Abstract
Articular cartilage damage remains an unsolved problem in orthopaedics. Insulin-like growth factor I (IGF-I) and fibroblast growth factor-2 (FGF-2) are anabolic and mitogenic for articular chondrocytes, and are candidates for the application of gene therapy to articular cartilage repair. We tested the hypothesis that the production of IGF-I and FGF-2 can be augmented by modulating vector designs and delivery methods used for gene transfer to articular chondrocytes. We developed a novel adeno-associated virus (AAV)-based plasmid (pAAV) to overexpress IGF-I and FGF-2 cDNAs in adult bovine articular chondrocytes. We found that the pAAV-based vectors generated significantly more growth factor than pcDNA vectors carrying the same cDNAs. Chondrocytes cotransfected with both IGF-I and FGF-2 cDNAs in two separate pAAV plasmids produced significantly more IGF-I and FGF-2 than cells transfected by a single pAAV plasmid carrying both cDNAs in a dicistronic cassette. These data indicate that pAAV vectors are more effective than pcDNA vectors for transfer of IGF-I and FGF-2 genes to articular chondrocytes. They further suggest that cotransfection may be an effective strategy for multiple gene transfer to these cells. These findings may be important in applying growth factor gene transfer to cell-based articular cartilage gene therapy.
Keywords: chondrocytes, gene transfer, insulin-like growth factor I (IGF-I), fibroblast growth factor-2 (FGF-2), adeno-associated virus (AAV)-based plasmid (pAAV)
Damaged articular cartilage has a poor intrinsic repair capacity and is responsible for considerable disability in the form of arthritis and joint trauma. Once articular cartilage substance is lost, the damage is generally permanent and is often progressive.1 Insulin-like growth factor I (IGF-I) and fibroblast growth factor-2 (FGF-2) are cell-regulatory molecules that promote anabolic and mitogenic activities by articular chondrocytes, and that may possess therapeutic potential.2–5 Such growth factors are candidates for the application of gene therapy to articular cartilage repair.6–12 Currently, the optimal transfection strategy for non-viral growth factor gene transfer into articular chondrocytes is unknown. The purpose of the present studies was to optimize growth factor production by articular chondrocytes. Specifically, we sought to identify specific features of plasmid design and delivery that may augment the results of growth factor gene transfer into articular chondrocytes.
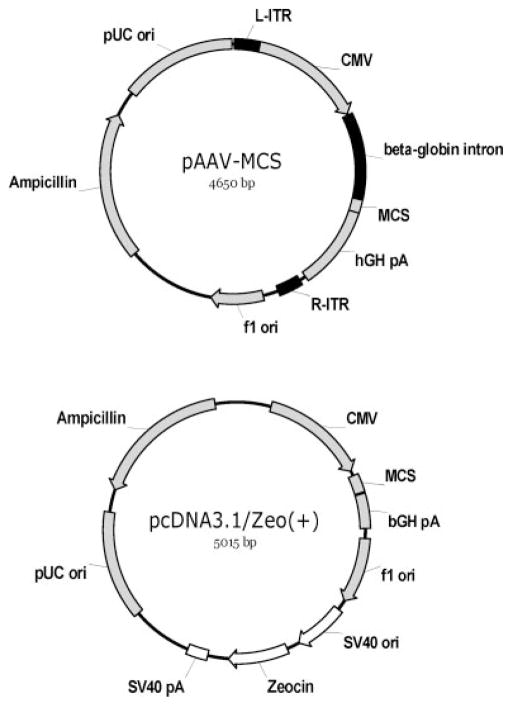
The pcDNA3.1/Zeo(+) (Invitrogen, Carlsbad, CA) plasmid (Fig. 1) has been used to construct IGF-I and FGF-2 overexpression vectors that successfully transfect primary chondrocyte cultures.13,14 The pAAV-MCS (Stratagene, Cedar Creek, TX) plasmid (Fig. 1) was originally designed to prepare recombinant adeno-associated virus vectors. We tested the hypothesis that pAAV-based constructs would yield higher growth factor transgene expression than pcDNA-based vectors. To test this hypothesis, primary monolayer cultures of isolated bovine articular chondrocytes were transfected with pAAV or pcDNA carrying the IGF-I or FGF-2 gene.
Figure 1.
