Abstract
Inflammatory conditions mediated by activated microglia lead to chronic neuro-degenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s diseases. This study was conducted to determine the effect of floridoside isolated from marine red algae Laurencia undulata on LPS (100 ng/ml) activated inflammatory responses in BV-2 microglia cells. The results show that floridoside has the ability to suppress pro-inflammatory responses in microglia by markedly inhibiting the production of nitric oxide (NO) and reactive oxygen species (ROS). Moreover, floridoside down-regulated the protein and gene expression levels of iNOS and COX-2 by significantly blocking the phosphorylation of p38 and ERK in BV-2 cells. Collectively, these results indicate that floridoside has the potential to be developed as an active agent for the treatment of neuro-inflammation. [BMB Reports 2013; 46(8): 398-403]
Keywords: Alzheimer’s disease, Floridoside, Laurencia undulata, Neuro-inflammation, Oxidative stress
INTRODUCTION
Microglia, a resident macrophage cell in the CNS, comprises about 10% of the total glial cells of the brain (1). Upon activation, microglia change their morphology from ramified to amoeboid shape, and secrete inflammatory cytokines and neurotoxic factors (2). However, the excessive production of inflammatory mediators such as interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), nitric oxide (NO), prostaglandin E2 (PGE2), and reactive oxygen species (ROS) could lead to the breakdown of CNS balance (3). Previous reports indicated that excessive activation of microglia caused chronic neuro-degeneration in patients with Alzheimer’s, Parkinson’s, and Huntington’s diseases (4).
Chronic neuro-degeneration could be mimicked by a wide variety of stimulators, such as lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), and hydrogen peroxide (H2O2). Exposure to LPS particularly activates microglia through TLR4 receptor, and induces the secretion of pro-inflammatory mediators, eventually causing neuron injury (5). NO and PGE2 are secreted by activated microglia have been reported as crucial factors for the progression of neuro-inflammation (6-8). NO is generated through nitric oxide synthases enzyme (iNOS), while PGE2 is produced by cyclooxygenase-2 enzyme (COX-2) (9). The expression of iNOS and COX-2 are regulated via mitogen activated protein kinase (MAPK) signaling pathways (10-12).
Recently, more attention has been given to explore natural metabolites as potential therapeutic agents against toxicities associated with chronically activated microglia (13). For example, hepitaondiol, pacifenol, stypotriol triacetate, and paeonol are natural marine substances that have been reported as potent neuro-protective agents (14,15). Marine organisms are a source of plentiful bioactive metabolites, and marine red algae are an especially well-known source of anti-bacterial and antioxidant metabolites (16,17). Floridoside, a natural glycerol galactoside, was initially isolated in 1930 from the red algae, Laurencia undulata (L. undulata), by Colin and Gueguen (18). The molecular formula of floridoside is C9H18O8, and it contains six carbon rings with 4 hydroxyl groups and one galactopyranosyl residue with 2 hydroxyl groups (19). In a previous study, the ROS scavenging ability of floridoside was identified (19). The present study was planned to investigate the protective effects of floridoside against neuro-inflammation in LPS-activated BV-2 microglia cells.
RESULTS
Selection of the stimulator
Microglia cells are activated by the stimulation of LPS, PMA, and H2O2 (20,21). These three stimulators were used to investigate the influence of different stimulators on NO production. BV-2 cells were treated with different concentrations (10, 50 and 100 ng/ml) of each stimulator for 24 h. As shown in Fig. 1B, LPS-treated BV-2 cells produced more NO over other stimulators. Therefore, LPS (100 ng/ml) was selected as the stimulator for this study.
