Abstract
Gene transfer is a promising approach to the delivery of chondrotrophic growth factors to promote cartilage repair. It is unlikely that a single growth factor transgene will optimally regulate these cells. The aim of this study was to identify those growth factor transgene combinations that optimally regulate aggrecan, collagen type II and collagen type I gene expression by articular chondrocytes. We delivered combinations of the transgenes encoding fibroblast growth factor-2, insulin-like growth factor I, transforming growth factor beta1, bone morphogenetic protein-2, and/or bone morphogenetic protein-7 and assessed chondrocyte responses by measuring changes in the expression of aggrecan, type II collagen and type I collagen genes. These growth factor transgenes differentially regulated the magnitude and time course of all three-matrix protein genes. In concert, the transgenes regulated matrix gene expression in an interactive fashion that ranged from synergistic to inhibitory. Maximum stimulation of aggrecan (16-fold) and type II collagen (35-fold) expression was with the combination of IGF-I, BMP-2, and BMP-7 transgenes. The results indicate that the optimal choice of growth factor genes for cell-based cartilage repair cannot be predicted from observations of individual transgenes. Rather, such gene therapy will require an empirically based selection of growth factor gene combinations.
Keywords: growth factors, aggrecan, collagen, articular chondrocytes, gene therapy
Damaged articular cartilage has a poor intrinsic repair capacity and is responsible for considerable disability in the form of arthritis and joint trauma. This remains an unsolved problem in orthopedics. A number of cell-regulatory molecules have been identified that promote anabolic activity by articular chondrocytes and may have therapeutic potential.1–4
These include fibroblast growth factor-2 (FGF-2)5,6 insulin-like growth factor I (IGF-I),6–10 transforming growth factor beta1 (TGF-β1),11,12 bone morphogenetic protein-2 (BMP-2),13,14 and bone morphogenetic protein-7 (BMP-7).15,16 Direct delivery of such factors into joints is hampered by the rapid clearance of the factors from the joint17 and their relatively slow diffusion through cartilage matrix.18 Efforts to overcome the difficulties of delivering these molecules to damaged articular cartilage have led to the application of gene transfer technology to the problem of articular cartilage repair.19–27 However, no one growth factor is likely to activate the chondrocyte functions needed for repair, and multiple growth factors are likely to interact in regulating these functions. To address these needs, we systematically investigated the effect of multiple growth factor gene transfer on the expression of the genes encoding aggrecan (ACAN), collagen type IIα1 (COL2A1), and collagen type Iα2 (COL2A2) by adult bovine articular chondrocytes. These growth factors were selected on the basis of their established chondrotrophic activity and their representation of both shared and distinct signaling mechanisms.
EXPERIMENTAL PROCEDURES
Vector Construction
Vectors pAAV-IGF-I, pAAV-FGF-2, pAAV-BMP-2, pAAV-BMP-7, and pAAV-TGF-β1 were constructed as previously described.26 Briefly, IGF-I and FGF-2 cDNAs with EcoR I site at 5′ ends and Bgl II site at 3′ ends were generated by polymerase chain reaction (PCR) using pCMVhIGF-I21 and pCMVhFGF-223 as PCR templates, respectively. The PCR products were cloned into pAAV-MCS to obtain pAAV-IGF-I and pAAV-FGF-2, respectively. Human BMP-2, BMP-7, and TGF-β1 cDNAs were obtained from human U-2 osteosarcoma cell total RNA. After reverse transcription, PCR was performed to clone human BMP-2, BMP-7, and TGF-β1 cDNAs. The PCR products were cloned into pCR-II-TOPO (Invitrogen) and sequenced. After sequence confirmation, the cDNA in pCR-II-TOPO was subcloned into pAAV-MCS (Stratagene, LaJolla, CA) to obtain pAAV-BMP-2, pAAV-BMP-7, and pAAV-TGF-β1, respectively. To improve the readability of the data presented, the transgenes carried by pAAV-IGF-I, pAAV-FGF-2, pAAV-BMP-2, pAAV-BMP-7, and pAAV-TGF-β1 are here designated tIGF-I, tFGF-2, tBMP-2, tBMP-7, and tTGF-β1, respectively.
