Abstract
In this paper, we firstly reported a C-type lectin cDNA clone of 1029 bps from the larvae of A. Pernyi (Ap-CTL) using PCR and RACE techniques. The full-length cDNA contains an open reading frame encoding 308 amino acid residues which has two different carbohydrate-recognition domains (CRDs) arranged in tandem. To investigate the biological activities in the innate immunity, recombinant Ap-CTL was expressed in E. coli with a 6-histidine at the amino-terminus (Ap-rCTL). Besides acted as a broad-spectrum recognition protein binding to a wide range of PAMPs and microorganisms, Ap-rCTL also had the ability to recognize and trigger the agglutination of bacteria and fungi. In the proPO activation assay, Ap-rCTL specifically restored the PO activity of hemolymph blocked by anti-Ap-rCTL antibody in the presence of different PAMPs or microorganisms. In summary, Ap-rCTL plays an important role in insect innate immunity as an pattern recognition protein. [BMB Reports 2013; 46(7): 358-363]
Keywords: Antheraea pernyi, C-type lectin, Innate immunity, Pattern recognition protein, Pro-PO activation
INTRODUCTION
A critical first step in any immune response is the recognition of an invading organisms as foreign (1). In the acquired immune system of vertebrates, antibody molecules and T cell receptors can recognize a variety of antigens and function as recognition molecules to trigger different immune responses (2). Because of a lack of such an adaptive immune system, invertebrates have to rely completely on innate immune systems which are activated by a group of pattern recognition receptors (PRRs) to initiate the host immune response through binding to pathogen-associated molecular patterns (PAMPs) (3-6). The PAMPs are highly conserved structures present on the surface of different microorganisms but absent in the host, such as lipopolysaccharide (LPS), peptidoglycan (PGN) and mannan, etc (7).
Many lectins have been purified from a multitude of species including plants, mammals, viruses and parasites (8-11). In recent years, an increasing number of C-type lectins from insects have been reported to be involved in various biological responses (12-14). By now, more than 30 encoded C-type lectin-like domains have been identified in the fruit fly Drosophila melanogaster (15). The first C-type lectin from the silkworm Bombyx mori called LPS-binding protein (BmLBP) was isolated and characterized in 1997 (16) and then its cDNA cloning was described in 1999 (17). And in Manduca sexta, four immulectins (IML1-4) have been reported to stimulate prophenoloxidase activation and promote hemocyte encapsulation as important PRRs (18-21).
To date, analysis has mainly focused on the mechanism of the recognition of LPS and the functions of two CRDs in C-type lectins, but few experiments have been carried out on either the types of PAMPs recognized by C-type lectins or the immune effect then induced directly after recognition in vivo. In this paper, we report the cDNA cloning, expression, characterization and functions of a novel C-type lectin in Antheraea pernyi for the first time. Through a series of characterization and functional experiments, we have arrived at the conclusion that C-type lectin in Antheraea pernyi plays an important role in the innate immunity as an pattern recognition protein.
RESULTS AND DISCUSSION
cDNA cloning and sequence alignment of A. Pernyi C-type lectin (Ap-CTL)
C-type lectins have been isolated from a series of insects including M. sexta, B. mori and H. cunea (22). In this paper, we report the cDNA sequence of a novel C-type lectin from the lepidoptera Antheraea pernyi (Ap-CTL) (Fig. 1A). A 786-bp fragment was amplified by RT-PCR from immune challenged 5th instar larvae fat body mRNA. With the attempt to obtain the 5' and 3'- ends of this protein, 5'- and 3'- RACE PCRs were carried out. A fragment containing a 5'- noncoding region of 29 bps and initiation codon, and a fragment with a termination codon and a 3'- untranslated sequence ending with a poly (A) tail were obtained, respectively. The full- length sequence of Ap-CTL is 1029 bps with an open reading frame of 924 bps encoding 308 amino acid residues. The first 15 amino acid residues were predicted as a signal peptide by using the signalP program (www.cbs.dtu.dk/). One potential O-linked glycosylation site at threonine residue 245 was predicted by NetOGlyc 2.0 and two putative N-linked glycosylation sites at asparagine residues 77 and 114 were predicted by NetNGlyc 1.0. The calculated molecular mass of the mature protein was 33333 Da, with a predicted pI of 5.03.
