Abstract
It is unknown how soluble pattern-recognition receptors in blood, such as mannose-binding lectin (MBL) and ficolins, modulate mast cell-mediated inflammatory responses. We investigate how mouse MBL-A or ficolin-A regulate mouse bone marrow-derived mast cells (mBMMCs)-derived inflammatory response against bacterial lipopolysaccharide (LPS) stimulation. LPS-mediated pro-inflammatory cytokine productions on mBMMCs obtained from Toll-like receptor4 (TLR4)-deficient mice, TLR2-defficient mice, and their wildtype, were specifically attenuated by the addition of either mouse MBL-A or ficolin-A in a dose-dependent manner. However, the inhibitory effects by mouse MBL-A or ficolin-A were restored by the addition of mannose or N-acetylglucosamine, respectively. These results suggest that mouse MBL-A and ficolin-A bind to LPS via its carbohydrate-recognition domain and fibrinogen-like domain, respectively, whereby cytokine production by LPSmediated TLR4 in mBMMCs appears to be down-regulated, indicating that mouse MBL and ficolin may have an inhibitory function toward mouse TLR4-mediated excessive inflammation on the mast cells. [BMB Reports 2013; 46(7): 376-381]
Keywords: Ficolin, Innate immunity, Mannose-binding lectin, Mast cell, Toll-like receptor
INTRODUCTION
Innate immunity provides the first line of host defence against the invasion of pathogenic microbes (1). Crucial host immune-surveillance cells, such as dendritic cells, macrophages, and mast cells, function as sentinels, by providing early warning signals, and by the activation of host defence responses against invading pathogenic bacteria (2,3). These innate immune responses can be achieved by the direct recognition of bacteria through pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and complement receptors, which are activated in response to the recognition of pathogen-associated molecular patterns (PAMPs), such as bacterial lipopolysaccharide (LPS), lipoproteins, and fungal 1,3-β-D-glucan (4-7). Mast cells have been reported to produce different types of cytokines and chemokines, including pro-inflammatory and anti-inflammatory mediators, in response to different types of PAMPs stimuli. Therefore, they are thought to play a role in linking innate defenses to adaptive immune responses, and in orchestrating the overall host immune responses (5,8).
Mannose-binding lectin (MBL) and ficolins are multifunctional soluble PRRs that are crucial in the initiation of lectin complement pathway upon binding to microbial PAMPs, leading to bacterial lysis and phagocytosis of opsonized microbes (9,10). MBL and ficolins are oligomeric proteins assembled from subunits consisting of two distinct functional domains: the collagen-like domain, and the carbohydrate-recognition domain (CRD for MBL)/fibrinogen-like domain (FBG for ficolins) (9,11). In general, MBL and ficolins are circulating proteins in the blood that exist as a complex with MBL-associated serine proteases (MASPs). These complexes, when bound to microbial cells through their CRD/FBG, promote the elimination of microbes through the activation of the lectin-complement pathway and the facilitation of opsonophagocytosis (9,12).
Both MBL and ficolins have been indentified in humans and rodents. Human MBL is produced in the liver and exists in only one form in serum, while mouse MBL occurs in two distinct forms in mouse serum MBL-A and MBL-C both of which enable the activation of the lectin-complement pathway (13). In humans, three ficolins have been identified: ficolin-1 (M-ficolin), -2 (L-ficolin) and -3 (H-ficolin). Ficolin-2 and ficolin-3 are serum proteins, and are mainly produced in the liver, whereas ficolin-3 is synthesized in both the liver and the lungs (14). Ficolin-1 is mainly secreted by immune cells, such as monocytes, neutrophils, and alveolar epithelial cells, and recently has been shown to be present in serum at low concentrations (15). In mice, two types of ficolins, ficolin-A and ficolin-B, have been identified. Ficolin-A is highly expressed in the liver and spleen, and is present in the serum, while ficolin-B is expressed in bone marrow and the spleen. Based on the structural and functional properties and phylogenetic analysis, ficolin-A resembles human ficolin-2, while ficolin-B is more homologous with human ficolin-1 (13).
