Abstract
Adhesion molecules such as ICAM-1 are important in the infiltration of leukocytes into the site of inflammation. In this study, we investigated the inhibitory effects of curcumin on ICAM-1 expression and monocyte adhesiveness as well as its underlying action mechanism in the TNF-α-stimulated keratinocytes. Curcumin induced expression of heme oxygenase-1 (HO-1) in the human keratinocyte cell line HaCaT. In addition, curcumin induced Nrf2 activation in dose- and time-dependent manners in the HaCaT cells. Curcumin suppressed TNF-α- induced ICAM-1 expression and subsequent monocyte adhesion, which were reversed by the addition of tin protoporphyrin IX (SnPP), a specific inhibitor of HO-1, or HO-1 knockdown using siRNA. Furthermore, Nrf2 knockdown using siRNA reversed the inhibitory effect of curcumin on the TNF-α-induced ICAM-1 expression and adhesion of monocytes to keratinocytes. These results suggest that curcumin may exert its anti-inflammatory activity by suppressing the TNF-α-induced ICAM-1 expression and subsequent monocyte adhesion via expression of HO-1 in the keratinocytes. [BMB Reports 2013;46(8): 410-415]
Keywords: Curcumin, HO-1, ICAM-1, Inflammation, Keratinocyte
INTRODUCTION
The infiltration of leukocytes into the skin is one of the critical steps involved in the development of inflammatory skin diseases such as atopic dermatitis (1). Up-regulation of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) on the surface of keratinocytes and dermal microvascular endothelial cells may increase infiltration of leukocytes into the area of inflamed skin (2). Upon stimulation with inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interferon-γ (IFN-γ), the epidermal keratinocyte can express adhesion molecules such as ICAM-1 (2-4). Modulation of the ICAM-1 expression in the epidermal keratinocyte provides a rationale for the development of therapeutic agents against various inflammatory skin diseases.
Curcumin, a bioactive polyphenol, is present in the rhizome of the plant Curcuma longa (5). Curcumin exerts various biological activities such as anti-oxidant, anti-tumor, and anti-inflammatory activities by multiple action mechanisms depending on the type of stimulus and cells (5). Curcumin has beneficial effects against various inflammatory skin diseases such as psoriasis and atopic dermatitis (6). Curcumin inhibits the production of proinflammatory cytokines such as IL-1β and IL-6 by inhibition of NF-κB and MAPK pathways in TNF-α-stimulated human keratinocytes (7). Curcumin inhibited expression of COX-2 and cytokines including IL-1β, IL-6 and TNF-α by inhibition of NF-κB or AP-1 and MAPK pathways in UVB-irradiated human keratinocytes (8,9). Curcumin inhibited FcεRI signaling to exert an anti-allergic effect by directly inhibiting Syk kinase activity in antigen-stimulated mast cells (10). In addition, curcumin suppressed degranulation and secretion of TNF-α and IL-4 in activated mast cells in a passive cutaneous anaphylaxis mice model (10). Taken together, these reports suggest that curcumin exerts its beneficial effect on inflammatory skin diseases by multiple distinct mechanisms.
Heme oxygenase-1 (HO-1), an inducible antioxidant enzyme, catalyzes the degradation of heme into ferrous iron, carbon monoxide and biliverdin. HO-1 expression exerts beneficial effects against inflammatory skin diseases (11) and inhibits the development of diseases such as atopic dermatitis (12-14). Curcumin induces HO-1 expression in human skin fibroblasts (15,16) and keratinocytes (17). However, the functional relevance between curcumin-induced HO-1 expression and the inflammatory immune response during skin inflammation has not been demonstrated.
