe-Leu-Phe-Gln-Pro-Gln-Arg-Phe amide (neuropeptide FF) and A18Famide (neuropeptide AF) are present in mammals. Inflammation increases the expression level of FMRFamide-like neuropeptides that enhance pain (41). FMRFamide and related peptides were thought to elicit their effects through G protein-coupled receptors (GPCRs), especially, opioid receptors. However, some effects of these peptides were insensitive to naloxone, which is an opioid inverse agonist (73). Recently, FMRFamide and related neuropeptides have been found to directly modulate the activity of ASICs. While FMRFamide triggered no current at normal pH, it potentiated the current amplitude of heterologously expressed ASIC1a by slowing the inactivation rate and generating sustained currents during acidification (73). Neuropeptide FF showed only slight effects on ASIC1a, but significantly potentiated ASIC3 currents (73).
Dynorphin: The dynorphin opioid peptides are endogenous neuropeptides, abundantly expressed in the CNS. They have antinociceptive effects at physiological concentrations. However, the expression level of dynorphin A and its metabolite increases under pathological conditions, and dynorphin A at micromolar concentration shows excitotoxic effects leading to neuronal death through opioid and NMDA receptor activation (74). Therefore, dynorphin peptides have either a protective or nociceptive effect on neurons depending on their concentration. Dynorphin A and big dynorphin potentiate the activity of ASIC1a in cortical neurons (39). Dynorphins directly interact with channels to increase ASIC1a activity by reducing steady-state desensitization. Steady-state desensitization shows that channels enter the desensitized state from the closed state during prolonged exposure to moderate acidic pH (75). Big dynorphin also promoted acidosis-induced neuronal death through inhibiting steady-state desensitization of ASIC1a in cortical neurons (76).
Phosphoinositides: ENaC has been well known to be regulated by phosphoinositides such as phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3]. PI(4,5)P2 is a cofactor for the function of ion channels including inwardly rectifying K+ (Kir) channels (77), KCNQ channels (78, 79), voltage-gated Ca2+ channels (VGCC) (80), and TRP channels (81). Several ion channels such as Kir1.1 channels, Kir6.2 channels (82), and transient receptor potential melastatin 4 (TRPM4) channels (83) have PI(3,4,5)P3 sensitivity, although they have different binding affinities to PI(3,4,5)P3. These are well described in a previous review (84). Phosphoinositides have been known to directly interact with ENaC to enhance the activity of channels (85). The negative-charged head groups of phosphoinositides putatively bind to conserved positive-charged residues in ENaC channels. The positive-charged residues in the intracellular C-terminus following the second transmembrane domain in β and γ ENaC are critical for PI(3,4,5)P3 sensitivity of ENaC, while the positive-charged residues in the extreme N-terminus of β and γ ENaC are important for the regulation of ENaC by PI(4,5)P2 (85). However, ASICs do not seem to have sensitivity to phosphoinositides. Supplementing PI(4,5)P2 analogue in the pipette solution had no effects on the activity of ASIC1a (86). Furthermore, activation of muscarinic receptors that hydrolyze endogenous membrane PI(4,5)P2 did not affect the amplitude of ASIC1a currents (86). This result conflicts with the previous study. The bath application of muscarinic receptor agonists strongly inhibited the amplitude of ASIC1a currents in Chinese hamster ovary (CHO) cells (87). Therefore, PI(4,5)P2 sensitivity of ASIC1a still needs to be clarified.
Phosphatidylinositol 3-kinase: Activation of tropomyosin-related kinase B (TrkB) by brain-derived neurotrophic factor (BDNF) induces pain hypersensitivity, although the underlying mechanism is not understood. A recent study found that TrkB activation increases the surface expression and the activity of ASIC1a via the phosphatidylinositol 3-kinase (PI3-K)/protein kinase B (PKB or Akt) pathway (88) (Fig. 2). BDNF-induced forward trafficking of ASIC1a is crucial for secondary mechanical hyperalgesia (88). Phosphorylation of Serine-25 at the N-terminus of ASIC1a is critical for BDNF-induced ASIC1a trafficking. Enhancement of ASIC1a surface expression by BDNF was abolished by the mutation of Serine-25 of ASIC1a to alanine (88). Therefore, ASIC1a could be a potent analgesic target for pain hypersensitivity.