Schematic illustration of plasmids used to construct IGF-I and FGF-2 expression vectors. Plasmids pcDNA3.1/Zeo(+) and pAAV-MCS both employ the human cytomegalovirus immediate-early (CMV) promoter and the bovine or human growth hormone polyadenylation signal (bGH pA or hGH pA) for the expression of the coding sequence inserted at multiple cloning site (MCS). In addition, plasmid pcDNA3.1/Zeo(+) contains an independent expression cassette for Zeocin resistance (SV40 promoter and origin, Zeocin resistance gene, and SV40 pA) while pAAV-MCS contains a beta-globin intron in the expression cassette and two AAV2 inverted terminal repeats (L-ITR and R-ITR) flanking the expression cassette.
Because many chondrocyte functions are involved in articular cartilage homeostasis and repair, the optimization of growth factor therapy for cartilage repair will likely necessitate the use of more than one growth factor. To test the hypothesis that growth factor production can be increased by adding a second copy of a growth factor cDNA, we constructed pAAV-based plasmids carrying both IGF-I and FGF-2 genes, separated by an internal ribosome entry site (IRES), in a dicistronic expression cassette15,16 and measured IGF-I and FGF-2 production. A second method of overexpressing two genes is to cotransfect target cells with two plasmids. We tested the hypothesis that cotransfection by two plasmids, each carrying a single cDNA, is more effective than transfection by a single plasmid carrying two cDNAs in a dicistronic cassette.
MATERIALS AND METHODS
IGF-I and FGF-2 pAAV Vector Construction
Plasmid pCMVhIGF-I and pCMVhFGF-2 were previously constructed from pcDNA3.1/Zeo (+) (Invitrogen) as described.13,14 IGF-I and FGF-2 cDNAs with EcoR I site at 5′ ends and Bgl II site at 3′ ends were generated by polymerase chain reaction (PCR) using pCMVhIGF-I and pCMVhFGF-2 as PCR templates and the primer pairs (Table 1), IGF-I forward primer 1 with reverse primer, and FGF-2 forward primer 1 with reverse primer, respectively. PCR products were cloned into pCR II-TOPO (Invitrogen). After sequencing, they were sub-cloned into pAAV-MCS at EcoR I and Bgl II sites to obtain pAAV-IGF-I and pAAV-FGF-2, respectively. To simultaneously express two genes using a single plasmid vector, plasmid pAAV-IRES was constructed by inserting an encephalomyocarditis virus IRES element17 into pAAV-MCS at BamH I and Sal I sites. The IRES element was generated by PCR using plasmid pAAV-IRES-hrGFP (Stratagene) as a template and the two IRES primers (Table 1). The IRES PCR product in pCR II-TOPO was sequenced and sub-cloned into pAAV-MCS to obtain pAAV-IRES. The IGF-I cDNA with EcoR I site at 5′ end and Bgl II site at 3′ end, generated as described above, was inserted into pAAV-IRES before IRES at the EcoR I and BamH I sites to obtain plasmid pAAV-IGF-I-IRES. One IGF-I cDNA with Sal I site at the 5′ end and Bgl II site at the 3′ end was generated by PCR using pCMVhIGF-I as the template and primers (Table 1): IGF-I FW primer 2 and IGF-I RV primer, and was inserted into pAAV-IGF-I-IRES after IRES at the Sal I and Bgl II sites to obtain plasmid pAAV-IGF-I-IRES-IGF-I. We also used this strategy to construct plasmids, pAAV-FGF-2-IRES, pAAV-FGF-2-IRES-FGF-2, pAAV-IGF-I-IRES-FGF-2, and pAAV-FGF-2-IRES-IGF-I.
Table 1.