In this study, we examined the possible role of curcumin-induced HO-1 expression in TNF-α-induced ICAM-1 expression and subsequent monocyte adhesiveness in a human keratinocyte cell line HaCaT. Curcumin induced HO-1 expression and Nrf2 activation in the HaCaT cells. Blockage of HO-1 activity by SnPP, an HO-1 inhibitor, or HO-1 knockdown using small interfering RNAs (siRNA) reversed the suppressive effect of curcumin on the TNF-α-induced expression of ICAM-1 and subsequent adhesion of monocytes to HaCaT cells. These results suggest that HO-1 expression contributes to the suppressive activity of curcumin on the TNF-α-induced expression of ICAM-1 and subsequent monocyte adhesion in the keratinocytes.
RESULTS
Curcumin induces HO-1 expression and Nrf2 activation in the HaCaT cells
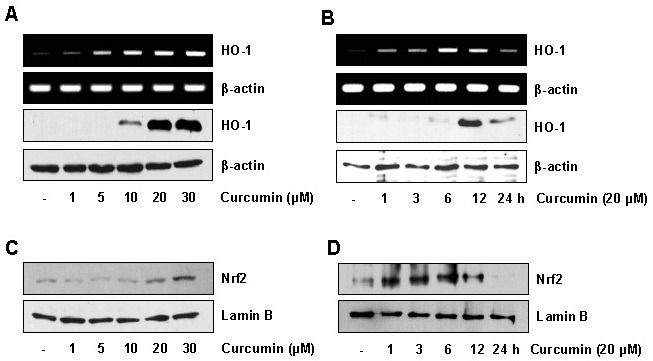
Although previous studies have demonstrated that curcumin induced HO-1 expression in endothelial cells (18), macrophages (19), and monocytes (20), the effect of curcumin on HO-1 expression was not analyzed in detail in keratinocytes. We investigated the effect of curcumin on HO-1 expression in HaCaT cells. HaCaT cells were treated with curcumin and then HO-1 mRNA and protein expression were measured by RT-PCR and immunoblot analysis, respectively. As shown in Fig. 1A and B, curcumin significantly induced mRNA and protein expression of HO-1 in dose- and time dependent manners in the HaCaT cells. Curcumin had no significant cytotoxic effect on the HaCaT cells at the concentrations tested (data not shown). Since expression of the HO-1 gene is regulated by various transcriptional factors such as Nrf2 (11), we next examined Nrf2 activation in the HaCaT cells stimulated with curcumin. HaCaT cells were treated with curcumin, and then translocation of Nrf2 into the nucleus was monitored by immunoblot analysis. Curcumin induced nuclear accumulation of Nrf2 in dose- and time-dependent manners in the HaCaT cells (Fig. 1C and D).
Fig. 1. Curcumin induces HO-1 expression and Nrf2 activation in HaCaT cells. (A) HaCaT cells were treated with various concentrations of curcumin for 1 h (for RNA) or 12 h (for protein). Total RNA and protein were analyzed by RT-PCR (upper panel) and immunoblotting (bottom panel), respectively. (B) HaCaT cells were incubated with 20 μM curcumin for varying times, and total RNA and protein were analyzed by RT-PCR (upper panel) and immunoblotting (bottom panel), respectively. (C) HaCaT cells were incubated with various concentrations of curcumin for 15 min. The nuclear extracts of cells were prepared and the levels of Nrf2 were determined by immunoblotting. (D) Activation of Nrf2 was measured after exposure to 20 μM curcumin for the indicated time. The levels of Nrf2 in the nuclear extracts of cells were determined by immunoblotting.