MODULATION OF ASICs BY GPCRs
Gi/o protein-coupled receptors
Cannabinoid-1 receptor: Cannabinoid-1 (CB1) receptors, which modulate nociceptive pain, are highly expressed in nociceptive primary sensory neurons (89,90). Activation of CB1 receptors, which belong to the Gi/o protein-coupled receptor family, induces the inhibition of adenylyl cyclase (AC) leading to the reduction of the cyclic AMP (cAMP) level. WIN55,212-2, an agonist of CB1 receptors reversibly inhibited ASIC currents in rat primary sensory neurons (91). Furthermore, the mean number of action potentials induced by acid stimulus decreased following activation of CB1 receptors (91). Suppression of ASIC currents by CB1 receptors was abolished by the application of cAMP analogue or the AC activator forskolin (91). These results indicate that analgesic effects of cannabinoids are mediated by the inhibition of AC/cAMP-dependent pathway through CB1 receptors. A-kinase anchoring protein 150 (AKAP150) has been reported to be co-localized with ASIC1a and ASIC2a in rat cortical neurons, and regulates the activity of these channels. It has also been reported that the inhibition of protein kinase A (PKA) binding to AKAP150 reduces the amplitude of ASIC currents, suggesting that AKAP150 mediates the PKA-dependent phosphorylation of ASICs (92). Therefore, the reduction of PKA activity may be involved in the CB1 receptor-mediated analgesic effects of cannabinoids (Fig. 2).
Gq/11 protein-coupled receptors
5-HT2 receptor: 5-HT (or serotonin), an important proinflammatory mediator, is released from platelets, mast cells, and endothelial cells during tissue injury accompanied by inflammation. 5-HT establishes pain sensation and hyperalgesia through sensitizing nociceptive afferents (93). Proinflammatory mediators such as 5-HT, bradykinin, NGF, and interleukin-1 are known to enhance the activity and the expression levels of ASICs. A mixture of these mediators enhanced the amplitude of ASIC-like currents and the number of neurons expressing ASICs in rat DRG neurons (94). Fourteen mammalian 5-HT receptor subtypes are divided into 7 subgroups of 5-HT receptors (5-HT1-7). 5-HT-induced hyperalgesia is mediated by 5-HT2 receptor subtype, which belongs to the Gq/11 protein-coupled receptor family. 5-HT2 agonists, excluding the 5-HT3 receptor agonists, significantly induced hyperalgesia (95). Another study supported the involvement of 5-HT2 receptors in 5-HT-induced hyperalgesia, which was inhibited by 5-HT2 antagonists in DRG neurons (96). Moreover, activation of 5-HT2 receptors potentiated the activity of ASICs in rat DRG neurons via protein kinase C (PKC)-dependent signaling pathways (93). ASICs have a PDZ-binding domain at their C-termini, and interact with PDZ-containing proteins. The combined proteins regulate the surface expression and the activity of ASICs (97). Protein interacting with C-kinase (PICK1), which was shown to co-localize with ASICs, directly interacts with ASICs through the PDZ-binding domain (98). Therefore, the PKC signaling pathway may be involved in the 5-HT2 receptor-mediated potentiation of ASICs (Fig. 2).
Neurokinin-1 receptor: Activation of neurokinin-1 (NK1) receptors by substance P, which is released from nociceptive nerve fibers, has an antinociceptive effect in acid-induced chronic muscle pain (99). Mice, lacking substance P and neurokinin A production by disrupting the tachykinin 1 (Tac1) gene or injection of a selective NK1 receptor antagonist, displayed persistent long-lasting hyperalgesia (99). Substance P significantly reduced ASIC3-mediated currents in muscle afferent DRG neurons. The inhibitory effect of substance P was restrictively observed in neurons expressing ASIC3. While NK1 receptor is a Gq/11 protein-coupled receptor, the inhibition of ASIC3-mediated currents by substance P is mediated by the unconventional G protein-independent, tyrosine kinase-dependent pathway (99). Replacing GTP with a non-hydrolysable analog in the internal pipette solution, had no effects on the inhibition of ASIC3-mediated currents by substance P (99). However, ASIC3-mediated currents were significantly diminished by a bath application of genistein, a phosphotyrosine kinase (PTK) inhibitor (99). Furthermore, M channel-like activity is also involved in the antinociceptive effect of substance P. During tissue acidosis in the muscle, ASIC3 channels are activated by protons, and depolarize the muscle afferent neurons, resulting in the firing of action potentials and the release of substance P. Substance P activates NK1 receptors, and the activation of NK1 receptors leads to the unconventional pathway, which activates tyrosine kinase and the M channel in the muscle afferent neurons. ASIC3-mediated action potentials are inhibited by M channel activation (99).