MBL and ficolins have been reported to recognize a wide range of clinically important microbes, including bacteria, fungi, and viruses, and then induce specific innate immune responses (9,10,16,17). It has been shown that MBL and ficolins bind to the LPS of bacteria, and then induce the activation of the TLR4 signalling pathway, leading to the mediation of inflammatory responses during infection (13,18). Several reports have shown that molecules like MBL and the analogous lung surfactant proteins-A (SP-A) or SP-D regulate inflammatory cellular responses on various immune cells by different mechanisms upon stimulation with PAMPs (19-22). Since the molecular regulatory mechanisms between mast cells, host MBL, and ficolins during bacterial infection are largely unknown, in this study, we investigate the effect of mouse MBL-A and ficolin-A on LPS-induced inflammatory cytokine production using mouse bone marrow stem cell-derived mast cells (mBMMCs).
RESULTS
Bacterial LPS induced IL-6 production on mouse mast cells via TLR4-dependent manner
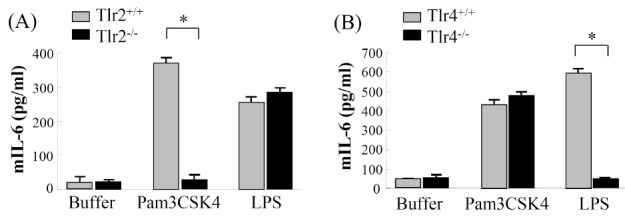
To examine the biological functions of mouse TLR2 and TLR4 during mast cell-mediated cytokine production in response to PAMP stimuli, we estimated the amounts of mouse IL-6 (mIL-6) production on mBMMCs prepared from Toll-like receptor 2 (TLR2)-deficient mice (Tlr2-/-), TLR4-deficient mice (Tlr4-/-), and their wild type (Tlr2+/+/ and Tlr4+/+). When these mast cells were stimulated with N-palmitoyl-S-dipalmitoylglyceryl-Cys-Scr-(Lys)4 (Pam3CSK4) and LPS, which are known to be typical ligand molecules of TLR2 and TLR4, respectively, Pam3CK4-treated mast cells produced mIL-6 vigorously, but the Tlr2-/- mast cells did not, while LPS did not show any significant difference (Fig. 1A). However, as expected, when we stimulated Tlr4+/+-derived mast cells or Tlr4-/--derived mast cells with LPS, Tlr4+/+-derived mast cells produced mIL-6, but the Tlr4-/- mast cells did not (Fig. 1B). These results clearly suggest that mouse mast cells produce IL-6 in response to Pam3CSK4 and LPS distinctly in a TLR2-dependent and TLR4-dependent manner, respectively.
Fig. 1. Cytokine production was induced through TLR4 and TLR2 on mouse mast cells. The mBMMCs from Tlr2-/- mice (A), Tlr4-/- mice (B), and their wild type (Tlr2+/+ or Tlr4+/+) were stimulated for 6 hr with Pam3CSK4 (2.5 μg/ml) or LPS (200 ng/ml, smooth type from E. coli serotype O4). As a negative control, buffer alone was used instead of PAMP stimuli. Mouse IL-6 levels in the supernatant were determined by ELISA. Data are represented as means ± S.D. from triplicate experiments. Results are representative of three independent experiments that yield similar results. Asterisk indicates the statistical significance versus controls: *P < 0.01.
Mouse MBL-A and ficolin-A attenuate LPS-induced inflammatory cytokine production on mBMMCs
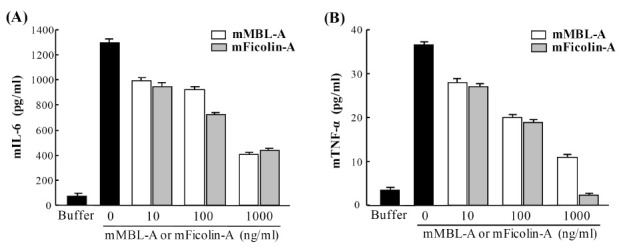
To determine whether MBL and ficolins can modulate LPSmediated inflammatory cytokine production in mast cells, mBMMCs were stimulated with LPS in the presence of mouse MBL-A or mouse ficolin-A. When the amounts of mIL-6 and mTNF-α were determined by ELISA, both proteins suppressed LPS-mediated mIL-6 and mTNF-α production on mBMMCs in a dose-dependent manner (Fig. 2A and 2B). Nevertheless, Pam3CSK4-mediated mIL-6 production was not affected by addition of mouse MBL-A or ficolin-A (data not shown). These results indicate that mouse MBL-A and ficolin-A specifically inhibit LPS-mediated inflammatory cytokine production in mouse mast cells.