HO-1 mediates the inhibitory effect of curcumin on TNF-α-induced ICAM-1 expression in the HaCaT cells
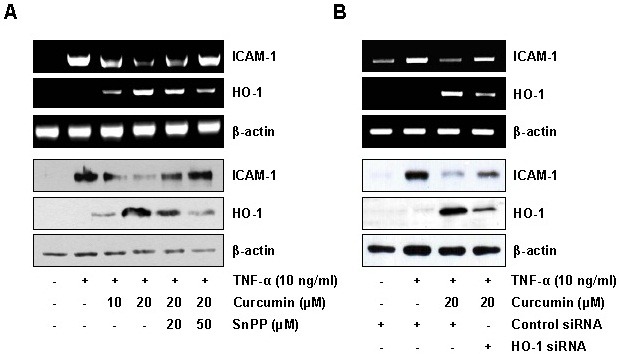
Previous studies have reported that curcumin inhibited the expression of TNF-α-induced ICAM-1 expression in various types of cells including endothelial cells (21,22). As shown in Fig. 2A, curcumin significantly inhibited ICAM-1 expression at the levels of mRNA and protein in TNF-α-stimulated HaCaT cells. To examine whether curcumin inhibits the TNF-α-induced expression of ICAM-1 via HO-1 expression, HaCaT cells were pretreated with a HO-1 inhibitor, SnPP, and the inhibitory effect of curcumin on the TNF-α-induced ICAM-1 expression was analyzed. SnPP reversed the inhibitory effect of curcumin on TNF-α-induced mRNA and protein expression of ICAM-1 (Fig. 2A). To further confirm the possible role of HO-1 expression in the inhibitory effect of curcumin on the TNF-α-induced ICAM-1 expression, we exploited the siRNA knockdown strategy against HO-1. HO-1 knockdown significantly reversed the inhibitory effect of curcumin on the TNF-α-induced ICAM-1 expression (Fig. 2B). These results suggest that HO-1 expression contributes to the inhibitory effects of curcumin on TNF-α-induced ICAM-1 expression in the keratinocytes.
Fig. 2. HO-1 induction is responsible for the inhibitory effect of curcumin on TNF-α-induced ICAM-1 expression in HaCaT cells. (A) HaCaT cells were incubated with curcumin for 6 h in the absence or presence of SnPP, and then exposed to TNF-α (10 ng/ml) for 1 h (for RNA) or 12 h (for protein). Total RNA and protein were analyzed by RT-PCR (upper panel) and immunoblotting (bottom panel), respectively. (B) HaCaT cells were transiently transfected with control or HO-1 siRNA. After 48 h, the transfected HaCaT cells were incubated with 20 μM curcumin for 6 h, and then exposed to TNF-α for 1 h (for RNA) or 12 h (for protein). Total RNA and protein were analyzed by RT-PCR (upper panel) and immunoblotting (bottom panel), respectively.
HO-1 expression induced by curcumin suppresses TNF-α-induced monocyte adhesion
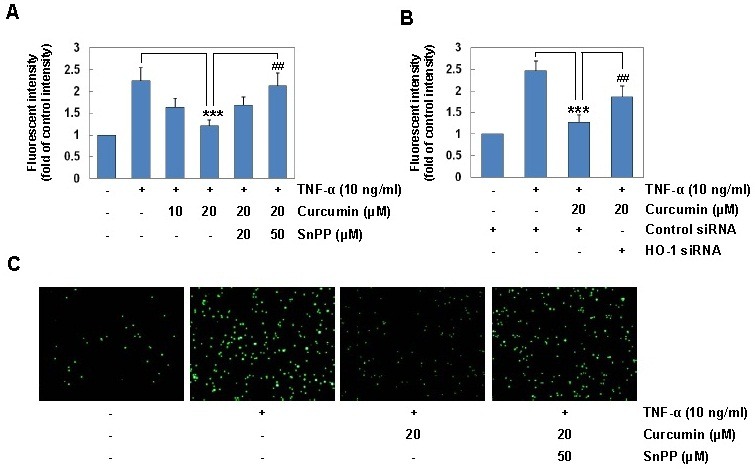
Since up-regulation of ICAM-1 leads to increased monocyte adhesion in human keratinocytes (23), we next examined the possible role of HO-1 expression in the inhibitory effect of curcumin on TNF-α-induced monocyte adhesion to HaCaT cells using a HO-1 inhibitor or siRNA knockdown. Treatment with curcumin suppressed TNF-α-induced monocyte adhesion to HaCaT cells in a dose-dependent manner (Fig. 3A). SnPP significantly reversed the inhibitory effect of curcumin on TNF-α-induced monocyte adhesion (Fig. 3A and C). In addition, HO-1 knockdown using siRNA significantly reversed the inhibitory effect of curcumin on TNF-α-induced monocyte adhesion. These results confirm that HO-1 expression mediates the inhibitory effect of curcumin on TNF-α-induced monocyte adhesion to HaCaT cells.