Chondrocyte Cell Culture and Transfection
Basal medium was prepared with DMEM, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine (Invitrogen, Carlsbad, CA), and 50 μg/ml ascorbic acid (Sigma, St. Louis, MO). Complete medium was prepared by supplementing basal medium with 10% FBS (Invitrogen). Bovine articular chondrocytes were isolated as previously described.26 Briefly, chondrocytes were isolated from the carpal joints of skeletally mature (growth plates closed) bovids, cultured at 3 × 105 cells/well in complete medium for 3 days, and transfected in fresh complete medium using FuGENE 6 (Roche Applied Science, Indianapolis, IN) and plasmid DNA. For single transfections, 2 μg of each plasmid DNA per well was used. For multiple transfections, 2 μg of each plasmid DNA per well was used together. After 4 h, transfection was stopped by replacing the medium with fresh complete medium. At daily intervals, triplicate samples of chondrocytes were lysed with lysis buffer RLT (RNeasy Mini kit, Qiagen, Valencia, CA), homogenized by passing six times through a 20-gauge needle and stored at −80°C for total RNA purification.
RNA Purification, Reverse Transcription, and Real-Time PCR Analysis
RNA purification and reverse transcription were performed as previously described.26 Briefly, total RNA was prepared using the RNeasy Mini kit (Qiagen). On-column DNase digestion was performed to remove any residual DNA. Reverse transcription was performed using the High-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and random primer. Reverse transcription was terminated by heating at 95°C for 20 min. cDNA samples were diluted 1:20 for real-time PCR analysis. Real-time PCR was performed as previously described.26 Briefly, aggrecan mRNA, type II collagen mRNA, type I collagen mRNA, and 18S rRNA were measured using a Prism 7000 Sequence Detector System and TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA). Primers were synthesized by Invitrogen and probes were 5′-end FAM and 3′-end MGB non-fluorescent quencher (MGBNFQ) labeled and synthesized by Applied Biosys-tems (Supplementary Table 1). The standard curve method was used to calculate the expression of target genes encoding aggrecan (ACAN), type II collagen (COL2A1), and type I collagen (COL1A2), and the content of 18S rRNA. Target gene mRNA levels were normalized to 18S rRNA levels. Fold changes of target gene expression are expressed as the ratio of growth factor gene transfected cells to cells transfected with empty vector (mock-transfected control). Three independent experiments were performed using different batches of articular chondrocytes obtained from different bovine joints at different times. Data are presented as the average of fold changes.
Statistical Analysis
Cell isolates from three different animals (n = 3) were used for all experiments. Analyses of aggrecan, type II collagen and type I collagen mRNA were performed on the ratios of transfected cells to mock-transfected control cells for the designated trangenes (dependent variables). The effects of tFGF-2, tIGF-I, tTGF-β1, tBMP-2, and tBMP-7 gene transfer and time on chondrocyte target gene expression after transfection were evaluated using analysis of variance (ANOVA). The ANOVA used terms for transgene, time, and the transgene-by-time interaction, as well as a random effect to correlate data from the same experimental run. A second analysis, also using ANOVA, directly tested whether simultaneous transfer generated synergistic or inhibitory effects compared to the transfer of individual genes. Synergistic effects were those for which the value of the combined growth factors was significantly greater than the sum of the effects of the individual growth factors. Inhibitory effects were those for which the value of the combined growth factors was significantly less than the sum of the effects of the individual growth factors. No adjustments were made for multiple comparisons. A 5% significance level was used for all comparisons.