Fig. 2. Mouse MBL-A and ficolin-A suppress LPS-mediated IL-6 and TNF-α production on mBMMCs. mBMMCs were stimulated with LPS (200 ng/ml, smooth type from E. coli serotype O4), which was pre-incubated with mouse MBL-A or ficolin-A. As a negative control, buffer alone was used instead of LPS. mIL-6 (A) and mTNF-α (B) levels in the supernatant were determined by ELISA. Data are represented as means ± S.D. from triplicate experiments. Results are representative of at least three independent experiments that yield similar results.
Mouse MBL-A and ficolin-A bind to LPS, not to extracellular domain of TLR4 in mast cells
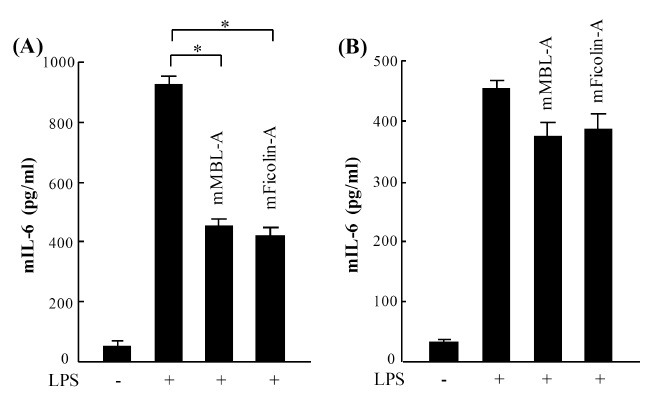
To examine the possibility of whether mouse MBL-A and ficolin-A bind to LPS via the CRD domain of MBL-A and the FBG domain of ficolin-A, LPS was pre-incubated with mouse MBL-A or ficolin-A prior to the stimulation of mast cells. As a control group, we prepared samples of directly incubated LPS with prepared mast cells before adding proteins. In accordance with previous results, pre-incubation of LPS with MBL-A or ficolin-A significantly reduced mIL-6 production on mBMMCs (Fig. 3A), whereas the pre-treatment of mast cells with the host lectin proteins did not clearly affect mIL-6 production (Fig. 3B), indicating that mouse MBL-A and ficolin-A attenuate LPS-mediated cytokine production through direct interaction with LPS, but not with TLR4 extracellular domain on mouse mast cells.
Fig. 3. Mouse MBL-A and ficolin-A attenuate LPS-mediated IL-6 production through direct binding to LPS, but not through TLR4 on mBMMCs. (A) LPS (200 ng/ml, smooth type from E. coli serotype O4) was pre-incubated with mouse MBL-A (1,000 ng/ml) or ficolin-A (1,000 ng/ml) prior to stimulation of mBMMCs. (B) Simultaneously, mBMMCs were pre-incubated with mouse MBL-A or ficolin-A before stimulation with LPS. mIL-6 levels in the supernatants were determined by ELISA. Data are represented as means ± S.D. from triplicate experiments. Results are representative of three independent experiments that yield similar results. Asterisk indicates the statistical significance versus controls: *P < 0.01.
Mannose and GlcNAc restored inhibitory effect of mouse MBL-A and ficolin-A toward LPS-mediated inflammatory cytokine production in mast cells
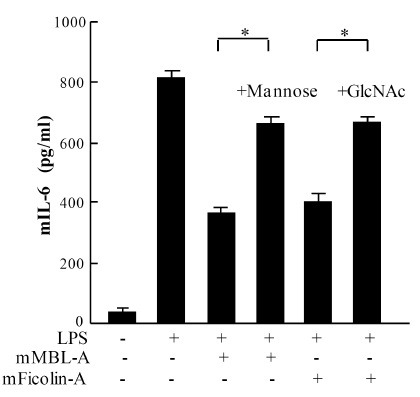
To further validate the obtained results, LPS was pre-incubated with mouse MBL-A or ficolin-A in the presence of mannose or GlcNAc prior to the stimulation of mast cells. By the addition of mannose or GlcNAc to the reaction mixture, LPS-mediated mIL-6 production was significantly reduced on mBMMCs (Fig. 4). However, mannose or GlcNAc alone did not show any influence on mast cell activation or on LPS-mediated cytokine production on mast cells (data not shown). These results strongly suggest that the binding of mouse MBL-A and ficolin-A to LPS occurs via the CRD domain of MBL-A and the FBG domain of mouse ficolin-A, respectively, and further supported our previous results that mouse MBL-A and ficolin-A inhibit LPS-mediated cytokine production on mast cells through binding to LPS.