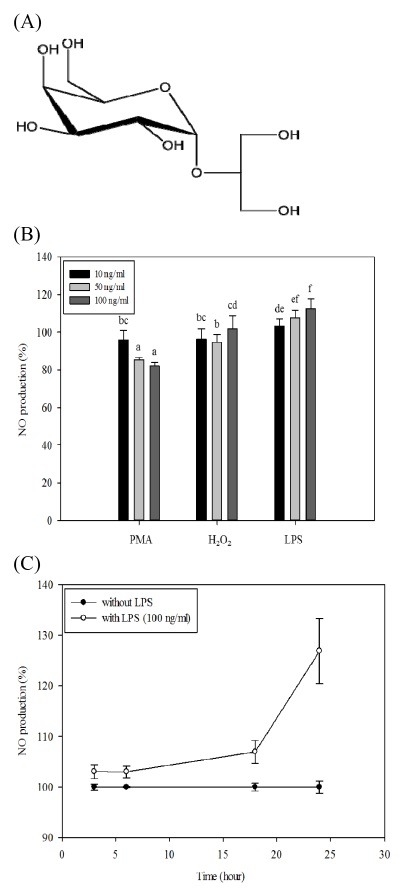
Fig. 1. The structure of floridoside and the effect of different stimulators on NO production of BV-2 cells. (A) Chemical structure of floridoside. (B) Effect of different stimulators on the NO production of BV-2 cells. Cells were maintained in serum-free medium for 1h and treated with different concentrations (10, 50, 100 ng/ml) of the stimulators for 24 h. Conditioned media was collected and NO production level was assessed by using Griess reagent. (C) Time dependent effect of LPS (100 ng/ml) on BV-2 cells. LPS was treated to BV-2 cells cultured in serum free media. Conditioned media was collected at different times (3, 6, 18, 24 and 50 h) and NO concentration was measured. Data are presented as means ± standard deviation (SD) of three independent experiments (n = 3 total). a-fMeans with different letters are significantly different (P < 0.05) according to Tukey b multiple range test.
To examine the effect of LPS with incubation time, the release levels of NO were tested in BV-2 cells treated with LPS (100 ng/ml) and incubated at different time intervals (3, 6, 18 and 24 h). As shown in Fig. 1C, NO secretion was significantly increased after 18 h. 24 h of incubation with LPS (100 ng/ml) was selected for BV-2 cell activation.
NO inhibitory effect of floridoside in activated BV-2 cells
Activated microglia cells lead to the generation of NO (22). Before the investigation of the NO inhibitory effect of floridoside on LPS-stimulated BV-2 cells, the cytotoxicity of floridoside was confirmed by MTT assay. BV-2 cells were treated with different concentrations of floridoside (1, 10 and 50 μM) for 24 h. As shown in Fig. 2A, all groups tested showed more than 90% cell viability. These results indicated that floridoside (1-50 μM) has no significant cytotoxicity on BV-2 cells (23-25).
Fig. 2. Effect of floridoside on the viability and activation of BV-2 cells. (A) Effect of floridoside on viability of BV-2 cells. Cells were serum starved for 1 h and treated with LPS (100 ng/ml) followed by the treatment with floridoside (1, 10 and 50 μM) 24 h. Cell viability was assessed by MTT assay. (B) Time dependent NO inhibitory effect of floridoside. Cells were cultured in serum-free media and treated with LPS (100 ng/ml) for 1 h and then incubated with floridoside (1, 10 and 50 μM) for 50 h. Conditioned media was collected at different times (18, 24 and 50 h) and NO production levels were compared. (C) Inhibitory effects of different concentrations (1, 10 and 50 μM) of floridoside on LPS-induced NO production in BV-2 cells. Conditioned medium was collected after 24 h and NO concentration was measured using the Griess reaction. Standard nitrite curve was used to quantify the NO production level. Data are presented as means ± standard deviation (SD) of three independent experiments (n = 3 total). a-eMeans with different letters are significantly different (P < 0.05) according toTukey b multiple range test.
As shown in Fig. 2B, LPS-induced NO production (% of control) of BV-2 cells was significantly decreased by floridoside at 18, 24 and 50 h of incubation. To quantify the effect of floridoside on NO production in activated microglia, BV-2 cells were exposed to LPS with floridoside for 24 h. As shown in Fig. 2C, floridoside dose-dependently suppressed NO production. Treatment with 50 μM of floridoside showed around 50% reduction in NO level in activated BV-2 cells.