Fig. 1. Sequence analysis and alignment of the cDNA cloning of A. pernyi CTL (Ap-CTL). (A) The numbers of nucleotide and deduced amino acid sequences (one-letter symbols) are shown on the left of the nucleotide sequence, respectively. The predicted signal peptide were assigned negative numbers and underlined. The start codon ATG is shown in bold and the termination cordon (TGA) is marked with an asterisk (*). Two potential N-linked glycosylation sites and one O-linked glycosylation site are marked with ▲ and ■, respectively. The putative polyadenylation sequence, TATAA, is double underlined and the two CRDs are divided by "||". (B) The CLUSTALW multiple sequence alignment program was used to align the A. pernyi CTL amino acid sequence with other 7 most similar proteins. Residues conserved in all sequences are marked with "*", less conservative amino acid subsitutions are marked with ":" and ".", respectively. Ap_CTL, A. pernyi CTL; lo_lectin 3, L. obliqua lectin 3; dp_ctl21, D. plexippus C-type lectin 21; ms_iml-2a, M. sexta immulectin-2a; ms_iml-2b, M. sexta immulectin-2b; pr_ctl, P. rapae C-type lectin; bm_ctl21, B. mori C-type lectin 21 precursor; bm_ctl19, B. mori C-type lectin 19 precursor.
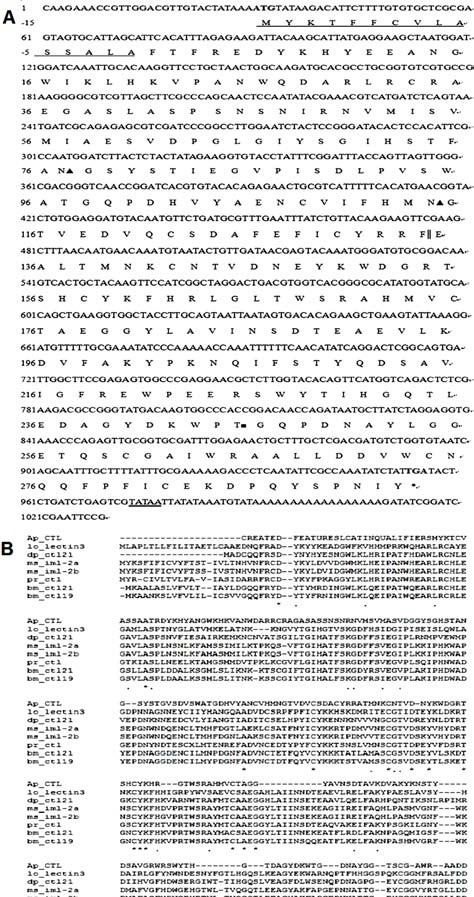
A BLAST search was performed using the mature amino acid sequence of Ap-CTL. This indicated that Ap-CTL was a novel member of the C-type lectin superfamily. Using the CLUSTALW multiple sequence alignment program, an alignment was performed with the deduced amino acid sequence of Ap-CTL and other 7 members of the C-type lectin superfamily of insects (Fig. 1B). The alignment result revealed that Ap-CTL contained two highly conserved tandem C-type carbohydrate-recognition domains (CRDs) and all the conservative cytosine residues found in insect C-type lectins. A phylogenetic tree was then constructed using the DNAMAN program which revealed that Ap-CTL was located separately from the other lectins, which indicated that Ap-CTL might be only distantly related to other insect C-type lectins (data not shown).