Fig. 4. Effect of mannose or GlcNAc onto mouse MBL-A and ficolin-A-mediated inhibition of IL-6 production on mBMMCs. LPS (200 ng/ml, smooth type from E. coli serotype O4) was pre-incubated with mouse MBL-A (1,000 μg/ml) and ficolin-A (1,000 ng/ml) in the presence of mannose (0.1 M) or GlcNAc (0.1 M) prior to stimulation of mBMMCs. mIL-6 levels were measured in culture supernatants. Data are represented as means ± S.D. from triplicate experiments. Asterisk indicates the statistical significance versus controls: *P < 0.01.
DISCUSSION
In this study, we have demonstrated that LPS-mediated mIL-6 and mTNF-α production on mBMMCs is attenuated by the addition of mouse MBL-A or ficolin-A. Several reports have shown a regulatory role of host lectins, such as MBL, SP-A, and SP-D, in response to invading pathogens (19-22). MBL has been reported to inhibit bacterial peptidoglycan (PGN)-mediated inflammatory cytokine secretion on phorbol myristate acetate-stimulated U937 cells, and also to promote chemokine production, suggesting the importance of MBL binding to the GlcNAc residues of PGN (19). In contrast, SP-A inhibits PGN-mediated TNF-α secretion in U937 cells and alveolar macrophages by direct interaction with the extracellular domain of TLR2, rather than binding to PGN (21). Gardai et al. have proposed dual functions of lung collectins in macrophage-mediated pro-inflammatory immune responses, because the interaction between the collagenous tail of SP-A or SP-D and calreticulin/CD91 enhances pro-inflammatory responses by stimulation with LPS, while the interaction between their globular heads and signal inhibitory regulatory protein α (SIRPα) blocks pro-inflammatory mediator production (23). Similarly, our previous study showed that the binding of MBL to LPS through its CRD domain enhances LPS-mediated cytokine and chemokine production on human endothelial cells, suggesting possible functional roles of host collectin family proteins, in that collectins might function as intermediate bridging molecules between LPS and the potential cell receptors to facilitate LPS transferring to intracellular TLR4/MD2 via the distinct role of their collagenous stalks and pattern-recognition domain (20). Moreover, SP-A, SP-D, and MBL were also reported to bind the membrane-anchored receptor CD14, which facilitates the interaction between LPS and TLR4 (24,25). Taken together, these studies again highlight the heterogeneity of host innate immune responses towards the various PAMP stimuli during infection and inflammation.We suppose that the roles of these collectin family proteins in inflammatory responses against bacterial infection will be distinguished in mast cells.
We have investigated the effects of mouse MBL-A and ficolin-A on mast cell activation in response to LPS under different conditions. Both mouse MBL-A and ficolin-A showed inhibitory effects on the LPS-mediated inflammatory response on mast cells. The pre-incubation of LPS with mouse MBL-A or ficolin-A ameliorated the LPS-mediated immune response, but pre-treatment of the cells with these lectins did not. Furthermore, the LPS-mediated immune responses generated by treatments of MBL-A or ficolin-A were restored by the addition of by mannose or GlcNAc, showing the binding specificities of mouse MBL-A and mouse ficolin-A to ligand molecules. We speculate that mouse MBL and ficolins bound to LPS intervene in the recognition of LPS by TLR4 on the surface of mast cells. Our results reveal that the interaction of MBL or ficolins with TLR4 receptor on mast cells is less likely in this case. TLR4-mediated human mast cell responses have been reported to be dependent on the serum components, relying on the presence of soluble CD14 (26). In our case, without soluble CD14, vigorous mBMMC-mediated cytokine expression was still produced by LPS, even at a relatively low concentration (200 ng/ml, Escherchia coli serotype O4) (27). The different responsiveness of mast cells to LPS could be explained by the different sources and purity of the LPS serotype used and the heterogeneity of mast cells in different animal species.