Fig. 3. HO-1 induction mediates the inhibitory effect of curcumin on TNF-α-induced monocyte adhesion in HaCaT cells. (A) HaCaT cells were incubated with 20 μM curcumin for 6 h in the absence or presence of SnPP, and then exposed to TNF-α (10 ng/ml) for 12 h. HaCaT cells were co-cultured with calcein-AM-labeled THP-1 monocytes for 1 h. The calcein-AM fluorescent intensity was measured by an ELISA plate reader. (B) HaCaT cells transfected with control or HO-1 siRNA were incubated with 20 μM curcumin for 6 h, and stimulated with TNF-α for 12 h. HaCaT cells were co-cultured with calcein-AM-labeled THP-1 monocytes for 1 h. The calcein- AM fluorescent intensity was measured by an ELISA plate reader. Results are means ± SD. Statistical significance: ***P <0.001 compared to the TNF-α alone, ##P < 0.01 compared to the TNF-α and curcumin. (C) Microphotographs were obtained using fluorescence microscopy (original magnification, ×40).
Nrf2 is responsible for the inhibitory effect of curcumin on TNF-α-induced ICAM-1 expression and monocyte adhesion
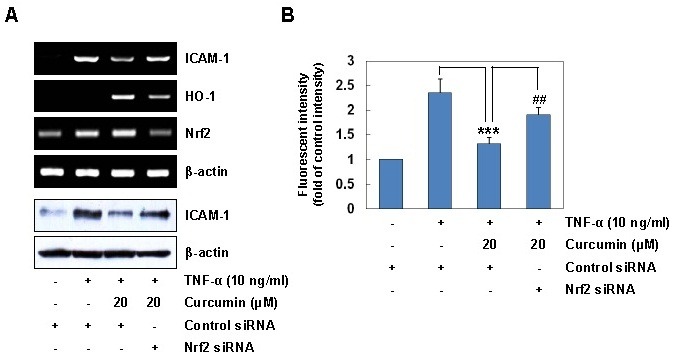
Since Nrf2 is a transcriptional factor involved in expression of the HO-1 gene (11), we further examined the functional relevance between Nrf2 activation and ICAM-1 expression. Nrf2 knockdown experiments using siRNA were performed to evaluate the suppressive function of curcumin on the TNF-α-induced ICAM-1 expression in HaCaT cells. Suppression of Nrf2 activation by Nrf2 siRNA significantly reversed the inhibitory effect of curcumin on the TNF-α-induced ICAM-1 expression at the levels of mRNA (Fig. 4A, upper panel) and protein (Fig. 4A, lower panel). In addition, siRNA knockdown of Nrf2 reversed the inhibitory effect of curcumin on TNF-α-induced monocyte adhesion to HaCaT cells (Fig. 4B). These results indicate that Nrf2 activation contributes to the inhibitory effect of curcumin on TNF-α-induced ICAM-1 expression and subsequent monocyte adhesiveness in HaCaT cells.
Fig. 4. siRNA knockdown of Nrf2 reverses the inhibitory effects of curcumin on TNF-α-induced ICAM-1 expression and monocyte adhesion in HaCaT cells. (A) HaCaT cells transfected with control or Nrf2 siRNA were incubated with 20 μM curcumin for 6 h, and stimulated with TNF-α for 1 h (for RNA) and for 12 h (for protein). Total RNA and protein were analyzed by RT-PCR (upper panel) and immunoblotting (bottom panel). (B) HaCaT cells transfected with control or Nrf2 siRNA were incubated with 20 μM curcumin for 6 h, and stimulated with TNF-α for 12 h. HaCaT cells were co-cultured with calcein-AM-labeled THP-1 monocytes for 1 h. The calcein-AM fluorescent intensity was measured by an ELISA plate reader. Results are means ± SD. Statistical significance: ***P < 0.001 compared to the TNF-α alone, ##P < 0.01 compared to the TNF-α and curcumin.