Identification of the mRNA transcription and protein expression of Ap-CTL
To examine whether the expression of Ap-CTL mRNA was inducible and where the expression location was, semi-quantitative RT-PCR was performed using gene specific primers which encoded a 720-bp fragment. Total RNA was extracted from a variety of tissues including hemocytes, fat body, midgut and integument of native and microbial challenged larvae. No band was detected in tissues of native larvae without injection (Fig. 2A) and larvae injected with insect saline only (data not shown). However, in the immune challenged group, a significant intense band in the fat body and a less intense band in the midgut were detected. The expression of mRNA in fat body challenged at different times is shown in Fig. 2B and bands were hardly detected until 12 h after injection. A peak was reached at 24-48 h post-injection, and then the band intensity began to fall since 72 h post-injection. These results indicate that Ap-CTL is an inducible protein following the invasion of an exogenous substance, and the fat body is the primary synthesis site of Ap-CTL like most insect hemolymph proteins. As a loading control, A. Pernyi ribosomal protein S3 was used and remained unchanged throughout the experiment.
Fig. 2. Analysis of the mRNA expression of Ap-CTL and SDS-PAGE. (A) Semi-quantitative RT-PCR was performed to detect the mRNA level of Ap-CTL in response to challenges. (A) mRNA expression of Ap-CTL in different tissues: 5 μg total RNA from different tissues was obtained, including fat body (Fb), hemocytes (Hc), midgut (Mg) and integument (Ig) of challenged larvae. Tissues from naive larvae were extracted as negative controls. The same experiment was performed when detecting the mRNA expression of Ap-CTL challenged for different times (0, 12, 24, 48, 72 h) in fat body (B) A. pernyi ribosomal protein S3 (rps3) was used as an internal reference control. The average relative expressions were representative of three independent repeats ± 1 SD. (C) SDS-PAGE and western blot analysis of Ap-CTL. Lane M, molecular weight markers (shown on the left); lane 1, SDS-PAGE and Commassie Blue staining of recombinant CTL purified by Ni2+ affinity chromatography under denaturing conditions (0.5 μg). Lane 2 and 3, Immunoblot analysis of natural Ap-CTL of larvae plasma (15 μg) and recombinant CTL (0.3 μg) using antibody against Ap-rCTL, respectively. The arrow indicates the 33kDa Ap-CTL.
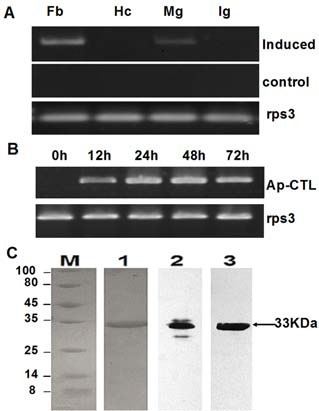
To obtain sufficient amount of protein for the functional study, Ap-CTL was expressed in E. coli with a 6-histidine at the amino-terminus, which was called Ap-rCTL. After sonification, the soluble recombinant protein was eluted with 200 mM imidazole using Ni2+-NTA affinity chromatography. The purified protein migrated as a single band at approximately 33 kDa on SDS-PAGE (Fig. 2C, lane 1), which was similar to that of the natural protein in A. Pernyi larvae hemolymph, detected by western blot with antibody against Ap-CTL (Fig. 2C, lane 2 and 3). However, there were another two bands bound to anti- Ap-rCTL antibody (Fig. 2C, lane 2). Besides the reason of non-specifical recognition by this polyclonal antibody, there were probably more than one kind of C-type lectin, or even two isoforms of Ap-CTL with similar amino acid sequences existing in the hemolymph. So, Edman degradation and MSLDI mass spectrometry needs to be performed in future studies.