In 1996, two reports described that mast cell-deficient mice show increased mortality after E. coli infection (2,3), suggesting a crucial role of mast cells in bacterial infection, and a minor contribution of complement activation by MBL. However, clinical studies also revealed that MBL-deficient individuals are more likely to develop systemic inflammatory response syndrome (SIRS) and to progress to septic shock (28), suggesting MBL may also be involved in the modulation of inflammatory responses. How MBL exerts a modulatory function in this disease remains unclear, suggesting that human MBL may have multiple mechanisms in vivo.
In this study, we have shown that murine MBL-A and ficolin-A have an anti-inflammatory function by interfering with the interaction between LPS and TLR4 on mast cells. These findings provide insight into the pathogenesis of the diseases, and also into how collagenous domain-containing host defense proteins respond to bacterial infection at a mast cell-distributed host-environment interface.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from Hyochang Science (Daegu, Korea). TLR4-deficient mice (Tlr4-/-, C3H/HeJ background) and its wild type (strain C3H/HeN) were purchased from Jackson Laboratory (Bar Harbor, Maine). TLR2-deficient mice (Tlr2-/-) and the corresponding wild type mice were kindly provided by Professor Shizuo Akira (Osaka University, Osaka, Japan). All mice were maintained in specific pathogen-free conditions at the Samsung Biomedical Research Center Facility. All animal experiments in this study were done in accordance with the institutional guidelines of the Samsung Medical Center.
Cell culture
Mouse bone marrow stem cell-derived mast cells (mBMMCs) were prepared and cultured as previously described (29,30). Briefly, mouse bone marrow stem cells were isolated from the femur and tibia of 6∼8-week-old mice, and cultured to a final density of 1 × 106 cells/ml in Iscove’s modified Dulbecco’s medium (IMDM) (Gibco) supplemented with L-glutamine (2 mM, Sigma), heat inactivated fetal calf serum (10%, Gibco), penicillin (100 IU/ml, Sigma), streptomycin (100 μg/ml, Sigma), murine stem cell factor (SCF) (30 ng/ml, Peprotech), and IL-3 (30 ng/ml, Peprotech) at 37℃, in 5% (v/v) CO2 air with 95% humidity. The cultural medium was changed every 5-7 days. Non-adherent cells were collected and transferred to fresh medium, which were maintained for approximately 8 weeks. The homogeneity of mast cells was determined by staining with toluidine blue and FACS analysis. Cells cultured for 7-9 weeks were used in the experiments.
Cytokine assay
Mouse MBL-A and ficolin-A were purified from mouse serum (Aleken Biologicals, Nash, TX) using Staphylococcus aureus PGN-coupled Sepharose 4B, as previously described (31). Smooth-type LPS from E. coli serotype O4 were kindly provided by the Borstel Research Center (Germany). Mast cells were harvested by centrifugation at 1,000 rpm for 5 min and suspended with basal medium in the absence of SCF and IL-3 for overnight starvation. For the induction of cytokines, mast cells were suspended with fresh basal medium and seeded into the wells of 96-well culture plates (SPL, Korea) (0.25 × 106 cells/150 μl of basal medium) and stimulated in triplicate with LPS (200 ng/ml) for 6 hr at 37℃, in 5% (v/v) CO2 air with 95% humidity. After stimulation, the supernatant from each well was collected by centrifugation at 1,000 rpm for 7 min and used for a subsequent cytokine assay. To measure the levels of mIL-6 and mTNF-α in each supernatant, mIL-6 and mTNF-α ELISA were performed according to the manufacturer’s instructions (DuoSet ELISA, R&D).
In some experiments, mast cells from Tlr4-/- , Tlr2-/-, and their wild type were used to determine the effect of TLR4 and TLR2 on mast cell-mediated IL-6 production in response to LPS and N-palmitoyl-S-dipalmitoylglyceryl Cys-Scr-(Lys)4 (Pam3CSK4) (EMC Microcollections GmbH), respectively. After the stimulation of these cells with LPS (200 ng/ml) or Pam3CSK4 (2.5 ug/ml), IL-6 levels in the supernatant were analyzed as described above.