Primers for PCR and Plasmid Construction
| IRES FW primer | 5′-TCGGATCCAGCAATTCCTCGACGACTGCATAGG-3′ |
| IRES RV primer | 5′-GAGTCGACCATGGTTGTGGCCATTATCATCGTG-3′ |
| IGF-I FW primer 1 | 5′-CAGAATTCACAATGGGAAAAATCAGCAGTCTTCC-3′ |
| IGF-I FW primer 2 | 5′-ACGTCGACACAATGGGAAAAATCAGCAGTCTTCC-3′ |
| IGF-I RV primer | 5′-CTAGATCTCTACATCCTGTAGTTCTTGTTTCCTG-3′ |
| FGF-2 FW primer 1 | 5′-TCGAATTCACCATGGCAGCCGGGAGCATCGCCACG-3′ |
| FGF-2 FW primer 2 | 5′-ACGTCGACATGGCAGCCGGGAGCATCACCACG-3′ |
| FGF-2 RV primer | 5′-CTAGATCTTCAGCTCTTAGCAGACATTGGAAG-3′ |
Chondrocyte Cell Culture and Transfection
Dulbecco’s minimum essential medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, and glutamine were from Invitrogen. Other reagents were from Sigma (St. Louis, MO). Basal medium was prepared with DMEM, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 50 μg/ml ascorbic acid. Complete medium was prepared with basal medium supplemented with 10% FBS. Cell lysis buffer was prepared as previously described.18
Bovine articular chondrocytes were isolated as previously described.19 Bovine articular cartilage was dissected from carpal joints of legs of ~1-year-old bovines. Isolated chondrocytes were washed, and suspended in complete medium, then placed in monolayer culture in 6-well plates at 3 × 105 cells/well in 4 ml of complete medium at 37°C in 5% CO2. After 3 days, the medium was replaced with 2 ml complete medium and cells were transfected using FuGENE 6 (Roche Applied Science, Indianapolis, IN) and plasmid DNA. Specifically, for single transfection, 2 μg of each plasmid DNA per well was used. For cotransfection, 2 μg of each different plasmid DNA per well was used together. The ratio of plasmid DNA (μg) to FuGENE 6 reagent (μl) was 1:3. After 4 h, transfection was stopped by replacing the medium with 4 ml of fresh complete medium.
Growth Factor Analysis
On day 2 and day 4 after transfection, conditioned medium (CM) was collected and replaced with basal medium. On day 6 after transfection, CM was collected and the culture was terminated. Following CM removal, the cell layer was sonicated in 0.5 ml cell lysis buffer. CM and cell lysate were stored at −20°C for growth factor analysis. Human IGF-I and human FGF-2 were measured by DuoSet ELISA kits from R&D Systems (Minneapolis, MN) with detection limits of 30 pg/ml and 16 pg/ml, respectively. IGF-I production by pAAV-IGF-I transfected chondrocytes, into the medium and cell layer, respectively, was 1077.40 ± 116.15 versus 64.74 ± 2.30 ng/well (day 2), 789.29 ± 102.51 versus 16.17 ± 1.19 ng/well (day 4), and 189.52 ± 31.53 versus 3.48 ± 0.87 ng/well (day 6). FGF-2 production by pAAV-FGF-2 transfected chondrocytes into the cell layer and medium, respectively, was 485.05 ± 27.47 versus 1.45 ± 0.16 ng/well (day 2), 167.38 ± 9.48 versus 13.48 ± 0.87 ng/well (day 4), and 82.97 ± 15.78 versus 3.07 ± 0.34 ng/well (day 6), respectively. Because more than 90% of IGF-I and FGF-2 were released into the medium and retained by the chondrocytes, respectively, IGF-I was measured in the medium and FGF-2 was measured in the cell layer.
Glyocosaminoglycan and DNA Analysis
Glycosaminoglycan (GAG) produced by chondrocytes and released into medium (free GAG) or retained with the cells (cell-associated GAG) were measured by dimethylmethlyene blue (DMB) assay of conditioned medium or proteinase k cell digest, respectively. Chondroitin sulfate A (Sigma) was used as the standard. Cell proliferation was assessed by DNA analysis of the cell layer digest by Picogreen dsDNA assay (Invitrogen). Pure phage λ DNA was used as the standard.
Statistical Analysis
Data are presented as mean ± SD of at least three independent experiments. A Student’s t-test was used to assess the statistical difference between groups. p-Values less than 0.05 were considered statistically significant differences.