Intracellular ROS scavenging effect of floridoside in activated BV-2 cells
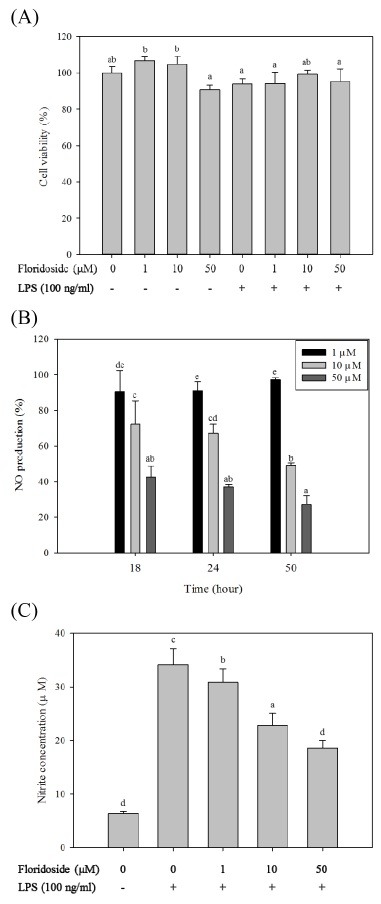
To analyze the intracellular ROS scavenging effect of floridoside, BV-2 cells were activated with H2O2 (500 μM). Intracellular ROS levels were measured by detecting the fluorescence intensity of the oxidant-sensitive probe DCFH-DA. BV-2 cells were treated with different concentrations of floridoside (1, 10 and 50 μM) for 1 h, followed by stimulation with H2O2 (500 μM). As shown in Fig. 3, floridoside dose-dependently reduced intracellular ROS levels. The ROS scavenging effect was dose dependent, and 50 μM of floridoside decreased the fluorescence intensity by up to 59.89% compared to the control group untreated with floridoside.
Fig. 3. ROS scavenging effect of floridoside in H2O2 (500 μM) activated BV-2 cells. Intracellular ROS levels were measured by detecting the fluorescence intensity of the oxidant-sensitive florescent probe DCFH-DA. Florescence intensity was recorded at different time intervals (0-120 min) in the presence of floridoside (1, 10 and 50 μM).
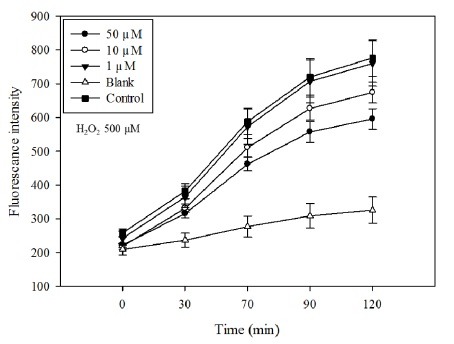
Inhibitory effect of floridoside on iNOS and COX-2 expression in activated BV-2 cells
Activated microglia induces the expression of inflammatory enzymes such as iNOS and COX-2. Western blot and RT-PCR analysis were used to measure the protein and gene expression levels of iNOS and COX-2. To determine whether floridoside inhibits LPS-induced iNOS and COX-2 expression in BV-2 cells, cells were treated with LPS in the presence or absence of floridoside. As shown in Fig. 4A, mRNA and protein expression of iNOS and COX-2 have increased in LPS-treated BV-2 cells. However, the cell groups treated with floridoside showed dose-dependent inhibition of iNOS and COX-2 expression. Moreover, the cells treated with 50 μM of floridoside showed significantly lower iNOS and COX-2 expressions. The floridoside-induced reduction in iNOS expression exhibits a similar pattern to that of the floridoside-mediated inhibition of NO production (Fig. 2B, 2C). These results indicate that floridoside has probably suppressed the NO production by reducing the protein and gene expression levels of iNOS.