To determine the effect of mouse MBL-A or ficolin-A on LPS-induced cytokine production in mast cells, LPS (200 ng/ml) was pre-incubated with or without each protein (10, 100, 1,000 μg/ml) for 80 min at room temperature prior to stimulation. To understand the involvement of TLR4 as well as LPS in the case described above, each protein (1,000 ng/ml) was pre-incubated with mast cells for 3 hr before stimulation with LPS (200 ng/ml), which was simultaneously compared with the previous setup, with pre-incubation of LPS (200 ng/ml) with each protein (1,000 ng/ml) for 80 min before stimulation. IL-6 levels in the supernatant were analyzed as described above.
In some experiments, to determine the effect of mannose or GlcNAc on the LPS-mediated immune responses described above, both proteins were pre-treated with LPS in the presence or absence of mannose (0.1M) or GlcNAc (0.1M) to inhibit their binding to LPS before the stimulation of mast cells. As a control, mast cells were only incubated with mannose or GlcNAc in the presence or absence of LPS. IL-6 levels in the supernatant were analyzed as described above.
Statistical analysis
Data are represented as the mean ±S.D. of at least three independent experiments. Statistical analysis was performed using Student’s t test. P < 0.01 was considered statistically significant between two sample means.
Acknowledgments
The authors are grateful to Professor Misao Matsushita (Tokai University, Kanagawa, Japan) for providing rat monoclonal antibodies against mouse MBL-A and ficolin-A, Professor Shizuo Akira (Osaka University, Osaka, Japan) for providing Tlr2-/- mice and corresponding wild type mice, and Professor Helmut Brade (Borstel Research Center, Borstel, Germany) for providing purified LPSs. This work was supported for two years by a Pusan National University Research Grant.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. (2007);449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Malaviya R., Ikeda T., Ross E., Abraham S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. (1996);381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 3.Echtenacher B., Mannel D. N., Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. (1996);381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G., Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. (2007);7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 5.Supajatura V., Ushio H., Nakao A., Akira S., Okumura K., Ra C., Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Invest. (2002);109:1351–1359. doi: 10.1172/JCI0214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R., Janeway C. A. Jr. Decoding the patterns of self and nonself by the innate immune system. Science. (2002);296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 7.Janeway C. A. Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. (2002);20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 8.Frossi B., De Carli M., Pucillo C. The mast cell: an antenna of the microenvironment that directs the immune response. J. Leukoc. Biol. (2004);75:579–585. doi: 10.1189/jlb.0603275. [DOI] [PubMed] [Google Scholar]
- 9.Endo Y., Matsushita M., Fujita T. Role of ficolin in innate immunity and its molecular basis. Immunobiology. (2007);212:371–379. doi: 10.1016/j.imbio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K., Ip W. E., Michelow I. C., Ezekowitz R. A. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr. Opin. Immunol. (2006);18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garred P., Honore C., Ma Y. J., Munthe-Fog L., Hummelshoj T. MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement. Mol. Immunol. (2009);46:2737–2744. doi: 10.1016/j.molimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Thiel S., Vorup-Jensen T., Stover C. M., Schwaeble W., Laursen S. B., Poulsen K., Willis A. C., Eggleton P., Hansen S., Holmskov U., Reid K. B., Jensenius J. C. A second serine protease associated with mannan- binding lectin that activates complement. Nature. (1997);386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 13.Fujita T., Matsushita M., Endo Y. The lectin- complement pathway-its role in innate immunity and evolution. Immunol. Rev. (2004);198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 14.Sallenbach S., Thiel S., Aebi C., Otth M., Bigler S., Jensenius J. C., Schlapbach L. J., Ammann R. A. Serum concentrations of lectin-pathway components in healthy neonates, children and adults: mannan-binding lectin (MBL), M-, L-, and H-ficolin, and MBL-associated serine protease-2 (MASP-2). Pediatr. Allergy Iimmunol. (2011);22:424–430. doi: 10.1111/j.1399-3038.