RESULTS
Mock Transfected Chondrocytes Produce Low Levels of Growth Factors
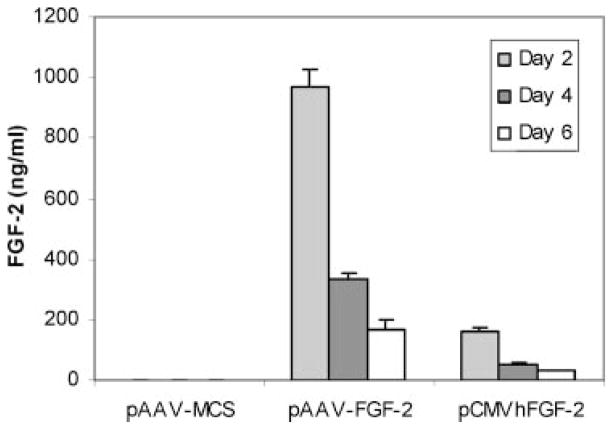
Articular chondrocytes transfected by an empty vector produced l.4 ± 0.4 ng/ml IGF-I during the first 2 days of culture and no detectable IGF-I at subsequent time points (Fig. 2). FGF-2 production into the cell layer was 3–4 ng/ml over the 6-day duration of the studies (Fig. 3). These data indicate that the growth factors produced by transfected chondrocytes reflect almost entirely the expression of the respective transgenes.
Figure 2.
IGF-I production following transfection by pAAV-IGF-I, pCMVhIGF-I, or pAAV-MCS (empty vector). Articular chondrocytes were transfected using the designated vectors, and IGF-I measurement was performed after each 48-h period ending 2, 4, and 6 days following transfection.
Figure 3.
FGF-2 production following transfection by pAAV-FGF-2, pCMVhFGF-2, or pAAV-MCS (empty vector). Articular chondrocytes were transfected using the designated vectors and FGF-2 measurement was performed after each 48-h period ending 2, 4, and 6 days following transfection.
pAAV Vectors Generate Higher Growth Factor Levels than Do pcDNA Vectors
Chondrocytes transfected by pAAV-IGF-I produced 26.3 (p <0.001), 107.9 (p <0.001), and 870.7 (p <0.001)-fold greater amounts of IGF-I than chondrocytes transfected by pCMVhIGF-I at days 2, 4, and 6, respectively (Fig. 2). Similarly, chondrocytes transfected with pAAV-FGF-2 produced 6.1 (p <0.001), 6.3 (p <0.001), and 5.7 (p <0.001)-fold more FGF-2 than chondrocytes transfected with pCMVhFGF-2 over these respective time periods (Fig. 3).
IGF-I and FGF-2 Are Differentially Expressed from a pAAV-Based Dicistronic Vector
We transfected articular chondrocytes using the dicistronic pAAV expression vector pAAV-IGF-I-IRES-FGF-2 or pAAV-FGF-2-IRES-IGF-I and measured IGF-I and FGF-2 production.
IGF-I
Cells transfected by pAAV-IGF-I-IRES-FGF-2 produced significantly more IGF-I than cells transfected by pAAV-FGF-2-IRES-IGF-I (p <0.001) at all time points tested (Fig. 4, sets 6 and 7). Compared to the single copy of IGF-I cDNA in pAAV-IGF-I-IRES, the addition of a second copy of IGF-I cDNA in pAAV-IGF-I-IRES-IGF-I did not affect IGF-I production at day 2 (p = 0.377), but did increase IGF-I production 2.4-fold at day 4 (p = 0.030) and 3.8-fold at day 6 (p = 0.037) (Fig. 4, sets 4 and 5). The two copies of IGF-I cDNA in pAAV-IGF-I-IRES-IGF-I failed to increase IGF-I production compared to the single copy of IGF-I cDNA in pAAV-IGF-I-IRES-FGF-2 at all time points tested (Fig. 4, sets 5 and 6). Taken together, these findings suggest that, in general, the first IGF-I cDNA is preferentially expressed in the dicistronic cassette used in these plasmids.
Figure 4.
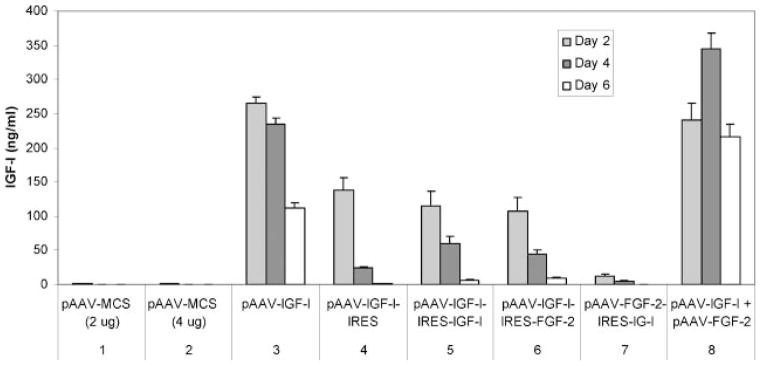
IGF-I production by chondrocytes transfected or cotransfected using pAAV-based vectors. Articular chondrocytes were transfected by the designated plasmids (sets 3–7), or cotransfected simultaneously by the designated plasmid pair (set 8). Transfection by pAAV-MCS (empty vector) at matching concentrations was used as a control for transfection (set 1) and cotransfection (set 2) groups.