RESULTS
Individual Trangenes
Aggrecan Gene Expression
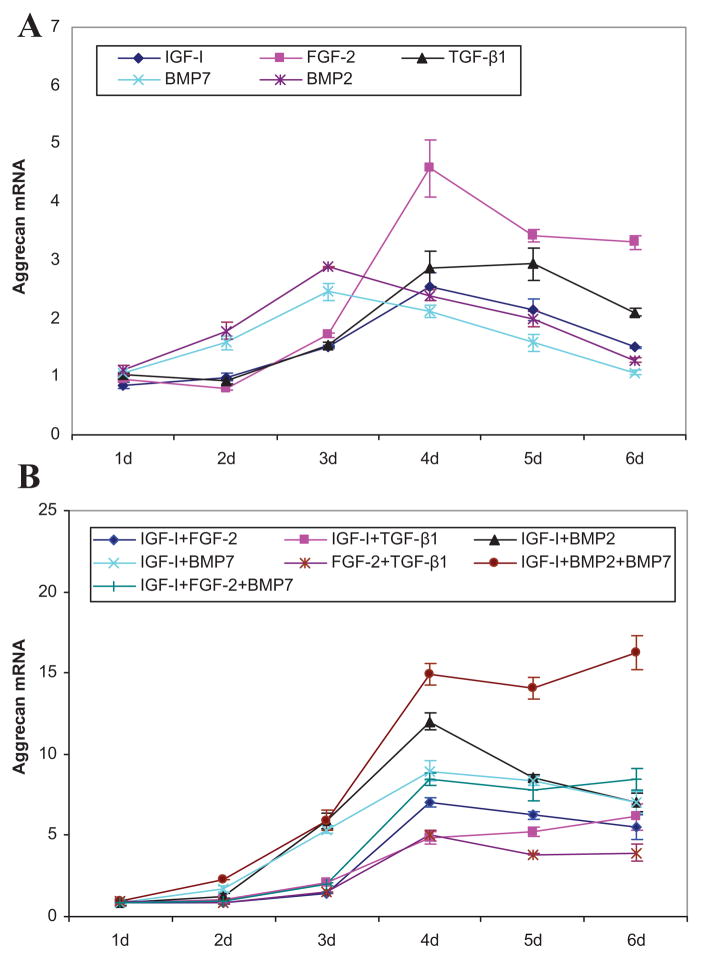
All growth factor transgenes increased ACAN expression compared to mock-transfected controls. All transgenes generated a biphasic pattern of ACAN expression, but with differing time courses. Specifically, maximal stimulation by tBMP-2 and tBMP-7 occurred at 3 days, while that for tIGF-I, tFGF-2, or tTGF-β1 was delayed to 4–5 days. The magnitude of the maximal effect ranged from 2.5-fold (p = 0.0014) by tBMP-7 to 4.6-fold by tFGF-2 (p = 0.0017) (Fig. 1).
Figure 1.
Aggrecan gene expression. Effects of the designated individual (A) and combinations of (B) growth factor transgenes at the designated times following transfection of articular chondrocytes. Data represent the ratio of the means of the treated and control groups ± SD from three independent experiments. Pair comparisons are provided in Supplementary Figures 1 and 2.
Type II Collagen Gene Expression
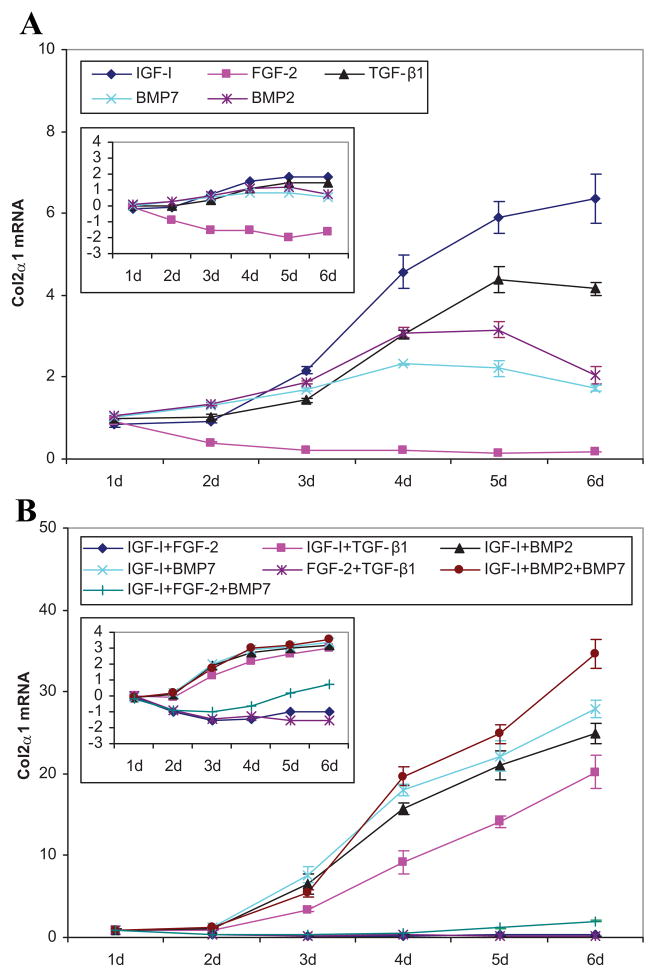
The IGF-I transgene stimulated COL2A1 expression starting at 3 days progressed to 6.3-fold (p = 0.0009) at 6 days. The effect of tBMP-2 and tBMP-7 was limited to 3.2-fold (p = 0.0009) and 2.3-fold (p < 0.0001), respectively, and their time course was biphasic, decreasing by 6 days. Stimulation by tTGF-β1 appeared at 3 days and increased to a plateau of ~4-fold (p ≤ 0.0008) after 4 days. In contrast to the other four growth factor transgenes, tFGF-2 rapidly and progressively decreased COL2A1 expression from 60% (p = 0.022) of control levels at 2 days to 79–86% at 4–6 days (all p ≤ 0.0005) (Fig. 2).