CONCLUSION
Acid-sensing ion channels are pH sensors for detecting a wide range of pH fluctuations during normal physiological processes and pathological conditions. The functions and the properties of ASICs have been investigated using various pharmacological tools and genetic techniques. ASICs are implicated in a number of physiological activities, including pain perception, synaptic plasticity, learning and memory, fear, ischemic neuronal injury, and mechanosensation. However, questions remain about the involvement of ASICs in mechanosensation and learning and memory. Many signaling molecules that regulate the activity of ASICs exist, and ASICs can be modulated by GPCRs. However, the regulation of ASICs remains poorly understood. Further investigation into the regulatory mechanisms of ASICs is necessary for developing effective therapeutic strategies for the treatment of pain and neurological diseases.
Acknowledgments
We are grateful to Dong-Il Kim, Jin-Young Jeong, Dasom Baek, and Jihye Yeon for their valuable discussions and comments on our manuscript. This work was supported by the DGIST R&D Program of the Ministry of Education, Science and Technology of Korea (13-BD-0403) and the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (No. 2012R1A1A2044699).
References
- 1.Basbaum A. I., Bautista D. M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. (2009);139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steen K. H., Issberner W., Reeh P. W. Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci. Lett. (1995);199:29–32. doi: 10.1016/0304-3940(95)12002-L. [DOI] [PubMed] [Google Scholar]
- 3.Jones N. G., Slater R., Cadiou H., McNaughton P., McMahon S. B. Acid-induced pain and its modulation in humans. J. Neurosci. (2004);24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steen K. H., Reeh P. W., Anton F., Handwerker H. O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J. Neurosci. (1992);12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M. L., Von Hanwehr R., Siesjo B. K. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J. Cereb. Blood Flow Metab. (1986);6:574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- 6.Zha X. M. Acid-sensing ion channels: trafficking and synaptic function. Mol. Brain. (2013);6:1. doi: 10.1186/1756-6606-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wemmie J. A., Price M. P., Welsh M. J. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends. Neurosci. (2006);29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Miesenbock G., De Angelis D. A., Rothman J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. (1998);394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 9.Magnotta V. A., Heo H. Y., Dlouhy B. J., Dahdaleh N. S., Follmer R. L., Thedens D. R., Welsh M. J., Wemmie J. A. Detecting activity-evoked pH changes in human brain. Proc. Natl. Acad. Sci. U. S. A. (2012);109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. (2007);449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 11.Noel J., Salinas M., Baron A., Diochot S., Deval E., Lingueglia E. Current perspectives on acid-sensing ion channels: new advances and therapeutic implications. Exp. Rev. Clin. Pharmacol. (2010);3:331–346. doi: 10.1586/ecp.10.13. [DOI] [PubMed] [Google Scholar]
- 12.Lingueglia E., de Weille J. R., Bassilana F., Heurteaux C., Sakai H., Waldmann R., Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. (1997);272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y., Chen Z., Li W. G., Cao H., Feng E. G., Yu F., Liu H., Jiang H., Xu T. L. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. (2010);68:61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Bohlen C. J., Chesler A. T., Sharif-Naeini R., Medzihradszky K. F., Zhou S., King D., Sanchez E. E., Burlingame A. L., Basbaum A. I., Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. (2011);479:410–414. doi: 10.1038/nature10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W. G., Yu Y., Huang C., Cao H., Xu T. L. Nonproton ligand sensing domain is required for paradoxical stimulation of acid-sensing ion channel 3 (ASIC3) channels by amiloride. J. Biol. Chem. (2011);286:42635–42646. doi: 10.1074/jbc.M111.289058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherwood T. W., Lee K. G., Gormley M. G., Askwith C. C. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J. Neurosci. (2011);31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoagland E. N., Sherwood T. W., Lee K. G., Walker C. J., Askwith C. C. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J. Biol. Chem. (2010);285:41852–41862. doi: 10.1074/jbc.M110.171330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yermolaieva O., Leonard A. S., Schnizler M. K., Abboud F. M., Welsh M. J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. U.S.A. (2004);101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., Wei W. L., MacDonald J. F., Wemmie J. A., Price M. P., Welsh M. J., Simon R. P. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. (2004);118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Samways D. S., Harkins A. B., Egan T. M. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. (2009);45:319–325. doi: 10.1016/j.ceca.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pignataro G., Simon R. P., Xiong Z. G. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. (2007);130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 22.Gao J., Duan B., Wang D. G., Deng X. H., Zhang G. Y., Xu L., Xu T. L. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. (2005);48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Chen X., Grunder S. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J. Physiol. (2007);579:657–670. doi: 10.1113/jphysiol.2006.120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escoubas P., De Weille J. R., Lecoq A., Diochot S., Waldmann R., Champigny G., Moinier D., Menez A., Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J. Biol. Chem. (2000);275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 25.Diochot S., Baron A., Rash L. D., Deval E., Escoubas P., Scarzello S., Salinas M., Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. (2004);23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voilley N., de Weille J., Mamet J., Lazdunski M. Nonsteroid Anti-Inflammatory Drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. (2001);21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dube G. R., Lehto S. G., Breese N. M., Baker S. J., Wang X., Matulenko M. A., Honore P., Stewart A. O., Moreland R. B., Brioni J. D. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. (2005);117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Diochot S., Baron A., Salinas M., Douguet D., Scarzello S., Dabert-Gay A. S., Debayle D., Friend V., Alloui A., Lazdunski M., Lingueglia E. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. (2012);490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- 29.Babinski K., Catarsi S., Biagini G., Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+. J. Biol. Chem. (2000);275:28519–58525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- 30.Wang W., Duan B., Xu H., Xu L., Xu T. L. Calcium-permeable acid-sensing ion channel is a molecular target of the neurotoxic metal ion lead. J. Biol Chem. (2006);281:2497–2505. doi: 10.1074/jbc.M507123200. [DOI] [PubMed] [Google Scholar]
- 31.Staruschenko A., Dorofeeva N. A., Bolshakov K. V., Stockand J. D. Subunit-dependent cadmium and nickel inhibition of acid-sensing ion channels. J. Neurobiol. (2007);67:97–107. doi: 10.1002/neu.20338. [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Yu Y., Xu T. L. Modulation of acid-sensing ion channels by Cu2+ in cultured hypothalamic neurons of the rat. Neuroscience. (2007);145:631–641. doi: 10.1016/j.neuroscience.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 33.De Weille J., Bassilana F. Dependence of the acid-sensitive ion channel, ASIC1a, on extracellular Ca2+ ions. Brain Res. (2001);900:277–281. doi: 10.1016/S0006-8993(01)02345-9. [DOI] [PubMed] [Google Scholar]
- 34.Chu X. P., Wemmie J. A., Wang W. Z., Zhu X. M., Saugstad J. A., Price M. P., Simon R. P., Xiong Z. G. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J. Neurosci. (2004);24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron A., Schaefer L., Lingueglia E., Champigny G., Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J. Biol. Chem. (2001);276:35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- 36.Immke D. C., McCleskey E. W. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. (2001);4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 37.Smith E. S., Cadiou H., McNaughton P. A. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience. (2007);145:686–698. doi: 10.1016/j.neuroscience.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Duan B., Wang Y. Z., Yang T., Chu X. P., Yu Y., Huang Y., Cao H., Hansen J., Simon R. P., Zhu M. X., Xiong Z. G., Xu T. L. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J. Neurosci. (2011);31:2101–2112. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherwood T., Franke R., Conneely S., Joyner J., Arumugan P., Askwith C. Identification of protein domains that control proton and calcium sensitivity of ASIC1a. J. Biol. Chem. (2009);284:27899–27907. doi: 10.1074/jbc.M109.029009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadiou H., Studer M., Jones N. G., Smith E. S., Ballard A., McMahon S. B., McNaughton P. A. Modulation of acid-sensing ion channel activity by nitric oxide. J. Neurosci. (2007);27:13251–13260. doi: 10.1523/JNEUROSCI.2135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Askwith C. C., Cheng C., Ikuma M., Benson C., Price M. P., Welsh M. J. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. (2000);26:133–141. doi: 10.1016/S0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- 42.Sherwood T. W., Askwith C. C. Endogenous arginine-phenylalanine-amide-related peptides alter steadystate desensitization of ASIC1a. J. Biol. Chem. (2008);283:1818–1830. doi: 10.1074/jbc.M705118200. [DOI] [PubMed] [Google Scholar]