FGF-2
Transfection of chondrocytes by pAAV-IGF-I-IRES-FGF-2 or by pAAV-FGF-2-IRES-IGF-I produced, in contrast to IGF-I, similar amounts of FGF-2 at days 2, 4, and 6 of culture (p = 0.212, p = 0.116, and p = 0.653, respectively) (Fig. 5, sets 6 and 7). The two copies of FGF-2 cDNA in pAAV-FGF-2-IRES-FGF-2 generated 1.5-fold (p = 0.003) more FGF-2 than the single copy in pAAV-FGF-2-IRES at day 2, but did not increase FGF-2 at day 4 (p = 0.203) or day 6 (p = 0.958) (Fig. 5, sets 4 and 5). However, replacing the IGF-I cDNA in pAAV-FGF-2-IRES-IGF-I with a second copy of FGF-2 cDNA to create pAAV-FGF-2-IRES-FGF-2, increased FGF-2 production 2.2-fold (p <0.001), 1.9-fold (p <0.001), and 1.4-fold (p = 0.022) at days 2, 4, and 6, respectively (Fig. 5, sets 5 and 6). These data suggest that, under most conditions tested, the position of the FGF-2 cDNA did not affect cDNA expression.
Figure 5.
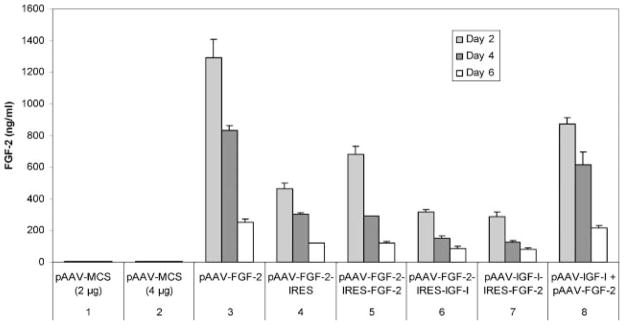
FGF-2 production by chondrocytes transfected or cotransfected using pAAV-based vectors. Articular chondrocytes were transfected by the designated plasmids (sets 3–7), or cotransfected simultaneously by the designated plasmid pair (set 8). Transfection by pAAV-MCS (empty vector) at matching concentrations was used as a control for transfection (set 1) and cotransfection (set 2) groups.
Cotransfection by Two Plasmids, Each Carrying a Single cDNA, Generates Higher Expression than Transfection by a Single Plasmid Carrying Two cDNAs
We cotransfected articular chondrocytes with both pAAV-IGF-I and pAAV-FGF-2 and compared growth factor production to that by cells transfected with the same cDNAs in a dicistronic cassette.
IGF-I
IGF-I production by articular chondrocytes was 2.3-fold (p = 0.002), 7.7-fold (p <0.001), and 25.5 (p <0.001)-fold greater at days 2, 4, and 6, respectively, when cotransfected by pAAV-IGF-I + pAAV-FGF-2 than when transfected by pAAV-IGF-I-IRES-FGF-2 (Fig. 4, sets 6 and 8). Cotransfected cells produced 20.2-fold (p <0.001), 67.9-fold (p <0.001), and 506.5-fold (p <0.001) more IGF-I than cells transfected by pAAV-FGF-2-IRES-IGF-I at the same time points (Fig. 4, sets 7 and 8).
FGF-2
Cotransfection by pAAV-IGF-I + pAAV-FGF-2 generated 2.8-fold (p = 0.001), 4.1 (p <0.001), and 2.6-fold (p <0.001) more FGF-2 than transfection by pAAV-FGF-2-IRES-IGF-I at days 2, 4, and 6, respectively (Fig. 5, sets 6 and 8). Cotransfected cells produced 3.0-fold (p <0.001), 4.9 (p <0.001), and 2.7-fold (p <0.001) more FGF-2 than pAAV-IGF-I-IRES-FGF-2 at the same time points (Fig. 5, sets 7 and 8).