Figure 2.
Type II collagen gene expression. Effects of the designated individual (A) and combinations of (B) growth factor transgenes at the designated times following transfection of articular chondrocytes. Data represent the ratio of the means of the treated and control groups ± SD from three independent experiments. The insert illustrates the same data expressed as the natural log of the ratio to depict ratios that are <1. Pair comparisons are provided in Supplementary Figures 3 and 4.
Type I Collagen Gene Expression
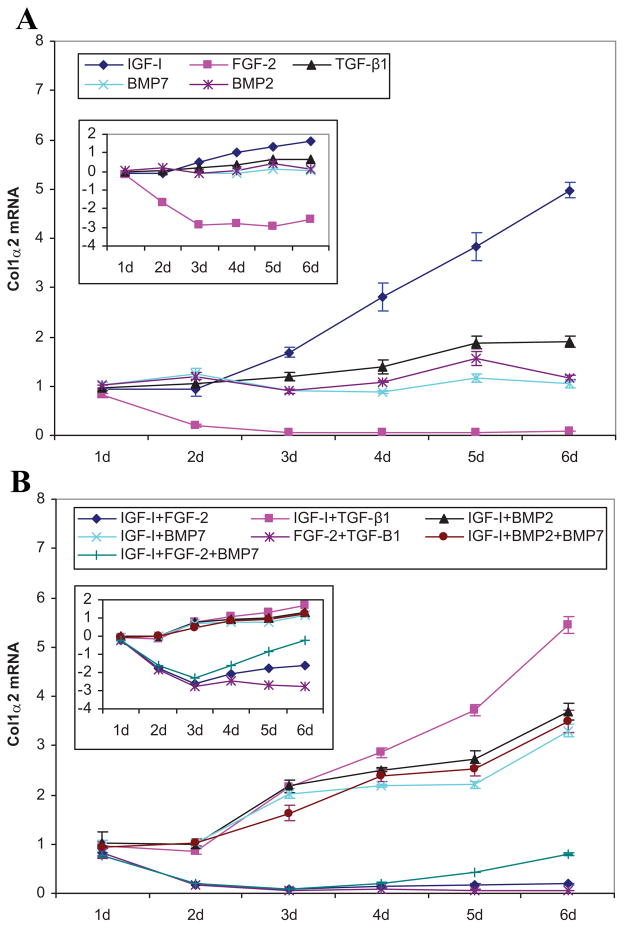
The patterns of the time course of COL1A2 expression in response to tIGF-I and tFGF-2 were nearly identical to those of COL2A1. Starting at 3 days, tIGF-I progressively increased COL1A2 expression to a maximum of 5.0-fold (p = 0.0001) at 6 days. The FGF-2 transgene rapidly and progressively decreased COL1A2 expression from 19% (p = 0.0250) at 1 days, to 81% (p = 0.0038) at day 2, to 92% (p = 0.0003) at 6 days (Fig. 3).
Figure 3.
Type I collagen gene expression. Effects of the designated individual (A) and combinations of (B) growth factor transgenes at the designated times following transfection of articular chondrocytes. Data represent the ratio of the means of the treated and control groups ± SD from three independent experiments. The insert illustrates the same data expressed as the natural log of the ratio to depict ratios that are <1. Pair comparisons are provided in Supplementary Figures 5 and 7.