Functions of Ap-rCTL as a pattern recognition protein
Enzyme-linked immunosorbent assay was performed to investigate the binding of Ap-rCTL to different PAMPs and microorganisms. As shown in Fig. 3A, with the protein concentration increased, Ap-rCTL bound to more PAMPs, when the concentration of protein reached 50 μg and more, the bound ability did not increase any more (data not shown) as the reason that the binding sites of Ap-rCTL were too saturated to bind more PAMPs. In the whole, it was shown that Ap-rCTL bound more strongly to LPS and laminarin than to mannan and DAP-PGN, and the weakest binding was to LTA and Lys-PGN. Similar results were obtained through the binding of Ap-rCTL to insoluble saccharides, including curdlan, Lys-PGN and DAPPGN (data not shown). As shown in Fig. 3B, Ap-rCTL bound to all these three types of microorganisms: G+ bacteria, G- bacteria and fungi. The saturated concentration of Ap-rCTL was 90 μg (data not shown) and the binding classified from strong to weak was: B. subtilis, C. albican, E. coli, S. cerevisiae, S. aureus, and M. luteus. In general, Ap-rCTL was found to be a broad-spectrum pattern recognition protein binding to both PAMPs and microbial cells.
Fig. 3. Binding and agglutination assay of Ap-rCTL. Different concentrations of Ap-rCTL was incubated with soluble PAMP- coated (A) and microbial-coated (B) microtiter plates to detect the binding ability of this recombinant protein in an ELISA experiment. The contents of soluble PAMPs and microorganisms were 10 μg/well and 107 cells/well, respectively. Wells coated with PBS only were used as a negative control. (C) To further identidy the agglutination of microorganisms, 10 μl bovine albumin (BSA, 100 μg/ml) or Ap-rCTL (100 μg/ml) was incubated with 10 μl EB dyed E. coli, S. aureaus or S. cerevisiae (2 × 108 cells/ml) in PBS. After incubation for 30 min at room temperature, cells were observed under a fluorescence microscopy. Aggregation was determined as the mean ± SEM, n = 3.
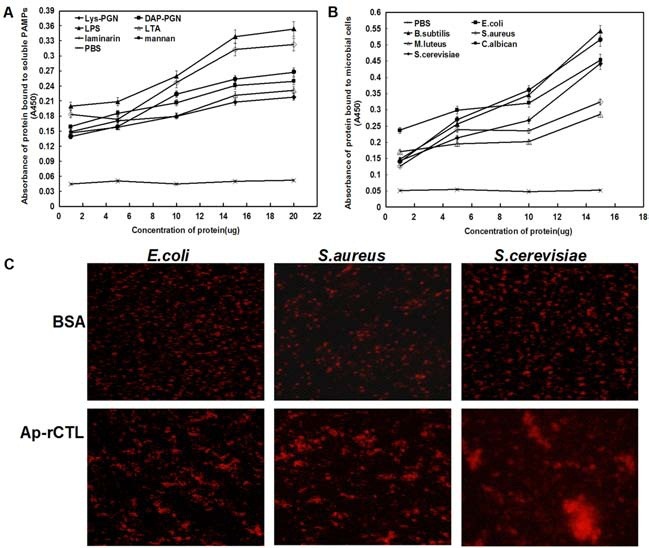
Comparing this two aspects of binding, we found that Ap-rCTL bound more strongly to microbial cells than to PAMPs at the same concentration. On one hand, microbial cell walls were composed of a variety of PAMPs, and the binding to microorganisms might be strengthen by the cooperative function of all PAMPs present on the cell walls, compared with the binding to a certain PAMP only. On the other hand, as is well known, proteins, especially glycoproteins, play an important role in signal transduction between cells, and this similar function might also exist in microorganism binding through the modulation to the binding between Ap-rCTL and PAMPs, or even through the binding to Ap-rCTL by proteins directly. On balance, Ap-rCTL acted as a broad-spectrum recognition protein binding to different PAMPs and microorganisms.
Since Ap-rCTL is able to bind to bacteria and fungi, an agglutination assay was performed to test whether the recombinant protein had the ability to produce aggregation of these microorganisms. When Ap-rCTL was incubated with EB dyed E. coli, S. aureus and S. cerevisiae in the presence of calcium, aggregation was observed under a fluorescence microscope. However, such an effect was not detected when a control protein, bovine serum albumin (BSA) was added at the same concentration to the microorganisms (Fig. 3C).