- 43.Alijevic O., Kellenberger S. Subtype-specific modulation of acid-sensing ion channel (ASIC) function by 2-guanidine-4-methylquinazoline. J. Biol. Chem. (2012);287:36059–36070. doi: 10.1074/jbc.M112.360487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wemmie J. A., Askwith C. C., Lamani E., Cassell M. D., Freeman J. H. Jr., Welsh M. J. Acid-sensing ion channel 1 is localized in brain Rregions with high synaptic density and contributes to fear conditioning. J. Neurosci. (2003);23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de la Rosa D. A., Krueger S. R., Kolar A., Shao D., Fitzsimonds R. M., Canessa C. M. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J. Physiol. (2003);546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zha X. M., Costa V., Harding A. M., Reznikov L., Benson C. J., Welsh M. J. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J. Neurosci. (2009);29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H. Jr., Welsh M. J. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. (2002);34:463–477. doi: 10.1016/S0896-6273(02)00661-X. [DOI] [PubMed] [Google Scholar]
- 48.Wu P. Y., Huang Y. Y., Chen C. C., Hsu T. T., Lin Y. C., Weng J. Y., Chien T. C., Cheng I. H., Lien C. C. Acid-sensing ion channel-1a is not required for normal hippocampal LTP and spatial memory. J. Neurosci. (2013);33:1828–1832. doi: 10.1523/JNEUROSCI.4132-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu X. P., Xiong Z. G. Acid-sensing ion channels in pathological conditions. Adv. Exp. Med. Biol. (2013);961:419–431. doi: 10.1007/978-1-4614-4756-6_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn Y. J., Kim H., Lim H., Lee M., Kang Y., Moon S., Kim H. S., Kim H. H. AMP-activated protein kinase: implications on ischemic diseases. BMB Rep. (2012);45:489–495. doi: 10.5483/BMBRep.2012.45.9.169. [DOI] [PubMed] [Google Scholar]
- 51.Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. (1988);1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 52.Wahlgren N. G., Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies--the need for new approaches. Cerebrovasc Dis. (2004);17(Suppl 1):153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- 53.Xiong Z. G., Pignataro G., Li M., Chang S. Y., Simon R. P. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr. Opin. Pharmacol. (2008);8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somjen G. G. Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res. (1984);311:186–188. doi: 10.1016/0006-8993(84)91416-1. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell W. G., Grubbs R. C. Inhibition of audiogenic seizures by carbon dioxide. Science. (1956);123:223–224. doi: 10.1126/science.123.3189.223. [DOI] [PubMed] [Google Scholar]
- 56.Velisek L., Dreier J. P., Stanton P. K., Heinemann U., Moshe S. L. Lowering of extracellular pH suppresses low-Mg2+-induces seizures in combined entorhinal cortex-hippocampal slices. Exp. Brain Res. (1994);101:44–52. doi: 10.1007/BF00243215. [DOI] [PubMed] [Google Scholar]
- 57.Ziemann A. E., Schnizler M. K., Albert G. W., Severson M. A., Howard M. A. 3rd, Welsh M. J., Wemmie J. A. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. (2008);11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friese M. A., Craner M. J., Etzensperger R., Vergo S., Wemmie J. A., Welsh M. J., Vincent A., Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. (2007);13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 59.Krishtal O. A., Pidoplichko V. I. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. (1981);6:2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- 60.Ikeuchi M., Kolker S. J., Burnes L. A., Walder R. Y., Sluka K. A. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. (2008);137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen N. J., Attwell D. Modulation of ASIC channels in rat cerebellar purkinje neurons by ischaemia-related signals. J. Physiol. (2002);543:521–529. doi: 10.1113/jphysiol.2002.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deval E., Noel J., Lay N., Alloui A., Diochot S., Friend V., Jodar M., Lazdunski M., Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. (2008);27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delmas P., Hao J., Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. (2011);12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 64.Chen C. C., Wong C. W. Neurosensory mechanotransduction through acid-sensing ion channels. J. Cell. Mol. Med. (2013);17:337–349. doi: 10.1111/jcmm.