Two Transgenes
Aggrecan Gene Expression
The addition of tIGF-I to any other growth factor transgene synergistically increased ACAN expression. Maximal synergy occurred with [tIGFI + tBMP-7] at 10.6-fold (p < 0.0001). The time course of the synergistic interaction varied, appearing at 3 days for [tIGF-I + tBMP-2] and [tIGF-I + tBMP-7], 4 days for [tIGF-I + tFGF-2], and 5 days for [tIGF-I + tTGF-β1]. Interestingly, [tIGF-I + tFGF-2] and [tIGF-I + tBMP-2] were inhibitory, with C/S ratios of 0.3 (p = 0.0010) and 0.4 (p < 0.0001), respectively, on the day before they became synergistic. The addition of tTGF-β1 as a second transgene prevented the late decline in ACAN expression in response to tFGF-2 and reversed the late decline in response to tIGF-I. Unlike transgene pairs containing tIGF-I, the combination [tFGF-2 + tTGF-β1] was generally inhibitory, yielding C/S ratios of 0.4–0.7 (all p ≤ 0.0011) from 3 to 5 days. Detailed comparative data are provided in Supplementary Tables 2–5 and detailed time course data are provided in Supplementary Figure 8 and Table 6.
Type II Collagen Gene Expression
The addition of tIGF-I to tTGF-β1, tBMP-2, or tBMP-7 synergistically increased COL2A1 expression to maxima of 2.3-, 3.7-, and 4.4-fold, respectively (all p < 0.0001) compared to the sum of their individual effects. This represents increases compared to controls of 20.1-, 24.9-, and 27.9-fold, respectively. The time course of the synergistic interaction was similar for COL2A1 and ACAN expression. In contrast, [tFGF-2 + tIGF-I] reduced COL2A1 expression to levels less than mock-transfected controls. This inhibition appeared at 2 days and persisted for the remainder of the 6-day duration of the study. A partial reversal by tIGF-I of the inhibitory effect of tFGF-2 occurred at 4–6 days, shifting the time course curve of [tIGF-I + tFGF-2] from that of tFGF-2 to that of tIGF-I. The interaction between tFGF-2 and tTGF-β1 became inhibitory at 3 days. Detailed comparative data are provided in Supplementary Tables 2–5 and detailed time course data are provided in Supplementary Figure 9 and Table 6.
Type I Collagen Gene Expression
The addition of tIGF-I to tTGF-β1, tBMP-2, or tBMP-7 generated an early synergistic increase in COL1A2 expression of 1.3-, 2.0-, and 1.8-fold, respectively (all p ≤ 0.005). This lasted only 1 day and was, in all cases, followed by inhibition to C/S ratios of 0.4–0.9 (all p ≤ 0.0004) which persisted for the remaining duration of the experiments. The addition of tFGF-2 to tTGF-β1 synergistically inhibited COL1A2 expression in a progressive, time-dependent fashion from 1.2-fold (p = 0.367) at 2 days to 48.1-fold (p < 0.0001) at 6 days. The addition of tFGF-2 to tIGF-I synergistically inhibited COL1A2 expression 3.6-fold (p < 0.0001) at 3 days, but after 3 days tIGF-I partially reversed the inhibitory effect of tFGF-2, shifting the time course curve of [tIGF-I + tFGF-2] from that of tFGF-2 to that of tIGF-I. Despite the partial recovery effected by tIGF-I, COL1A2 expression by [tIGF-I + tFGF-2] remained below mock-transfected control levels. Detailed comparative data are provided in Supplementary Tables 2–5 and detailed time course data are provided in Supplementary Figure 10 and Table 6.