Taken together, these data indicate that simultaneous cotransfection by two vectors, each carrying a single cDNA, generates substantially more transgene protein product than transfection by a single vector carrying the two cDNAs in a dicistronic cassette.
Cotransfection by Two Plasmids Differentially Affects IGF-I and FGF-2 Transgene Expression
IGF-I
Chondrocytes cotransfected with the two plasmids, pAAV-IGF-I + pAAV-FGF-2, produced similar amounts of IGF-I as cells transfected with pAAV-IGF-I alone at day 2 (240.9 ± 24.0 ng/ml vs. 265.2 ± 8.4 ng/ml, p = 0.173). In contrast, chondrocytes cotransfected with the two plasmids produced 1.5-fold (p = 0.002) and 1.9-fold (p <0.001) more IGF-I than cells transfected with pAAV-IGF-I alone at days 4 and 6, respectively (Fig. 4, sets 3 and 8). These data suggest that IGF-I transgene expression achieved by cotransfection with two plasmids is similar or greater in magnitude, and is at least as durable, as that obtained with the pAAV-IGF-I plasmid alone.
FGF-2
Chondrocytes cotransfected with pAAV-IGF-I + pAAV-FGF-2 produced 67.4% (p = 0.004), 74.3% (p = 0.011), and 85.9% (p = 0.065) as much FGF-2 as cells transfected with pAAV-FGF-2 alone at days 2, 4, and 6, respectively. These data suggest that, unlike IGF-I, FGF-2 transgene expression by cotransfected chondrocytes is less than that obtained with the pAAV-FGF-2 plasmid alone (Fig. 5, sets 3 and 8).
DNA and Glycosaminoglycan Production
Both pcDNA-based and pAAV-based vectors carrying the IGF-I or FGF-2 gene increased DNA content (Supplementary Fig. 1) and both released and cell-associated GAG (Supplementary Fig. 2). Stimulation of DNA and cell associated GAG was generally significantly greater by pAAV-based vectors than by pcDNA-based vectors. Stimulation of released GAG by the two vector types in time course studies was initially similar, but pAAV-based vectors became more stimulatory than pcDNA-based vectors over time (Supplementary Fig. 2).
DISCUSSION
Our data indicate that, for the growth factor transgenes tested, the pAAV-based vector design generated higher growth factor production than the pcDNA vectors. The reason for the difference in transgene expression between the two plasmid types is uncertain. The insertion of an intron sequence into a plasmid within the transcription unit has been reported to increase protein expression from cDNAs.20–22 However, many cDNAs have been efficiently expressed from vectors in the absence of any intron sequences. Whether inclusion of a heterologous intron sequence within the transcription unit increases protein expression from a cDNA may depend on the specific cDNA sequences and/or on the cell types which are used to express the cDNAs. It is also possible that the L-ITR and R-ITR, present in the pAAV-based vectors, may contribute to their function. Due to its position, the Zeocin expression cassette in pcDNA3.1/Zeo(+) is unlikely to affect expression of the growth factor cDNAs.
Prior studies using viral23–28 and nonviral10,13,24,29,30 methods to transfer the IGF-I gene into articular chondrocytes have reported IGF-I production up to 120 ng/ml over 2 days. Although differences in experimental design preclude a direct comparison between these studies, the IGF-I production of >250 ng/ml over 2 days obtained with pAAV-based vectors has, to our knowledge, not been previously achieved. Fewer studies have reported FGF-2 gene transfer into articular chondrocytes7,12,14,31,32 and, to our knowledge, none has investigated cell-associated FGF-2. Though the studies are not directly comparable, the FGF-2 levels reported here compare favorably with those of prior studies.