Fig. 4. Effect of floridoside on the expression of signaling molecules in LPS activated BV-2 cells. (A) Floridoside mediated inhibition of protein and mRNA expression of COX-2 and iNOS. Protein and mRNA levels of COX-2 and iNOS were analyzed by Western blot and RT-PCR, respectively. β-actin and β-tubulin expressions were used as an internal controls. (B) Effect of floridoside on inhibition of MAPK molecules, p38, JNK and ERK1/2. Protein expression levels were analyzed by Western blot. The expression levels were quantified and presented in graphical form. Data are presented as means ± standard deviation (SD) of three independent experiments (n = 3 total). a-fMeans withdifferent letters are significantly different (P < 0.05) according to Tukey b multiple range test.
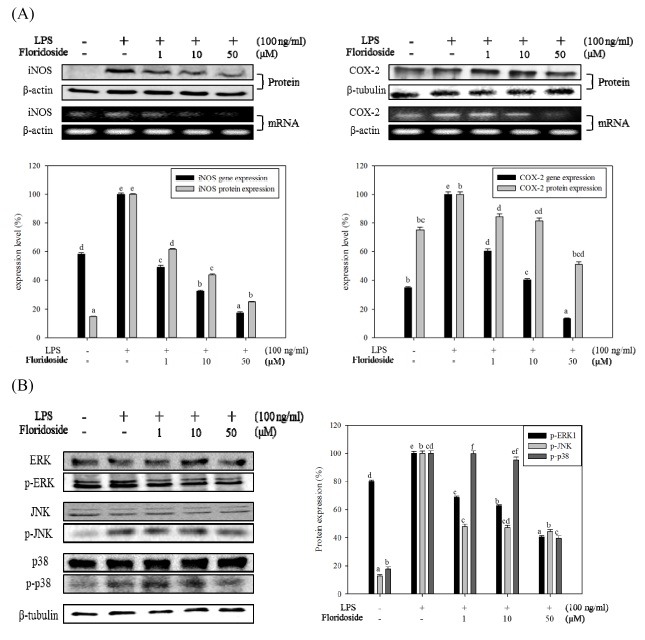
Effect of floridoside on MAPK protein expressions in activated BV-2 cells
To assess the effect of floridoside on MAPK signaling pathway molecules, Western blot analysis was used. BV-2 cells were prepared in the same way as described earlier. As shown in Fig. 4B, floridoside did not inhibit the LPS-induced phosphorylation levels of JNK, while ERK1/2 and p38 were evidently inhibited by floridoside treatment. Phospho-ERK1 expression levels were gradually decreased, while the phosphorylation of p38 was markedly inhibited at a concentration of 50 μM.
DISCUSSION
Microglia residing in the CNS is involved in regulating immune responses. However, the uncontrolled activation of microglia leads to progressive neurodegenerative disorders. Microglia are activated due to various stress factors. Upon activation, both immediate protein kinase responses and subsequent changes in the expression of hundreds of target genes lead to a neuro-inflammatory response (26). A number of reports have shown that activated microglia release excessive inflammatory mediators such as NO, ROS, and cytokines (27). Therefore, regulating microglia activation is considered a major target in the treatment of neuro-degenerative diseases.
In the current study, the effect of floridoside isolated from marine red algae Laurencia undulata against LPS-mediated BV-2 microglia activation was analyzed. Our results indicated that LPS stimulation elevates NO production via up-regulating iNOS expression more efficiently than other stimulators such as H2O2 and PMA (Fig. 1B). Moreover, it was observed that NO production was highest at 24 hours of LPS (100 ng/ml) stimulation compared with non-stimulated microglia cells (Fig. 1C). From these results, it was identified that microglia cells activate and generate NO effectively by LPS (100 ng/ml) treatment for 24 hours.
Recently, many reports have focused on the identification of preventive agents from marine sources for neuro-inflammatory diseases (14,15). In a previous study, it was found that floridoside shows a significant ROS scavenging effect on macrophage cells (19). Therefore, in this study, focus was centered on evaluating whether floridoside has the ability to control inflammatory responses in activated microglia. Under inflammatory conditions generated by 100 ng/ml of LPS, floridoside inhibited the NO and ROS production levels, dose- and time-dependently (Fig. 2B, 2C, 3).