Three Transgenes
Aggrecan Gene Expression
The addition of tBMP-7 to [tIGF-I + tBMP-2], or of tBMP-2 to [tIGF-I + tBMP-7] to create the three-transgene combination [tIGF-I + tBMP-2 + tBMP-7] generated similar effects on ACAN expression. Both were inhibitory (C/S ratio 0.8, p < 0.0001) at 3 days and became progressively synergistic to 2.5- and 2.4-fold (both p < 0.0001), respectively, at 6 days. This represents an increase in ACAN expression by [tIGF-I + tBMP-2 + tBMP-7] of 16.3-fold above mock-transfected controls. In contrast, tFGF-2 eliminated the stimulatory effect of [tIGF-I + tBMP-7] at 2 and 3 days, bringing ACAN expression down to the level of tFGF-2 alone until the interaction disappeared at 6 days. The effect of adding tFGF-2 to [tIGF-I + tBMP-7] differed from that of adding tBMP-7 to [tIGF-I + tFGF-2]. Specifically, the interaction of tBMP-7 with [tIGF-I + tFGF-2] had little effect until 5 and 6 days when it became synergistic to 1.2-fold, (p = 0.0032) and 1.6-fold (p < 0.0001), respectively.
Type II Collagen Gene Expression
The addition of tBMP-7 to [tIGF-I + tFGF-2] gradually, but completely, overcame the inhibitory effect of [tIGF-I + tFGF-2] on COL2A1 expression after 2 days (Supplementary Fig. 6B). The addition of tFGF-2 to [tIGF-I + tBMP-7] nearly abolished the strong, early stimulation by [tIGF-I + tBMP-7] of COL2A1 expression. This inhibition had been largely overcome by 6 days. The addition of tBMP-7 to [tIGF-I + tBMP-2], or of tBMP-2 to [tIGF-I + tBMP-7], initially inhibited COL2A1 expression but became synergistic to 1.4- and 1.2-fold (both p < 0.0001) by 6 days. This represents a 34.5-fold stimulation compared to mock-transfected control.
Type I Collagen Gene Expression
The addition of tBMP-7 to [tIGF-I + tFGF-2] slowly, and incompletely, overcame the inhibitory effect of [tIGF-I + tFGF-2] on COL1A2 expression. The addition of tFGF-2 to [tIGF-I + tBMP-7] abolished the increase in COL1A2 expression by [tIGF-I + tBMP-7], reducing COL1A2 expression to levels lower than the mock-transfected control. The addition of tBMP-7 to [tIGF-I + tBMP-2], or of tBMP-2 to [tIGF-I + tBMP-7] each inhibited COL1A2 expression to a C/S ratio of 0.1 (both p ≤ 0.0062) at 2 days, but had minimal or no effect thereafter.
Detailed comparisons of the effect and time course of each transgene combination are provided in Supplementary Data (Supplementary Figs. 1–9).
DISCUSSION
Dual gene transfer has been previously applied to articular chondrocytes. These genes include IGF-I plus BMP-2 or tTGF-β1,28 IGF-I plus IL-4,29 FGF-2 plus Sox9,30 and IGF-I plus FGF-2.26,31 IGF-I plus bFGF gene transfer has also been performed with the IL-1Ra gene.32 When tested, these studies generally demonstrated additive effects of these transgenes on chondrocyte aggrecan and collagen expression. To our knowledge, no systematic analysis of the effect of multiple growth factor gene transfer on the regulation of articular chondrocyte aggrecan or collagen gene expression has been reported.
Of the individual transgenes tested, tFGF-2 had the greatest stimulatory effect on aggrecan gene expression, while tIGF-I generated the maximal stimulation of type II collagen gene expression. These data suggest that combining these transgenes might augment the expression of both these essential matrix components. This was not the case. The FGF-2 transgene markedly depressed the expression of COL2A1 and almost completely abrogated the stimulatory effect of tIGF-I on it. Interestingly, the combination of tIGF-I and tFGF-2 did synergistically stimulate the expression of ACAN, but this only achieved a sevenfold increase compared to control. The data suggest that a more effective transgene pair would be [tIGF-I + tBMP-2] or [tIGF-I + tBMP-7]. These transgene pairs increased ACAN expression 12.0- and 9.0-fold, respectively, and increased COL2A1 expression 24.9- and 27.9-fold, respectively.
In the context of articular cartilage repair, the expression of COL1A2 is undesirable because it reflects a fibroblastic rather than chondrocytic phenotype. A limitation of tIGF-I in this respect is its stimulatory effect on both COL2A1 and COL1A2 expression. The addition of either tBMP-7 or tBMP-2 reduces this effect of tIGF-I on COL1A2 while increasing its effect on COL2A1. It is noteworthy that all interactions among these transgenes inhibited type I collagen gene expression.