DISCUSSION
During inflammation, the epidermal keratinocytes can be stimulated to express adhesion molecules such as ICAM-1, which facilitate leukocyte infiltration. Expression of adhesion molecules on the keratinocytes is induced by several stimuli including cytokines (2-4). Modulation of the ICAM-1 expression provides a target for the development of therapeutic agents against inflammatory skin diseases. Many naturally occurring polyphenols are considered promising therapeutic agents against inflammatory skin diseases (24). Due to its free radical scavenging and antioxidant properties, curcumin has attracted significant attention as a potential effective anti-inflammatory agent. Curcumin exerts anti-inflammatory effects in both in vitro and in vivo models (7,9,10), even though the relevant anti-inflammatory mechanisms are not fully understood. In this study, we show that curcumin significantly suppressed the TNF-α-induced ICAM-1 expression and subsequent monocyte adhesion via HO-1 expression in the keratinocytes.
Since previous studies have shown that curcumin strongly induced HO-1 expression and exerted cytoprotective effects in various types of cells including endothelial cells (18, 25), macrophages (19), monocytes (20) and skin fibroblasts (15, 16), we examined whether curcumin can induce the HO-1 expression in keratinocytes. As shown in Fig. 1, treatment with curcumin significantly induced the mRNA and protein expression of HO-1 in time- and dose-dependent manners in the HaCaT cells, indicating that curcumin is an inducer of HO-1 expression. Although previous studies reported that curcumin induced HO-1 expression in human skin fibroblasts (15) and keratinocytes (17), the functional roles of HO-1 expression in the suppressive effects of curcumin on the expression of adhesion molecules such as ICAM-1 in keratinocytes were not clarified. Using a pharmacological HO-1 inhibitor and siRNA knockdown against HO-1, we demonstrated that HO-1 expression mediates the suppressive effects of curcumin on the TNF-α-induced ICAM-1 expression and subsequent monocyte adhesiveness to the HaCaT cells (Fig. 2 and 3). These results provide evidence that suggest the functional consequence of the curcumin-induced HO-1 expression. Consistent with our results, several reports demonstrated that HO-1 expression exerts a regulatory effect on the process of inflammatory skin diseases such as atopic dermatitis-like lesions and contact hypersensitivity in mice (12-14). In addition, HO-1 expression inhibits T cell-dependent skin inflammation (12). Although the mechanisms by which HO-1 induction by curcumin exerts its anti-inflammatory activities are unclear, the by-products of HO-1 activity, including carbon monoxide and bilirubin, may contribute to the inhibitory effect of curcumin (11).
Since Nrf2 is a transcriptional factor responsible for HO-1 expression (11), we further analyzed the role of Nrf2 in the curcumin-induced ICAM-1 expression and subsequent monocyte adhesiveness in TNF-α-stimulated HaCaT cells. Knockdown of Nrf2 using siRNA significantly suppressed curcumin- induced HO-1 expression and prevented curcumin from suppressing TNF-α-induced ICAM-1 expression (Fig. 4A). In addition, the suppressive effect of curcumin on TNF-α-induced monocyte adhesion to HaCaT cells was significantly reversed by Nrf2 knockdown (Fig. 4B), suggesting the potential role of Nrf2 activation in the anti-inflammatory effects of curcumin. Recently, we reported that celastrol induced HO-1 expression via Nrf2 activation which was responsible for suppression of the IFN-γ-induced ICAM-1 expression and subsequent monocyte adhesion in the keratinocytes (26,27). These results support the position that Nrf2 is an important regulator to express various cellular defense enzymes such as HO-1 against oxidative stress and plays a critical role in regulating anti-inflammatory responses (28).