The unregulated production of NO and ROS by activated microglia cells could damage or mediate the death of nearby neurons, by inhibiting mitochondrial respiration in neuron cells (28). In addition, COX-2 also plays an important role in mediating PGE2 production, which is related to the neuro-inflammatory response (29). Our results showed that floridoside significantly attenuated both the LPS-induced up-regulation of iNOS, COX-2 mRNA, and protein expressions, and thereby inhibited NO and PGE2 production levels.
Recent studies have revealed that marine compounds can attenuate signalling pathways, such as MAPK pathways - - JNK, p38, and ERK1/2 - - which are connected to the regulation of the neuro-inflammatory response (13,30). In this study, it was shown that the LPS-activated phosphorylation of p38 and ERK1/2 were inhibited by floridoside. However, the expression level of phospho-JNK did not inhibit by floridoside treatment (Fig. 4B). The p38 and ERK1/2 signalling pathways are known to regulate the transcription of pro-inflammatory enzymes likes iNOS and COX-2. Moreover, recent research has also shown that NO production in microglia cell is suppressed via blocking ERK and p38 signaling pathways (31). Therefore, it could be suggested that the neuro-inflammatory response in activated microglia and the subsequent production of ROS and NO via overexpressed iNOS and COX-2 were inhibited by floridoside treatment, most probably through the suppression of p38 and ERK1/2 signalling pathways. Collectively, it can be suggested that floridoside has the potential to act against progressive neuron damage via regulating microglia activation.
The active focus of floridoside is ascribed to the six carbon rings with 4 hydroxyl groups and one galactopyranosyl residue with 2 hydroxyl groups (Fig. 1A). Most of the bioactive compounds show strong antioxidative activity by reacting as hydrogen-or electron-donating agents, and metal ion chelating properties of their phenolic groups. The results presented suggest that the floridoside with hydroxyl groups has higher protection against inflammation and oxidative stress. In conclusion, this study has revealed that floridoside effectively attenuates the inflammatory response in LPS-activated microglia cells via blocking p38 and ERK MAPK signalling pathways. Therefore, it could be suggested that floridoside could be developed as a candidate therapeutic agent against neuro-inflammation- mediated neuro-degeneration.
MATERIALS AND METHODS
Chemicals and preparation
Floridoside isolated from the red seaweed L. undulata was obtained from our previous study (19). BV-2 mouse microglia cells were purchased from Gibco BRL, Life Technologies (Grand Island, NY). Cell culture medium [DMEM], penicillin/streptomycin, FBS, and other materials required for culturing cells were purchased from Gibco BRL, Life Technologies (Grand Island, NY). LPS of Escherichia coli 026:B6, Griess reagent, and MTT were acquired from Sigma (St. Louis, US). Specific antibodies used for western blot analysis were purchased from Santa Cruz Biotechnology Inc. and Amersham Pharmacia Biosciences (Piscataway, US). Other chemicals and reagents used in this study were analytical grade.
Cell culture and viability assay
BV-2 cells were maintained in a 5% CO2 humidified atmosphere at 37℃ in DMEM supplemented with 5% heat-inactivated FBS, 100 μg/ml of streptomycin, and 100 U/ml of penicillin. The cells were cultured via two to three passages per week. Before the experiment, the cells were conditioned at least 1 h in serum-free medium.
Cell viability was determined by MTT reduction assay, as described by Hansen et al. (32). In brief, the cells were pre-incubated overnight in 96-well plates and pre-treated with 100 ng/ml concentrations of LPS for 1 h. Then, the cells were treated with different concentrations (1, 10 and 50 μM) of floridoside and incubated at 37℃ for 24 h. After the culture supernatants were removed, the resulting purple formazan were dissolved with DMSO (33). Absorbance values were read at 540 nm on a GENios microplate reader (Tecan Austria GmbH). Relative cell viability was calculated relative to the absorbance of the LPS untreated group.