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price M. P., Lewin G. R., Mcllwrath S. L., Cheng C., Xie J., Heppenstall P. A., Stucky C. L., Mannsfeldt A. G., Brennan T. J., Drummond H. A., Qiao J., Benson C. J., Tarr D. E., Hrstka R. F., Yang B., Williamson R. A., Welsh M. J. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. (2000);407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 66.Drew L. J., Rohrer D. K., Price M. P., Blaver K. E., Cockayne D. A., Cesare P., Wood J. N. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J. Physiol. (2004);556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farooqui A. A., Ong W. Y., Horrocks L. A. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol. Rev. (2006);58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 68.Liu L., Rittenhouse A. R. Effects of arachidonic acid on unitary calcium currents in rat sympathetic neurons. J. Physiol. (2000);525:391–404. doi: 10.1111/j.1469-7793.2000.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chyb S., Raghu P., Hardie R. C. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. (1999);397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 70.Meves H. Arachidonic acid and ion channels: an update. Br. J. pharmacol. (2008);155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson L. S., Kim K., Memmott J. E., Li C. FMRFamide-related gene family in the nematode, Caenorhabditis elegans. Brain. Res. Mol. Brain. Res. (1998);58:103–111. doi: 10.1016/S0169-328X(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 72.Schneider L. E., Taghert P. H. Isolation and characterization of a Drosophila gene that encodes multiple neuropeptides related to Phe-Met-Arg-Phe-NH2 (FMRFamide). Proc. Natl. Acad. Sci. U. S. A. (1988);85:1993–1997. doi: 10.1073/pnas.85.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gayton R. J. Mammalian neuronal actions of FMRFamide and the structurally related opioid Met-enkephalin-Arg6-Phe7. Nature. (1982);298:275–276. doi: 10.1038/298275a0. [DOI] [PubMed] [Google Scholar]
- 74.Hauser K. F., Foldes J. K., Turbek C. S. Dynorphin A (1-13) neurotoxicity in vitro: opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp. Neurolo. (1999);160:361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Babini E., Paukert M., Geisler H. S., Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J. Biol. Chem. (2002);277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 76.Sherwood T. W., Askwith C. C. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J. Neurosci. (2009);29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hilgemann D. W., Ball R. Regulation of cardiac Na+, Ca2+ Exchange and KATP Potassium Channels by PIP2. Science. (1996);273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 78.Suh B. C., Hille B. Recovery from muscarinic modulation of M current channels requires Phosphatidylinositol 4,5-Bisphosphate synthesis. Neuron. (2002);35:507–520. doi: 10.1016/S0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 79.Suh B. C., Inoue T., Meyer T., Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. (2006);314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suh B. C., Leal K., Hille B. Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. (2010);67:224–238. doi: 10.1016/j.neuron.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohacs T., Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers. Archiv. (2007);455:157–168. doi: 10.1007/s00424-007-0275-6. [DOI] [PubMed] [Google Scholar]
- 82.Rohacs T., Lopes C. M., Jin T., Ramdya P. P., Molnar Z., Logothetis D. E. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. U. S. A. (2003);100:745–750. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z., Okawa H., Wang Y., Liman E. R. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J. Biol. Chem. (2005);280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]
- 84.Suh B. C., Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. (2008);37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pochynyuk O., Tong Q., Medina J., Vandewalle A., Staruschenko A., Bugaj V., Stockand J. D. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J. Gen. Physiol. (2007);130:399–413. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li T., Yang Y., Canessa C. M. Impact of recovery from desensitization on acid-sensing ion channel-1a (ASIC1a) current and response to high frequency stimulation. J. Biol. Chem. (2012);287:40680–40689. doi: 10.1074/jbc.M112.418400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dorofeeva N. A., Karpushev A. V., Nikolaev M. V., Bolshakov K. V., Stockand J. D. Muscarinic M1 modulation of acid-sensing ion channels. Neuroreport. (2009);20:1386–1391. doi: 10.1097/WNR.0b013e3283318912. [DOI] [PubMed] [Google Scholar]