Functions of Ap-rCTL as a pattern recognition protein in proPO activation assay
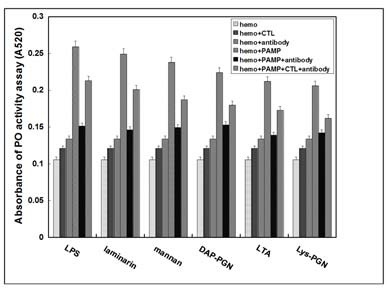
Since the activation of proPO cascade starts with recognition of invading materials as foreign substances, a proPO activation assay of native A. Pernyi larvae cell-free hemolymph was performed to observe whether Ap-rCTL was involved in this activation through acting as a pattern recognition protein (Fig. 4). Take it as an example that LPS was performed as the PAMP involving in the proPO activation assay, neither Ap-rCTL nor anti-Ap-rCTL antibody stimulated the proPO activation of native hemolymph (columns2and3) compared with LPS which induced the activation dramatically (column4). However, when LPS was added to the hemolymph incubated with anti-Ap-rCTL antibody in advance, the degree of activation decreased (column5), while the reduced activation could be partly restored when supererogatory Ap-rCTL was added to the mixture (column6). Similar results were also observed in the presence of other soluble PAMPs (Fig. 4) and microorganisms (data not shown). From the results of all of these experiments, we were able to conclude that C-type lectin is endogenously expressed in Antheraea pernyi larvae hemolymph and can induce the activation of the proPO cascade by recognizing PAMPs and microorganisms.
Fig. 4. ProPO activation assay of Ap-rCTL. To further understand the function of Ap-rCTL in the proPO pathway after binding PAMPs or microorgnisms, a proPO activitation assay was performed as described under "MATERIALS AND METHODS". The results of the proPO activitation assays are shown as the mean ± SEM (n = 3) and the analysis of variance and Newman-Keuls test is P < 0.05.
In short, Ap-rCTL acts as a broad-spectrum recognition protein involved in A. pernyi innate immunity through activating the proPO pathway and aggregation of the invading pathogen.
MATERIALS AND METHODS
Insects and injections
A. pernyi larvae were purchased from Shenyang Agricultural University and reared on a natural diet of quercus mongolica leaves at 27℃. On day 8, the 5th instar larvae were chilled on ice for 5 min, and immune challenged by a mixture of formalderhyde-killed E. coli, S. aureus, C. albican and S. cerevisiae (2 × 106 cells/ml, respectively) in 10 μl insect saline (ISL) using a 27-gauge needle. Larvae without injection or injected only with 10 μl ISL were used as negative controls. Then, 24 h after injection, hemolymph was collected as described by Lees (23).
Chemicals and microorganisms
LPS (L2880), laminarin (L9634), LTA (L3265) and mannan (M7504) were purchased from Sigma-Aldrich (USA). Curdlan (insoluble β-1,3-glucan) was purchased from Wako Pure Chemicals (Osaka). PGN was prepared from bacterial walls. Both Lys-PGN (M. luteus) and DAP-PGN (B. subtilis) were obtained as previously described (24).
cDNA cloning of Ap-CTL
Total RNA was extracted from challenged fat body using Trazol reagent (TAKARA), and then first-strand cDNA was synthesized with an MLV- reverse transcription kit (KEYGENTIC, China). C-F1/R1 (5'-TGGCARGARGCNCGNCTNCGNTGYC-3'/5'-AGRCTRTTNGGRTCYTTYTCRCADAT-3'), primers for cDNA fragment amplication of Ap-CTL were designed based on highly conserved amino acid sequences of the C-type lectin superfamily. The purified PCR product was ligated into the clone vector, pMD-18T (TAKARA), and then transformed into E. coli stain DH5α. After identification, positive clones were sequenced using T7 primers (GENSCRIPT, China). To obtain the 5' and 3' open reading frames of Ap-CTL, RACE PCRs were performed based on the manufacture of 5' RACE System for Rapid Ampliation of cDNA Ends and 3' RACE System for Rapid Ampliation of cDNA Ends (Invitrogen), respectively. Both 5' RACE and 3' RACE products were purified, subcloned into pMD-18T vectors, and then sequenced. The full-length cDNA sequence of Ap-CTL had been assembled by the Seqman program of DNASTAR.