ROS assay
Intracellular ROS levels were measured by detecting the fluorescence intensity of the oxidant-sensitive DCFH-DA. In brief, DCFH-DA is diffused into the cells, and is de-acetylated by cellular esterase to non-fluorescent 2’, 7’-Dichlorodihydrofluorescin (DCFH), which is rapidly oxidized to highly fluorescent 2’, 7’-Dichlorodihydrofluorescein (DCF) by ROS. BV-2 cells grown in fluorescence microtiter 96-well black plates were labeled with 20 μM DCFH-DA in HBSS, and incubated for 30 min in the dark at 37℃. Then, the cells were treated with different concentrations of floridoside and incubated for an additional 1 h. After the cells were washed with PBS 4 times, 500 μM H2O2 (100 μl) was added. The intensity of the fluorescence signal was detected time-dependently with an excitation wavelength of 485 nm and emission wavelength of 535 nm using a GENios microplate reader. The dose-dependent and time-dependent effects of treatment groups were plotted and compared with the fluorescence intensity of the control (H2O2 treated) and blank (H2O2 untreated) groups.
NO assay
NO production of the culture supernatants were measured by the Griess reaction, as described earlier by Coker and Laurent (34). In brief, nitrite is detected and analyzed by the formation of red pink color upon mixing NO2- containing conditioned media with Griess reagent. The cells were pre-treated with 100 ng/ml concentrations of LPS for 1 h. The cells were treated with different concentrations of floridoside (1, 10, and 50 μM) and incubated at 37℃ for 24 h. Then, 50 μl of culture supernatants from each sample were mixed with the same volume of Griess reagent, and the absorbance values were read after 15 min at 440 nm on a GENios microplate reader (Tecan Austria GmbH).
Western blot analysis
Standard procedures were used for the Western blotting. In brief, BV-2 cells pre-treated with LPS (100 ng/ml) for 1 h and treated with different concentrations of floridoside (1, 10 and 50 μM) for 24 h in 37℃ incubator were lysed in RIPA buffer (sigma aldrich, USA), for 1 min. The remaining cell debris was removed by centrifugation. The protein concentrations of the cell lysates were determined using BCA method. Cell lysates containing equal amounts of proteins (≈ 20 μg of total protein) were separated by SDS-PAGE gel electrophoresis and electro-blotted onto a nitrocellulose membrane. The membranes were blocked with 5% BSA and then incubated with desired primary and secondary antibodies (primary antibodies; ERK, p-ERK, JNK, p-JNK, p38, p-p38, COX-2, iNOS, β-actin, β-tubulin (Santa Cruz)). The protein expressions were detected by chemiluminescent ECL assay kit (Amersham Pharmacia Biosciences), according to the manufacturer’s instructions. Blots were visualized using an LAS3000Ⓡ Luminescent image analyzer, and the protein expression levels were quantified by Multi Gauge V3.0 software (Fujifilm Life Science, Tokyo, Japan).
RNA extraction and Reverse transcription (RT)-PCR analysis
Total RNA was extracted from BV-2 cells treated with LPS in the presence or absence of floridoside using TRIzolⓇ reagent, as reported in the manufacturer’s manual. The cDNA synthesized from mRNA was then incubated for a further 1 h at 42℃. PCR was carried out in an automatic Whatman thermocycler (Biometra, Kent, UK). Single-stranded cDNA was amplified by PCR with specific primers for iNOS, COX-2, and β-actin, and primer sequences were used to amplify the desired cDNA fragment as follows: iNOS forward and reverse primers: 5'-CCCTTCCGAAGTTTCTGGCAGCAGC-3' and 5'-GGCTGTCAGAGCCTCGTGGCTTTGG-3'; COX-2 forward and reverse primers: 5'-GGGGTACCTTCCAGCTGTCAAAATCTC-3' and 5'-GAAGATCTCGCCAGGTACTCACCTG-3'; β-actin forward and reverse primers: 5'-CCACAGCTGAGAGGGAAATC-3' and 5'-AAGGAAGGCTGGAAAAGAGC-3'. The following PCR conditions were applied for all amplifications: 30 cycles of denaturation at 94℃ for 30 s, annealing at 57℃ for 30 s, and extended at 72℃ for 30 s. The resulting cDNA was separated by electrophoresis on 1% agarose gel for 15 min at 100 V, followed by visualization under UV light after ethidium bromide staining. Band intensities were quantified with Multi gauge software (Fujifilm Life Science, Tokyo, Japan), and the bands of specific genes were normalized using β-actin as references.