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 15.Honore C., Rorvig S., Munthe-Fog L., Hummelshoj T., Madsen H. O., Borregaard N., Garred P. The innate pattern recognition molecule Ficolin-1 is secreted by monocytes/macrophages and is circulating in human plasma. Mol. Immunol. (2008);45:2782–2789. doi: 10.1016/j.molimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y. J., Doni A., Skjoedt M. O., Honore C., Arendrup M., Mantovani A., Garred P. Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J. Biol. Chem. (2011);286:3405–3417. doi: 10.1074/jbc.M110.190637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y. J., Doni A., Hummelshoj T., Honore C., Bastone A., Mantovani A., Thielens N. M., Garred P. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J. Biol. Chem. (2009);284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neth O., Jack D. L., Dodds A. W., Holzel H., Klein N. J., Turner M. W. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. (2000);68:688–693. doi: 10.1128/IAI.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadesalingam J., Dodds A. W., Reid K. B., Palaniyar N. Mannose-binding lectin recognizes peptidoglycan via the N-acetyl glucosamine moiety, and inhibits ligand- induced proinflammatory effect and promotes chemokine production by macrophages. J. Immunol. (2005);175:1785–1794. doi: 10.4049/jimmunol.175.3.1785. [DOI] [PubMed] [Google Scholar]
- 20.Kang H. J., Lee S. M., Lee H. H., Kim J. Y., Lee B. C., Yum J. S., Moon H. M., Lee B. L. Mannosebinding lectin without the aid of its associated serine proteases alters lipopolysaccharide-mediated cytokine/chemokine secretion from human endothelial cells. Immunology. (2007);122:335–342. doi: 10.1111/j.1365-2567.2007.02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami S., Iwaki D., Mitsuzawa H., Sano H., Takahashi H., Voelker D. R., Akino T., Kuroki Y. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J. Biol. Chem. (2002);277:6830–6837. doi: 10.1074/jbc.M106671200. [DOI] [PubMed] [Google Scholar]
- 22.Yamada C., Sano H., Shimizu T., Mitsuzawa H., Nishitani C., Himi T., Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J. Biol. Chem. (2006);281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 23.Gardai S. J., Xiao Y. Q., Dickinson M., Nick J. A., Voelker D. R., Greene K. E., Henson P. M. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. (2003);115:13–23. doi: 10.1016/S0092-8674(03)00758-X. [DOI] [PubMed] [Google Scholar]
- 24.Sano H., Chiba H., Iwaki D., Sohma H., Voelker D. R., Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J. Biol. Chem. (2000);275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- 25.Chiba H., Sano H., Iwaki D., Murakami S., Mitsuzawa H., Takahashi T., Konishi M., Takahashi H., Kuroki Y. Rat mannose-binding protein a binds CD14. Infect. Immun. (2001);69:1587–1592. doi: 10.1128/IAI.69.3.1587-1592.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadaradjalou S., Feger F., Thieblemont N., Hamouda N. B., Pleau J. M., Dy M., Arock M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur. J. Immunol. (2003);33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 27.McCurdy J. D., Lin T. J., Marshall J. S. Tolllike receptor 4-mediated activation of murine mast cells. J. Leuko. Biol. (2001);70:977–984. [PubMed] [Google Scholar]
- 28.Fidler K. J., Wilson P., Davies J. C., Turner M. W., Peters M. J., Klein N. J. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intensive Care Medicine. (2004);30:1438–1445. doi: 10.1007/s00134-004-2303-8. [DOI] [PubMed] [Google Scholar]
- 29.Huber M., Helgason C. D., Damen J. E., Liu L., Humphries R. K., Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. U.S.A. (1998);95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Y. J., Kim C. H., Ryu K. H., Kim M. S., So Y. I., Lee K. J., Garred P., Lee B. L. Adenosine derived from Staphylococcus aureus-engulfed macrophages functions as a potent stimulant for the induction of inflammatory cytokines in mast cells. BMB Rep. (2011);44:335–340. doi: 10.5483/BMBRep.2011.44.5.335. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y. G., Cho M. Y., Zhao M., Park J. W., Matsushita M., Fujita T., Lee B. L. Human mannose-binding lectin and L-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J. Biol. Chem. (2004);279:25307–25312. doi: 10.1074/jbc.M400701200. [DOI] [PubMed] [Google Scholar]