Sequence analysis and alignment of Ap-CTL
For analysis of the characterization of protein sequences, many sequence analytic tools were used. SignalP and computer pI/MW were used to analyze the deduced Ap-CTL protein sequence. The probable N- and O-glycosylation sites were predicted by NetNGlyc 1.0 and NetOGlyc 2.0 prediction servers, respectively. BLASTX was performed to compare the Ap-CTL amino acid sequences with the non-reductant public sequence database in NCBI, and CLUSTALW was used to align Ap-CTL with similar proteins. A phylogenetic tree was generated from CLUSTALW guide tree data using the DNAMAN program.
Expression of recombinant Ap-CTL in the prokaryotic expression system
A gene fragment encoding the mature Ap-CTL was amplified with primers containing NcoI and EcoR I restriction enzyme sites at the 5' end, respectively. After digestion, products were ligated into the same sites of pET-28a (+) expression vector and transformed into E. coli BL21 (DE3) so that the six-histidine tag was expressed at the amino-terminus of the protein. Following sequencing, the positive bacterial colony was selected and cultured in LB medium containing 60 μg/ml kanamycin. 1.0 mM IPTG (final concentration) was added to induce the expression of Ap-rCTL. Bacteria were harvested by centrifugation and sonicated. The soluble recombinant protein was purified by Ni2+ affinity chromatography and evaluated by SDS-PAGE. The concentration was determined by the Bradford assay. For biological analysis, Ap-rCTL was used as an antigen to produce rabbit antiserum.
Expression of Ap-CTL in response to microbial challenges
The tissue specificity of Ap-CTL gene transcript expression was determined by semi-quantitative RT-PCR: 5 μg total RNA was extracted from different tissues of 24 h-challenged larvae using a mixture of formaldehyde-killed microorganisms, including hemocytes, fat body, midgut and integument. Following reverse transcription, the first-strand cDNA was amplified using pfu polymerase (TAKARA). A pair of rps3 primers (5'-GCCGTT CTTGCCCTGTT-3'/5'-CGCGAGTTGACTTCGGT-3') was used as an internal reference control for normalization. Naive and ISL-injected larvae were used as negative controls. The expression of the Ap-CTL gene was monitored over time. The total RNA was extracted from the fat body of challenged larvae at different times (0, 12, 24, 48 and 72 h), and the analytical method was the same as that used for tissue specificity detection.
Binding assay of Ap-rCTL to PAMPs and microbial cells
10 μg of six soluble polysaccharides in 100 μl PBS was respectively applied to coat wells of 96-well microplates. After air drying and fixation, the plates were blocked with 200 μl BSA (1 mg/ml) in PBS at 37℃ for 2 h. After washing with 200 μl TPBS (PBS with 0.1% v/v Tween 20) for 15 min, different concentrations of Ap-rCTL in 100 μl PBS were added and incubated at room temperature for 3 h, respectively. Washing was then carried out as described and 100 μl 1:500 diluted anti-(His)6 monoclonal antibody in PBS was added, followed by incubation with 100 μl 1:2,000 diluted goat-anti-mouse Ig G conjugated to horseradish peroxidase (HRP). After the last washing, 50 μl substrate was added to each well and incubation was carried out at 37℃ for 30 min. At last, 100 μl 2M H2SO4 was used to stop the reaction and OD450nm was monitored with MULTISKAN FC (THERMO). To examine the binding specificity, the wells coated with PBS and those incubated with PBS instead of Ap-rCTL were performed as negative controls. To examine the binding to microbial cells, 100 μl samples of six microorganisms were used to coat wells (107 cells/well) and the protocols were the same as those described above, except for the microplates not being fixed at 60℃ for 1 h.
Agglutination of microbial cells by Ap-rCTL
For this, 10 μl Ap-rCTL (100 μg/ml) in PBS was incubated with 10 μl EB dyed microorganisms (2 × 108 cells/ml) at room temperature for 30 min in the presence of 0.1 mM Ca2+. Samples were then applied to microscope slides, and the aggregation of cells was observed under an Olympus BH-2 fluorescence microscope.