Statistical analysis
All data are presented as means ± standard deviation (SD). The mean values were calculated based on data from at least three independent experiments that were conducted on separate days using freshly prepared reagents. Data were analyzed using the analysis of variance (ANOVA) test of the statistical package for the social sciences (SPSS). Significance differences between treatment groups were determined using Tukey b multiple range tests. The significance of differences was defined at the P < 0.05 level.
Acknowledgments
This research was supported by a Grant from the Marine Bioprocess Research Center of the Marine Biotechnology Program, funded by the Ministry of Land, Transport, and Maritime Affairs, Republic of Korea.
References
- 1.Yokoyama A., Yang L., Itoh S., Mori K., Tanaka J. Microglia, a potential source of neurons, astrocytes, and oligodendrocytes. Glia. (2004);45:96–104. doi: 10.1002/glia.10306. [DOI] [PubMed] [Google Scholar]
- 2.Vilhardt F. Microglia: phagocyte and glia cell. Int. J. Biochem. Cell Biol. (2005);37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Matyszak M. K. Inflammation in the CNS: balance between immunological privilege and immune responses. Prog. Neurobiol. (1998);56:19–35. doi: 10.1016/S0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 4.Lull M. E., Block M. L. Microglial activation and chronic neurodegeneration. Neurotherapeutics. (2010);7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehnardt S., Massillon L., Follett P., Jensen F. E., Ratan R., Rosenberg P. A., Volpe J. J., Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. U. S. A. (2003);100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett J. A., Sweetland M. A., Wang J. L., Lancaster J. R., Jr., McDaniel M. L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc. Natl. Acad. Sci. U. S. A. (1993);90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvemini D., Seibert K., Masferrer J. L., Misko T. P., Currie M. G., Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J. Clin. Invest. (1994);93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vane J. R., Mitchell J. A., Appleton I., Tomlinson A., Bishop-Bailey D., Croxtall J., Willoughby D. A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. (1994);91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae D. S., Kim Y. H., Pan C. H., Nho C. W., Samdan J., Yansan J., Lee J. K. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep. (2011);45:108–113. doi: 10.5483/BMBRep.2012.45.2.108. [DOI] [PubMed] [Google Scholar]
- 10.Thalhamer T., McGrath M. A., Harnett M. M. MAPKs and their relevance to arthritis and inflammation. Rheumatology. (2008);47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 11.Takebe K., Nishiyama T., Hayashi S., Hashimoto S., Fujishiro T., Kanzaki N., Kawakita K., Iwasa K., Kuroda R., Kurosaka M. Regulation of p38 MAPK phosphorylation inhibits chondrocyte apoptosis in response to heat stress or mechanical stress. Int. J. Mol. Med. (2011);27:329–335. doi: 10.3892/ijmm.2010.588. [DOI] [PubMed] [Google Scholar]
- 12.Kim S. B., Jung E. S., Shin S. W., Kim M. H., Kim Y. S., Lee J. S., Park D. H. Anti-inflammatory activity of Camellia Japonica oil. BMB Rep. (2012);45:177–182. doi: 10.5483/BMBRep.2012.45.3.177. [DOI] [PubMed] [Google Scholar]
- 13.Park H. Y., Han M. H., Park C., Jin C. Y., Kim G. Y., Choi I. W., Kim N. D., Nam T. J., Kwon T. K., Choi Y. H. Anti-inflammatory effects of fucoidan through inhibition of NF-kappaB, MAPK and Akt activation in lipopolysaccharideinduced BV2 microglia cells. Food Chem. Toxicol. (2011);49:1745–1752. doi: 10.1016/j.fct.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Gil B., Ferrandiz M. L., Sanz M. J., Terencio M. C., Ubeda A., Rovirosa J., San-Martin A., Alcaraz M. J., Paya M. Inhibition of inflammatory responses by epitaondiol and other marine natural products. Life Sci. (1995);57:25–30. doi: 10.1016/0024-3205(95)00260-D. [DOI] [PubMed] [Google Scholar]