The only test condition that (i) increased ACAN expression, (ii) increased COL2A1 expression, and (iii) decreased COL1A2 expression was the three-trans-gene combination [tIGF-I + tFGF-2 + tBMP-7]. However, this combination generated only 8.4- and 2.0-fold stimulation of ACAN and COL2A1 expression, respectively. Further, it had lost much of its inhibitory effect on COL1A2 expression by 6 days following transfection. The maximal effect observed in these studies on both ACAN and COL2A1 expression was achieved by the three-transgene combination [tIGF-I + tBMP-2 + tBMP-7]. These amounted to 16.2- and 34.5-fold, respectively, compared to control. The maximal stimulation of COL1A2 by this combination was 3.5-fold. Thus, this combination strongly favored the expression of cartilage-specific genes. Taken together, these observations suggest that, to simultaneously achieve maximum expression of both ACAN and COL2A1 and to minimize COL1A2, the [IGF-I + BMP-2 +BMP-7] transgene combination is superior to any individual transgene and to any other transgene combination tested in this study. The growth factors studied act by both shared (BMP-2 and BMP-7),33 similar (BMP’s and TGF-β1),34 and distinct (TGF-β family,34 IGF-I,35 and FGF-2)36 signaling pathways.
The synergistic interactions identified in this study suggest that these pathways can differentially augment or inhibit each other in regulating both aggrecan and collagen gene expression. The data further suggest, as in the case of BMP-2 and BMP-7, that key pathways regulating these matrix genes may be shared, but not saturated, by the degree of growth factor overexpression achieved by each transgene alone. Additionally, the results may reflect, in part, variations in the timing and robustness of the transgene expression. Further studies will be required to elucidate the mechanisms underlying these interactions.
The pAAV vector employed in this study was selected for its ability to simultaneously transfect articular chondrocytes26 and to generate physiologically relevant concentrations of all the growth factor transgene products tested. This vector compares favorably with other plasmid vectors21,31 and rAAV vectors37 and, it lacks the potential safety and immunological limitations of adenoviral vectors. The concentrations of growth factor protein produced from these vectors, and the effect of transgene number on the trangene products are provided in Supplementary Data (Supplementary Fig. 10 and Table 1).
The similar direction of effect of these transgenes on COL1A2 and COL2A1 expression suggests that the differential expression of these two molecules, and hence the distinction between hyaline cartilage and fibrocartilage, is determined at least in part, by cell-regulatory factors not tested here. However, the marked differences in the magnitude of effect on COL1A2 and COL2A1 expression by all the tested growth factors indicate that these factors play an important role in defining the functional phenotype of articular chondrocytes. Also important to the function of these cells is the regulation of the catabolic factors. Additional studies will be required to determine the effect of growth factor gene transfer on this side of the chondrocyte homeostatic equation.
The present data demonstrate that multiple growth factor gene transfer generates substantial alterations in the gene expression of the two principal components of articular cartilage matrix. The data further demonstrate that the regulation of aggrecan and type II collagen gene expression is governed by complex interactions and is highly dependent on the specific transgenes employed. These findings may help guide the application of growth factor genes to cell-based articular cartilage repair.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIAMS grant AR047702 and the Department of Veterans Affairs.
Footnotes
Disclosure: Stephen B. Trippel is a paid consultant to Biomeasures. The other authors have no financial interests to disclose.
This article is a US Government work and as such is in the public domain in the United States of America
Additional Supporting Information may be found in the online version of this article.