The present study suggests that curcumin-induced HO-1 expression via Nrf2 activation is one mechanism responsible for its anti-inflammatory activity. Activation of Nrf2-HO-1 pathway using pharmacological or genetic approaches might be a way to develop a therapeutic agent for inflammatory skin diseases.
MATERIALS AND METHODS
Cell culture and reagents
The immortalized human keratinocyte cell line, HaCaT, was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin) at 37℃ in a humidified incubator containing 5% CO2 and 95% air. Human THP-1 monocytic cells were maintained in RPMI 1640 medium supplemented with 2 mM L-glutamine and 10% fetal bovine serum. Tin protoporphyrin IX (SnPP) was purchased from Calbiochem (La Jolla, CA, USA). Calcein acetoxymethyl ester (calcein-AM) was purchased from Molecular Probe (Eugene, OR, USA). HO-1 specific siRNA, primary antibodies against ICAM-1, HO-1 and actin (Santa Cruz, CA, USA) were obtained commercially. Curcumin, HRP-conjugated anti-rabbit or goat antibodies were supplied by Sigma (St. Louis, MO, USA).
Immunoblot analysis
Cell lysates were prepared by incubating cells in a lysis buffer (125 mM Tris-HCl pH 6.8, 2% SDS, 10% v/v glycerol) at 4℃ for 30 min. Equal amounts of cell lysates (30 μg of total protein) were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane by electroblotting. Immunoreaction was performed with the indicated antibodies, and the immunoreactive bands were detected by enhanced chemiluminescence (ECL; Amersham) as recommended by the manufacturer (29).
RT-PCR analysis
Total RNA was obtained from HaCaT cells using a Trizol reagent kit (Invitrogen, Gaithersburg, MD, USA) according to the manufacturer’s instructions. The RNA (2 μg) was reverse transcribed into cDNA with 10,000 U of reverse transcriptase and 0.5 μg/μL oligo-(dT)15 primer (Promega, Madison, WI, USA). PCR amplification of cDNA was performed using specific primers, as described previously (30). The following forward and reverse primers (5'->3') were used: ICAM-1 forward, GGT GAC GCT GAA TGG GGT TCC; ICAM-1 reverse, GTC CTC ATG GTG GGG CTA TGA CTC; HO-1 forward, GCG CAG CAT GCC CCA GGA TTT G; and HO-1 reverse, AGC TGG ATG TTG AGC AGG A; beta-actin forward, GCG GGA AAT CGT GCG TGA CAT T; and beta-actin reverse, GAT GGA GTT GAA GGT AGT TTC GTG. PCR products were resolved on a 1% agarose gel and visualized with UV light after ethidium bromide.
siRNA knockdown of HO-1 and Nrf2
To perform the knockdown experiments by siRNA, HaCaT cells were transfected with siRNA specifically targeting HO-1, Nrf2 or control siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions (27). Two days after transfection, HaCaT cells were stimulated with TNF-α in the presence or absence of curcumin. Cells were analyzed for ICAM-1 expression and monocyte adhesiveness.
Cell adhesion assay
Adherence of THP-1 cells to HaCaT cells was assayed using a cell-cell adhesion assay as previously described (31). Briefly, the calcein-AM labeled THP-1 cells (7.0 × 105/well) were co-cultured with HaCaT cells for 1 h at 37℃. The nonadherent THP-1 cells were removed from the monolayers by washing three times with PBS. For the adhesion quantification, the fluorescent intensity of each well was measured at λ = 485 nm excitation and λ = 538 nm emission by a Fluoroskan ELISA plate reader (Ani Labsystems Ltd. Oy. Finland). The fluorescence images were obtained at λ = 485 nm excitation and λ = 538 nm emission using a SPOT II digital camera-attached fluorescence microscope with Spot II data acquisition software (Diagnostic Instrument, Livingston, Scotland).
Statistical analysis
The results were expressed as the mean ± SEM from at least three independent experiments. The values were evaluated via one-way ANOVA, followed by Duncan’s multiple range tests using GraphPad Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA). If P values were <.05 null hypotheses of no difference were rejected.