We found that placing the IGF-I cDNA before the IRES (pAAV-IGF-I-IRES-FGF-2) produced significantly more IGF-I than placing the same cDNA after the IRES (pAAV-FGF-2-IRES-IGF-I). This is consistent with prior studies of the effect of IRES on gene expression.33 In contrast, the FGF-2 cDNA in pAAV-IGF-I-IRES-FGF-2 or pAAV-FGF-2-IRES-IGF-I yielded similar amounts of FGF-2. These results suggest that the IGF-I gene is highly position-dependent, while expression of the FGF-2 gene is relatively insensitive to its position within the cassette. The relatively high FGF-2 expression from the second position in the cassette may be specific to the cDNAs or cell type employed in these studies. This observation also suggests that, to achieve simultaneous overexpression of both IGF-I and FGF-2 using a dicistronic expression cassette, pAAV-IGF-I-IRES-FGF-2 is superior to pAAV-FGF-2-IRES-IGF-I because the former increases both IGF-I and FGF-2 while the latter generates primarily FGF-2.
Cotransfection by two plasmids, each carrying one gene, generated significantly more growth factor than transfection by a single plasmid containing the two genes. Since all the transfection conditions employed the same cDNAs and the same amount of cDNA, the observed differences suggest that cotransfection with two pAAV vectors enables more efficient expression than does transfection with the same cDNAs in the dicistronic cassette. The difference between cotransfection and single transfection was not as great for FGF-2 as for IGF-I. Furthermore, while cotransfection generated significantly more FGF-2 than delivery in a dicistronic cassette, the difference did not continue to increase with time as it did for IGF-I (Fig. 4, sets 6, 7, and 8; Fig. 5, sets 6, 7, and 8). When chondrocytes were cotransfected by the two vectors, pAAV-IGF-I + pAAV-FGF-2, IGF-I transgene expression was generally greater and FGF-2 transgene expression was generally lower than when the cells were transfected by each vector alone (Fig. 4, sets 3 and 8; Fig. 5, sets 3 and 8). The differential effect of cotransfection on IGF-I and FGF-2 production may reflect a cellular response to cotransfection, or regulation of one transgene by overexpression of the other. Further studies will be required to distinguish between these potential mechanisms.
The expression of both IGF-I and FGF-2 generally decreased with time, consistent with the presumed episomal location of the plasmids. However, production of both growth factors remained at biologically relevant levels for the duration of the experiments.
The observation that pAAV-based vectors were generally more effective than pcDNA-based vectors in stimulating cell proliferation and GAG production suggests that the growth factors being produced by the cells are biologically active. Further studies will be required to define the range of actions of these factors.
Taken together, the data demonstrate that gene transfer using pAAV-based vectors enables adult articular chondrocytes to simultaneously produce relevant amounts of two chondrotrophic growth factors, and that pAAV-based cotransfection using two different growth factor genes is a potential approach to the application of gene transfer to cell-based articular cartilage repair.
Supplementary Material
Acknowledgments
The work was supported by NIH grant AR47702 (S. B. T.) and the Department of Veterans Affairs.
Footnotes
The authors have no competing interests regarding the content of this manuscript.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 2.Fortier LA, Mohammed HO, Lust G, et al. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg [Br] 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 3.Shuler FD, Georgescu HI, Niyibizi C, et al. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res. 2000;18:585–592. doi: 10.1002/jor.1100180411. [DOI] [PubMed] [Google Scholar]
- 4.Trippel SB. Growth factors as therapeutic agents. Instr Course Lect. 1997;46:473–476. [PubMed] [Google Scholar]
- 5.Trippel SB, Ghivizzani SC, Nixon AJ. Gene-based approaches for the repair of articular cartilage. Gene Ther. 2004;11:351–359. doi: 10.1038/sj.gt.3302201. [DOI] [PubMed] [Google Scholar]
- 6.Baragi VM, Renkiewicz RR, Jordan H, et al. Transplantation of transduced chondrocytes protects articular cartilage from interleukin 1-induced extracellular matrix degradation. J Clin Invest. 1995;96:2454–2460. doi: 10.1172/JCI118303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucchiarini M, Madry H, Ma C, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Doherty PJ, Zhang H, Tremblay L, et al. Resurfacing of articular cartilage explants with genetically-modified human chondrocytes in vitro. Osteoarthritis Cartilage. 1998;6:153–159. doi: 10.1053/joca.1998.0107. [DOI] [PubMed] [Google Scholar]