- 88.Duan B., Liu D. S., Huang Y., Zeng W. Z., Wang X., Yu H., Zhu M. X., Chen Z. Y., Xu T. L. PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J. Neurosci. (2012);32:6351–6363. doi: 10.1523/JNEUROSCI.4479-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahluwalia J., Urban L., Capogna M., Bevan S., Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. (2000);100:685–688. doi: 10.1016/S0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 90.Hohmann A. G., Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. (1999);90:923–931. doi: 10.1016/S0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y. Q., Qiu F., Qiu C. Y., Cai Q., Zou P., Wu H., Hu W. P. Cannabinoids inhibit acid-sensing ion channel currents in rat dorsal root ganglion neurons. PLoS One. (2012);7:e45531. doi: 10.1371/journal.pone.0045531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chai S., Li M., Lan J., Xiong Z. G., Saugstad J. A., Simon R. P. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J. Biol. Chem. (2007);282:22668–22677. doi: 10.1074/jbc.M703624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu F., Qiu C. Y., Liu Y. Q., Wu D., Li J. D., Hu W. P. Potentiation of acid-sensing ion channel activity by the activation of 5-HT2 receptors in rat dorsal root ganglion neurons. Neuropharmacology. (2012);63:494–500. doi: 10.1016/j.neuropharm.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 94.Mamet J., Baron A., Lazdunski M., Voilley N. ProInflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J. Neurosci. (2002);22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tokunaga A., Saika M., Senba E. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. (1998);76:349–355. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 96.Lin S. Y., Chang W. J., Lin C. S., Huang C. Y., Wang H. F., Sun W. H. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J. Neurosci. (2011);31:1410–1418. doi: 10.1523/JNEUROSCI.4682-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lingueglia E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. (2007);282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 98.Duggan A., Garcia-Anoveros J., Corey D. P. The PDZ domain protein PICK1 and the sodium channels BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J. Biol. Chem. (2002);277:5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- 99.Lin C. C., Chen W. N., Chen C. J., Lin Y. W., Zimmer A., Chen C. C. An antinociceptive role for substance P in acid-induced chronic muscle pain. PNAS. (2012);109:E76–E83. doi: 10.1073/pnas.1108903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benson C. J., Xie J., Wemmie J. A., Price M. P., Henss J. M., Welsh M. J., Snyder P. M. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. U. S. A. (2002);99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sutherland S. P., Benson C. J., Adelman J. P., McCleskey E. W. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. U. S. A. (2001);98:711–716. doi: 10.1073/pnas.98.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. (1997);386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 103.Waldmann R., Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr. Opin. Neurobiol. (1998);8:418–424. doi: 10.1016/S0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 104.Baron A., Voilley N., Lazdunski M., Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J. Neurosci. (2008);28:1498–1508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ettaiche M., Deval E., Cougnon M., Lazdunski M., Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J. Neurosci. (2006);26:5800–5809. doi: 10.1523/JNEUROSCI.0344-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walder R. Y., Rasmussen L. A., Rainier J. D., Light A. R., Wemmie J. A., Sluka K. A. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J. Pain. (2010);11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ettaiche M., Guy N., Hofman P., Lazdunski M., Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J. Neurosci. (2004);24:1005–1012. doi: 10.1523/JNEUROSCI.4698-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ugawa S., Minami Y., Guo W., Saishin Y., Takatsuji K., Yamamoto T., Tohyama M., Shimada S. Receptor that leaves a sour taste in the mouth. Nature. (1998);395:555–556. doi: 10.1038/26882. [DOI] [PubMed] [Google Scholar]
- 109.Lu Y., Ma X., Sabharwal R., Snitsarev V., Morgan D., Rahmouni K., Drummond H. A., Whiteis C. A., Costa V., Price M., Benson C., Welsh M. J., Chapleau M. W., Abboud F. M. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. (2009);64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