Acknowledgments
This work was supported by the Priority Research Centers Program Grant (2009-0093812) and by a Grant (2012 R1A1A4A01007173) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology. This work was also supported by a Grant (HRF-201209-018) from Hallym University.
References
- 1.Gröne A. Keratinocytes and cytokines, Vet. Immunol. Immunopathol. (2002);88:1–12. doi: 10.1016/S0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 2.Dustin M. L., Singer K. H, Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J. Exp. Med. (1988);167:1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker J. N., Sarma V., Mitra R. S., Dixit V. M., Nickoloff B. J. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J. Clin. Invest. (1990);85:605–608. doi: 10.1172/JCI114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krutmann J., Kock A., Schauer E., ParlParlow F., Moller A., Kapp A., Forster E., Schopf E., Luger T. A. Tumor necrosis factor beta and ultraviolet radiation are potent regulators of human keratinocyte ICAM-1 expression. J. Invest. Dermatol. (1990);95:127–131. doi: 10.1111/1523-1747.ep12477839. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H., Beevers C. S., Huang S. The targets of curcumin. Curr. Drug. Targets. (2011);12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thangapazham R. L., Sharma A., Maheshwari R. K. Beneficial role of curcumin in skin diseases. Adv. Exp. Med. Biol. (2007);595:343–357. doi: 10.1007/978-0-387-46401-5_15. [DOI] [PubMed] [Google Scholar]
- 7.Cho J. W., Lee K. S., Kim C. W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NFkappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. (2007);19:469–474. [PubMed] [Google Scholar]
- 8.Cho J. W., Park K., Kweon G. R., Jang B. C., Baek W. K., Suh M. H., Kim C. W., Lee K. S., Suh S. I. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp. Mol. Med. (2005);37:186–192. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- 9.Grandjean-Laquerriere A., Gangloff S. C., Le Naour R., Trentesaux C., Hornebeck W., Guenounou M. Relative contribution of NF-kappaB and AP-1 in the modulation by curcumin and pyrrolidine dithiocarbamate of the UVB-induced cytokine expression by keratinocytes. Cytokine. (2002);18:168–177. doi: 10.1006/cyto.2002.0888. [DOI] [PubMed] [Google Scholar]
- 10.Lee J. H., Kim J. W., Ko N. Y., Mun S. H., Her E., Kim B. K., Han J. W., Lee H. Y., Beaven M. A., Kim Y. M., Choi W. S. Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J. Allergy Clin. Immunol. (2008);121:1225–1231. doi: 10.1016/j.jaci.2007.12.1160. [DOI] [PubMed] [Google Scholar]
- 11.Ryter S. W., Alam J., Choi A. M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. (2006);86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 12.Listopad J., Asadullah K., Sievers C., Ritter T., Meisel C., Sabat R., Döcke W. D. Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Exp. Dermatol. (2007);16:661–670. doi: 10.1111/j.1600-0625.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirino M., Kirino Y., Takeno M., Nagashima Y., Takahashi K., Kobayashi M., Murakami S., Hirasawa T., Ueda A., Aihara M., Ikezawa Z., Ishigatsubo Y. Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: implications for human disease. J. Allergy. Clin. Immunol. (2008);122:290–297. doi: 10.1016/j.jaci.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Pae H. O., Ha Y. A., Chai K. Y., Chung H. T. Heme oxygenase-1 attenuates contact hypersensitivity induced by 2,4-dinitrofluorobenzene in mice. Immunopharmacol Immunotoxicol. (2008);30:207–216. doi: 10.1080/08923970801946824. [DOI] [PubMed] [Google Scholar]
- 15.Rattan S. I., Fernandes R. A., Demirovic D., Dymek B., Lima C. F. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response. (2009);7:90–103. doi: 10.2203/dose-response.08-014.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima C. F., Pereira-Wilson C., Rattan S. I. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: relevance for anti-aging intervention. Mol. Nutr. Food Res. (2011);55:430–442. doi: 10.1002/mnfr.201000221. [DOI] [PubMed] [Google Scholar]