- 15.Himaya S. W., Ryu B., Qian Z. J., Li Y., Kim S. K. 1-(5-bromo-2-hydroxy-4-methoxyphenyl) ethanone [SE1] suppresses pro-inflammatory responses by blocking NF-kappaB and MAPK signaling pathways in activated microglia. Eur. J. Pharmacol. (2011);670:608–616. doi: 10.1016/j.ejphar.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kelman D., Posner E. K., McDermid K. J., Tabandera N. K., Wright P. R., Wright A. D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs. (2012);10:403–416. doi: 10.3390/md10020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu N., Fan X., Yan X., Li X., Niu R., Tseng C. K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry. (2003);62:1221–1224. doi: 10.1016/S0031-9422(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 18.Colin H., Guéguen E. The constitution of the principle sugar of Rhodymenia palmata. Cr Hebd. Seances Acad. Sci. (1930);191:163–164. [Google Scholar]
- 19.Li Y. X., Li Y., Lee S. H., Qian Z. J., Kim S. K. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and D-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food Chem. (2010);58:578–586. doi: 10.1021/jf902811j. [DOI] [PubMed] [Google Scholar]
- 20.Smith M. E., van der Maesen K., Somera F. P. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J. Neurosci. Res. (1998);54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Brown G. C., Neher J. J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. (2010);41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi H., Zhang J., Koike M., Nishioku T., Okamoto Y., Kominami E., von Figura K., Peters C., Yamamoto K., Saftig P., Uchiyama Y. Involvement of nitric oxide released from microglia-macrophages in pathological changes of cathepsin D-deficient mice. J. Neurosci. (2001);21:7526–7533. doi: 10.1523/JNEUROSCI.21-19-07526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y. Y., Hung S. L., Pai S. F., Lee Y. H., Yang S. F. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J. Endod. (2007);33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Krisanapiboon A., Buranapanitkit B., Oungbho K. Biocompatability of hydroxyapatite composite as a local drug delivery system. J. Orthop. Surg. (2006);14:315–318. doi: 10.1177/230949900601400315. [DOI] [PubMed] [Google Scholar]
- 25.Li Q., Verma I. M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. (2002);2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 26.Ekdahl C. T., Kokaia Z., Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. (2009);158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 27.Peterson L. J., Flood P. M. Oxidative stress and microglial cells in Parkinson's disease. Mediators Inflamm. (2012);2012:1–12. doi: 10.1155/2012/401264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beal M. F. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann. Neurol. (1998);44:S110–114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 29.Claria J. Cyclooxygenase-2 biology. Curr. Pharm. Des. (2003);9:2177–2190. doi: 10.2174/1381612033454054. [DOI] [PubMed] [Google Scholar]
- 30.Himaya S. W. A., Ryu B., Qian Z. J., Kim S. K. Paeonol from Hippocampus kuda Bleeler suppressed the neuro- inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicology in Vitro. (2012);26:878–887. doi: 10.1016/j.tiv.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G., HE J. L., Xie X. Y., Yu C. LPS-induced iNOS expression in N9 microglial cells is suppressed by geniposide via ERK, p38 and nuclear factor-κB signaling pathways. Int. J. Mol. Med. (2012);30:561–568. doi: 10.3892/ijmm.2012.1030. [DOI] [PubMed] [Google Scholar]
- 32.Hansen M. B., Nielson S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. (1989);119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 33.Rajapakse N., Kim M. M., Mendis E., Kim S. K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase- mediated nuclear factor-kappaB signaling. Immunology. (2008);123:348–357. doi: 10.1111/j.1365-2567.2007.02683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coker R. K., Laurent G. J. Pulmonary fibrosis: cytokine in the balance. Eur. Respir. J. (1998);11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]