- 9.Evans CH, Ghivizzani SC, Smith P, et al. Using gene therapy to protect and restore cartilage. Clin Orthop Relat Res. 2000;379(Suppl):214–219. doi: 10.1097/00003086-200010001-00027. [DOI] [PubMed] [Google Scholar]
- 10.Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz EM. The adeno-associated virus vector for orthopaedic gene therapy. Clin Orthop Relat Res. 2000;379 (Suppl):31–39. doi: 10.1097/00003086-200010001-00005. [DOI] [PubMed] [Google Scholar]
- 12.Yokoo N, Saito T, Uesugi M, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 13.Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–1449. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- 14.Madry H, Emkey G, Zurakowski D, et al. Over-expression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J Gene Med. 2004;6:238–245. doi: 10.1002/jgm.488. [DOI] [PubMed] [Google Scholar]
- 15.Davies MV, Kaufman RJ. Internal translation initiation in the design of improved expression vectors. Curr Opin Biotechnol. 1992;3:512–517. doi: 10.1016/0958-1669(92)90079-x. [DOI] [PubMed] [Google Scholar]
- 16.Dirks W, Wirth M, Hauser H. Dicistronic transcription units for gene expression in mammalian cells. Gene. 1993;128:247–249. doi: 10.1016/0378-1119(93)90569-o. [DOI] [PubMed] [Google Scholar]
- 17.Jang SK, Krausslich HG, Nicklin MJ, et al. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1998;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanning PJ, Emkey G, Smith RJ, et al. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J Biol Chem. 2003;278:50940–50948. doi: 10.1074/jbc.M305107200. [DOI] [PubMed] [Google Scholar]
- 19.Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7:286–291. doi: 10.1038/sj.gt.3301086. [DOI] [PubMed] [Google Scholar]
- 20.Hamer DH, Leder P. Splicing and the formation of stable RNA. Cell. 1979;18:1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- 21.Hermening S, Kugler S, Bahr M, et al. Increased protein expression from adenoviral shuttle plasmids and vectors by insertion of a small chimeric intron sequence. J Virol Methods. 2004;122:73–77. doi: 10.1016/j.jviromet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Xia W, Bringmann P, McClary J, et al. High levels of protein expression using different mammalian CMV promoters in several cell lines. Protein Expr Purif. 2006;45:115–124. doi: 10.1016/j.pep.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Brower-Toland BD, Saxer RA, Goodrich LR, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 24.Capito RM, Spector M. Effect of expansion medium on ex vivo gene transfer and chondrogenesis in type II collagen-glycosaminoglycan scaffolds in vitro. Osteoarthritis Cartilage. 2006;14:1203–1213. doi: 10.1016/j.joca.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Gelse K, Jiang QJ, Aigner T, et al. Fibroblast-mediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis Rheum. 2001;44:1943–1953. doi: 10.1002/1529-0131(200108)44:8<1943::AID-ART332>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Nixon AJ, Brower-Toland BD, Bent SJ, et al. Insulinlike growth factor-I gene therapy applications for cartilage repair. Clin Orthop Relat Res. 2000;379(Suppl):201–213. doi: 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- 27.Smith P, Shuler FD, Georgescu HI, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156–1164. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Sung LY, Lo WH, Chiu HY, et al. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials. 2007;28:3437–3447. doi: 10.1016/j.biomaterials.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Capito RM, Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14:721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Capito RM, Spector M. Delivery of plasmid IGF-1 to chondrocytes via cationized gelatin nanoparticles. J Biomed Mater Res A. 2007;84:73–83. doi: 10.1002/jbm.a.31372. [DOI] [PubMed] [Google Scholar]
- 31.Kaul G, Cucchiarini M, Arntzen D, et al. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibro-blast growth factor 2 (FGF-2) in vivo. J Gene Med. 2006;8:100–111. doi: 10.1002/jgm.819. [DOI] [PubMed] [Google Scholar]
- 32.Schmal H, Mehlhorn AT, Zwingmann J, et al. Stimulation of chondrocytes in vitro by gene transfer with plasmids coding for epidermal growth factor (hEGF) and basic fibroblast growth factor (bFGF) Cytotherapy. 2005;7:292–300. doi: 10.1080/14653240510027253. [DOI] [PubMed] [Google Scholar]
- 33.Hennecke M, Kwissa M, Metzger K, et al. Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mRNAs. Nucleic Acids Res. 2001;29:3327–3334. doi: 10.1093/nar/29.16.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.