- 17.Berge U., Kristensen P., Rattan S. I. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp. Gerontol. (2008);43:658–662. doi: 10.1016/j.exger.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Motterlini R., Foresti R., Bassi R., Green C. J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. (2000);28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 19.Kim A. N., Jeon W. K., Lee J. J., Kim B. C. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic. Biol. Med. (2010);49:323–331. doi: 10.1016/j.freeradbiomed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Hsu H. Y., Chu L. C., Hua K. F., Chao L. K. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. J. Cell Physiol. (2008);215:603–612. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A., Dhawan S., Hardegen N. J., Aggarwal B. B. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem. Pharmacol. (1998);55:775–783. doi: 10.1016/S0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 22.Gupta B., Ghosh B. Curcuma longa inhibits TNF-alpha induced expression of adhesion molecules on human umbilical vein endothelial cells. Int. J. Immunopharmacol. (1999);21:745–757. doi: 10.1016/S0192-0561(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 23.Bito T., Roy S., Sen C. K., Packer L. Pine bark extract pycnogenol downregulates IFN-gamma-induced adhesion of T cells to human keratinocytes by inhibiting inducible ICAM-1 expression. Free Radic. Biol. Med. (2000);28:219–227. doi: 10.1016/S0891-5849(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 24.Kostyuk V., Potapovich A., De Luca C. The promise of plant polyphenols as the golden standard skin anti-inflammatory agents. Curr. Drug. Metab. (2010);11:414–424. doi: 10.2174/138920010791526033. [DOI] [PubMed] [Google Scholar]
- 25.Olszanecki R., Gebska A., Korbut R. The role of haem oxygenase-1 in the decrease of endothelial intercellular adhesion molecule-1 expression by curcumin. Basic Clin. Pharmacol. Toxicol. (2007);101:411–415. doi: 10.1111/j.1742-7843.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 26.Seo W. Y., Ju S. M., Song H. Y., Goh A. R., Jun J. G., Kang Y. H., Choi S. Y., Park J. Celastrol suppresses IFN-gamma-induced ICAM-1 expression and subsequent monocyte adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. Biochem. Biophys. Res. Commun. (2010);398:140–145. doi: 10.1016/j.bbrc.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 27.Seo W. Y., Goh G. R., Ju S. M., Song H. Y., Kwon D. J., Jun J. G., Kim B. C., Choi S. Y., Park J. Celastrol induces expression of heme oxygenase-1 through ROS/Nrf2/ARE signaling in the HaCaT cells. Biochem. Biophys. Res. Commun. (2011);407:535–540. doi: 10.1016/j.bbrc.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 28.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. (2010);80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Song H. Y., Ju S. M., Goh A. R., Kwon D. J., Choi S. Y., Park J. Suppression of TNF-alpha-induced MMP-9 expression by a cell-permeable superoxide dismutase in keratinocytes. BMB Rep. (2011);44:462–467. doi: 10.5483/BMBRep.2011.44.7.462. [DOI] [PubMed] [Google Scholar]
- 30.Lee S. H., Kim D. W., Eom S. A., Jun S. Y., Park M., Kim D. S., Kwon H. J., Kwon H. Y., Han K. H., Park J., Hwang H. S., Eum W. S., Choi S. Y. Suppression of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation in mice by transduced Tat-Annexin protein. BMB Rep. (2012);45:354–359. doi: 10.5483/BMBRep.2012.45.6.036. [DOI] [PubMed] [Google Scholar]
- 31.Park L. J., Ju S. M., Song H. Y., Lee J. A., Yang M. Y., Kang Y. H., Kwon H. J., Kim T. Y., Choi S. Y., Park J. The enhanced monocyte adhesiveness after UVB exposure requires ROS and NF-kappaB signaling in human keratinocyte. J. Biochem. Mol. Biol. (2006);39 doi: 10.5483/bmbrep.2006.39.5.618. [DOI] [PubMed] [Google Scholar]