ProPO activation assay
For this, 20 μl Ap-rCTL (200 μg/ml) in 20 mM Tris-HCl, 50 mM NaCl, pH 8.0, was incubated with 20 μl cell-free hemolymph and 5 μl soluble PAMP at room temperature for 20 min, then 450 μl substrate solution (1 mM 4-methylcatechol, 2 mM 4-hychoxyproline ethylester in the same buffer) was added. The PO activity was determined by monitoring OD520nm at 1-min intervals. To identify the function of Ap-rCTL in pro-PO activation, 10 μl rabbit antiserum (anti-Ap-rCTL antibody) was pre-incubated with naive hemolymph for 20 min at 4℃ to block endogenous C-type lectin, then Ap-rCTL and PAMP were added and the PO activity induced by recombinant protein only was observed. As a negative control, samples without Ap-rCTL, PAMP or both (replaced by appropriate buffer) were examined.
Acknowledgments
We would like to thank Dr. Xu for providing bacterial and fungi stains used in this study. This work was supported by grants from the National Natural Science Foundation of China (No. 30972770 and No. 31100647), the Natural Science Foundation of Liaoning Province (No. 20072066), the Project of Education Department of Liaoning Province (No. 2008S219) and the Talent Supporting Program of Liaoning Province (LJQ2011108).
References
- 1.Yu X. Q., Zhu Y. F., Ma C., Fabrick J. A., Kanost M. R. Pattern recognition proteins in Manduca sexta plasma. Insect. Biochem. Mol. Biol. (2002);32:1287–1293. doi: 10.1016/S0965-1748(02)00091-7. [DOI] [PubMed] [Google Scholar]
- 2.Yu X. Q., Tracy M. E., Ling E., Scholz F. R., Trenczek T. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem. Mol. Biol. (2005);35:285–295. doi: 10.1016/j.ibmb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen C., Durrant J., Newton R. P., Ratcliffe N. A. A study of novel lectins ad their involvement in the activation of the prophenoloxidase system in Blaberus discoidalis. Biochem. J. (1995);310:23–31. doi: 10.1042/bj3100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y. D., Fu L. D., Jia Y. P., Du X. J., Wang Q., Wang Y. H., Zhao X. F., Yu X. Q., Wang J. X. A hepatopancreas-specific C-type lectin from the Chinese shrimp Fenneropenaeus chinensis exhibits antimicrobial activity. Mol. Immunol. (2008);45:348–361. doi: 10.1016/j.molimm.2007.06.355. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Song L. S., Li C., Zhao J., Zhang H., Ni D., Xu W. Cloning and characterization of a novel C-type lectin from Zhikong scallop Chlamys farreri. Mol. Immunol. (2007);44:722–731. doi: 10.1016/j.molimm.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Fabrick J. A., Baker J. E., Kanost M. R. cDNA cloning, purification, propeiities, and function of a β-1,3-glucan recognition protein from a pyralid moth, Plodia interpunctella. Insect Biochem. Mol. Biol. (2003);33:579–594. doi: 10.1016/S0965-1748(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R., Janeway C.A. Innate immunity:the virtues of a nonclonal system of recognition. Cell. (1997);91:295–298. doi: 10.1016/S0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L., Song L. S., Xu W., Qian P. Y. Molecular cloning and immune responsive expression of a novel C-type lectin gene from bay scallop Argopecten irradians. Fish Shellfish Immun. (2008);25:231–238. doi: 10.1016/j.fsi.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Stratton L., Wu S., Richards R. C., Vanya E. K. Oligomerisation and carbohydrate binding in an Atlantic salmon serum C-type lectin consistent with non-self recognition. Fish Shellfish Immun. (2004);17:315–323. doi: 10.1016/j.fsi.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Song X., Wang L., Kong P., Yang J., Liu L., Qiu L., Zhang Y., Qiu L., Song L. AiCTL-6, a novel C-type lectin from bay scallop Argopecten irradians with a long C-type lectin-like domain. Fish Shellfish Immun. (2011);30:17–26. doi: 10.1016/j.fsi.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Jomori T., Natori S. Function of the lipopolysaccharide-binding protein of Periplaneta americana as an opsonin. FEBS Lett. (1992);6:283–296. doi: 10.1016/0014-5793(92)80305-Z. [DOI] [PubMed] [Google Scholar]