References
- 1.Chopra R, Anastassiades T. Specificity and synergism of polypeptide growth factors in stimulating the synthesis of proteoglycans and a novel high molecular weight anionic glycoprotein by articular chondrocyte cultures. J Rheumatol. 1998;25:1578–1584. [PubMed] [Google Scholar]
- 2.Guerne PA, Sublet A, Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994;158:476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- 3.Trippel SB. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995;43:129–132. [PubMed] [Google Scholar]
- 4.Trippel SB, Coutts RD, Einhorn TA, et al. Growth factor actions on articular cartilage. J Bone Joint Surg. 1996;78A:1272–1286. [Google Scholar]
- 5.Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Sah RL, Chen AC, Grodzinsky AJ, et al. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 7.Fortier LA, Mohammed HO, Lust G, et al. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 8.Luyten FP, Hascall VC, Nissley SP, et al. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 9.Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19:26–32. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 10.Eleswarapu SV, Leipzig ND, Athanasiou KA. Gene expression of single articular chondrocytes. Cell Tissue Res. 2007;327:43–54. doi: 10.1007/s00441-006-0258-5. [DOI] [PubMed] [Google Scholar]
- 11.Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- 12.Rosier RN, O’Keefe RJ, Crabb ID, et al. Transforming growth factor beta: an autocrine regulator of chondrocytes. Connect Tissue Res. 1989;20:295–301. doi: 10.3109/03008208909023900. [DOI] [PubMed] [Google Scholar]
- 13.Grunder T, Gaissmaier C, Fritz J, et al. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004;12:559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Reddi AH. Cartilage morphogenetic proteins: role in joint development, homoeostasis, and regeneration. Ann Rheum Dis. 2003;62(Suppl 2):ii73–ii78. doi: 10.1136/ard.62.suppl_2.ii73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chubinskaya S, Hakimiyan A, Pacione C, et al. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2007;15:421–430. doi: 10.1016/j.joca.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flechtenmacher J, Huch K, Thonar EJ, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–1904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell JR, Offenberg HL, Ramanathan-Girish S, et al. A safety, tolerability and pharmacokinetic study of intra-articular recombinant human insulin-like growth factor I (rhIGF-I) in patients with severe osteoarthritis (OA) of the knee. Arthritis Rheum. 2000;43:S223. [Google Scholar]
- 18.Garcia AM, Szasz N, Trippel SB, et al. Transport and binding of insulin-like growth factor I through articular cartilage. Arch Biochem Biophys. 2003;415:69–79. doi: 10.1016/s0003-9861(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 19.Brower-Toland BD, Saxer RA, Goodrich LR, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 20.Cucchiarini M, Madry H, Ma C, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–1449. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- 22.Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 23.Madry H, Emkey G, Zurakowski D, et al. Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J Gene Med. 2004;6:238–245. doi: 10.1002/jgm.488. [DOI] [PubMed] [Google Scholar]
- 24.Shuler FD, Georgescu HI, Niyibizi C, et al. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res. 2000;18:585–592. doi: 10.1002/jor.1100180411. [DOI] [PubMed] [Google Scholar]
- 25.Yokoo N, Saito T, Uesugi M, et al. Repair of articular cartilage defect by autologous transplantation of basic fibro-blast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 26.Shi S, Mercer S, Trippel SB. Effect of transfection strategy on growth factor overexpression by articular chondrocytes. J Orthop Res. 2010;28:103–109. doi: 10.1002/jor.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans CH, Ghivizzani SC, Smith P, et al. Using gene therapy to protect and restore cartilage. Clin Orthop Relat Res. 2000;379:S214–S219. doi: 10.1097/00003086-200010001-00027. [DOI] [PubMed] [Google Scholar]
- 28.Smith P, Shuler FD, Georgescu HI, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156–1164. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Manning K, Rachakonda PS, Rai MF, et al. Co-expression of insulin-like growth factor-1 and interleukin-4 in an in vitro inflammatory model. Cytokine. 2010;50:297–305. doi: 10.1016/j.cyto.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Cucchiarini M, Terwilliger EF, Kohn D, et al. Remodelling of human osteoarthritic cartilage by FGF-2, alone or combined with Sox9 via rAAV gene transfer. J Cell Mol Med. 2009;13:2476–2488. doi: 10.1111/j.1582-4934.2008.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orth PKG, Cucchiarini M, Zurakowski D, et al. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg Sports Traumatol Arthrosc. 2011;19:2119–2130. doi: 10.1007/s00167-011-1448-6. [DOI] [PubMed] [Google Scholar]
- 32.Chen B, Qin J, Wang H, et al. Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med. 2010;42:684–695. doi: 10.3858/emm.2010.42.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ten Dijke P, Korchynskyi O, Valdimarsdottir G, et al. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 35.Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Szebenyi G, Fallon JF. Fibroblast growth factors as multifunctional signaling factors. Int Rev Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- 37.Madry H, Cucchiarini M, Terwilliger EF, et al. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14:393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.