- 12.Yu X. Q., Kanost M. R. Manduca sexta lipopolysaccharide-specific immulectin-2 protects larvae from bacterial infection. Dev. Comp. Immunol. (2003);27:189–196. doi: 10.1016/S0145-305X(02)00099-X. [DOI] [PubMed] [Google Scholar]
- 13.Jomori T., Natori S. Molecular cloning of cDNA for lipopolysaccharide binding protein from the hemolymph of the American cockroach Periplaneta americana. J. Biol. Chem. (1991);266:13318–13323. [PubMed] [Google Scholar]
- 14.Wang X. W., Zhang X. W., Xu W. T., Zhao X. F., Wang J. X. facilitates the clearance of Vibrio anguillarum in vivo in Chinese White shrimp. Dev. Comp. Immunol. (2009);33:1039–1047. doi: 10.1016/j.dci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Brown A. C., Harrison L. M., Kapulkin W., Jones B. F., Sinha A., Savage A., Villalon N., Cappello M. Molecular cloning and characterization of a C-type lectin from Ancylostoma ceylanicum: Evidence for a role in hookworm reproductive physiology. Mol. Biochem. Parasit. (2007);151:141–147. doi: 10.1016/j.molbiopara.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe A., Miyazawa S., Kitami M., Tabunoki H., Ueda K., Sato R. Characterizaion of a novel C-Type lectin, bombyx mori multibinding protein, from the B. mori hemolymph: mechanism of wide-range microorganism recognition and role in immunity. Immunol. J. (2006);177:4594–4604. doi: 10.4049/jimmunol.177.7.4594. [DOI] [PubMed] [Google Scholar]
- 17.Takase H., Watanabe A., Yoshizawa Y., Kitami M., Sato R. Identification and comparative ananlysis of three novel C-type lectins from the silkworm with functional implications in pathogen recognition. Dev. Comp. Immunol. (2009);33:789–800. doi: 10.1016/j.dci.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Yu X. Q., Kanost M. R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J. Biol. Chem. (2000);275:37373–37381. doi: 10.1074/jbc.M003021200. [DOI] [PubMed] [Google Scholar]
- 19.Yu X. Q., Gan H., Kanost M. R. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem. Mol. Biol. (1999);29:585–597. doi: 10.1016/S0965-1748(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 20.Yu X. Q., Ma Y. Calcium is not required for immulectin-2 binding, but protects the protein from proteinase digestion. Insect Biochem. Mol. Biol. (2006);36:505–516. doi: 10.1016/j.ibmb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Ling E., Yu X. Q. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. (2006a);30:289–299. doi: 10.1016/j.dci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Chai L. Q., Tian Y. Y., Yang D. T., Wang X. J., Zhao X. F. Molecular cloning and characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev. Comp. Immunol. (2008);32:71–83. doi: 10.1016/j.dci.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Moon H. J., Lee S. Y., Kyrata S., Lee B. L. Purification and molecular cloning of cDNA from an inducible antibacterial protein from larvae of the colepteran, Tenebrio molitor. J. Biochem. (1994);116:53–58. doi: 10.1093/oxfordjournals.jbchem.a124502. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y., Park J. W., Kwon H. M., Hwang H. O., Jang I. H., Masuda A., Kurokawa K., Nakayama H., Lee W. J., Dohmae N., Zhang J., Lee B. L. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. J. Biol. Chem. (2010);M110:144014. doi: 10.1074/jbc.M110.144014. [DOI] [PMC free article] [PubMed] [Google Scholar]


