Abstract
Purpose of review
Although insulin is life-saving an sustaining for those with Type 1 diabetes (T1D) curing the disease will be much more complex than simple replacement of this hormone. T1D is be an autoimmune disease orchestrated by T cells, and includes many arms of the immune response. Tremendous effort has gone into understanding its underlying immune, genetic and environmental causes, and this progress has led to immunologically-based clinical trials in T1D. This review will focus primarily on the clinical trials of the past decade that have attempted to translate these fundamental findings.
Recent findings
It is known that powerful, non-specific immune suppressants can temporarily slow the course of newly diagnosed T1D, yet are too toxic for long-term use, especially in children. Recent clinical trials to reverse T1D have used newly developed therapies which target specific components of the immune process believed to be involved with T1D. Although well justified and designed, no recent approach has resulted in clinical remission and few have had any effect on disease course.
Summary
Advances in our fundamental understanding of how the human diabetes immune response is activated and regulated coupled with lessons that have been learnt from the most recent era of completed trials are guiding us toward development of more effective, multipronged therapies to ablate diabetes autoimmunity, restore immune tolerance, preserve beta cells, and, ultimately, improve the lives of patients with T1D.
Keywords: Type 1 diabetes, T cells, autoantibodies, regulatory T cells, autoantigens, immune therapy
Introduction
Type 1 diabetes (T1D) affects up to 3 million North Americans, is primarily a disease of childhood, and is increasing in incidence, especially in young children.(1-3) It is an autoimmune disease specific for the insulin-producing beta cells in the pancreas.(4) Generally, all, or nearly all, beta cells are destroyed and individuals are left with the inability to produce insulin, with life-threatening consequences. Insulin, discovered almost 100 years ago, is life-saving but is required daily and even with the best-managed regimens, T1D patients have increased risks for morbidity and mortality, reaffirming that insulin is not a cure for this disease.(4, 5)
Steady progress since the 1970s has led to the recognition that T1D is an autoimmune disease with an underlying genetic component and one or more unidentified environmental triggers.(6-9) The current paradigm for initiation of T1D is that genetically susceptible individuals encounter an environmental trigger that activates the beta-cell autoimmune response, which expands over months or years and results in near-total beta cell loss.(10-12) Recent studies have also suggested that individuals prone to T1D have heightened markers of beta cell stress, although it is uncertain if these reflect inherent defects with repair of cellular damage or are due to excess metabolic demands.(13, 14) It remains a possibility that depending on the underlying genetics, different individuals may be susceptible to different triggers.(15) The event or antigen that incites T1D has remained elusive, and it is currently not possible to identify individuals prior to the onset of beta cell autoimmunity.
One of the significant successes in T1D over the past decades has been the identification of autoantibodies to beta cell antigens.(16-18) These autoantibodies are required for the diagnosis of T1D but it is unclear what role they play in T1D pathogenesis.(17) Nevertheless, the presence of autoantibodies significantly predates the clinical onset of disease, suggesting they may play a role in disease progression.(10, 16, 19) The odds of developing and the time to clinical disease can be predicted in asymptomatic individuals depending on the number of positive autoantibodies, which is now part of the entry criteria in T1D preventative trials.
The area that has experienced the greatest advances and has provided the foundation for the most promising clinical trials to prevent or reverse T1D is the study of the contribution of T cells to T1D.(20-22) It is apparent that beta-cell antigen-specific T cells orchestrate other components of the immune response to beta cells and are directly involved in beta cell killing.(23, 24) In humans, both CD4 and CD8 T cells are found infiltrating islets in newly diagnosed T1D.(25) In rodent models, either CD4 or CD8 T cells can adoptively transfer disease. CD8 T cells likely are directly involved with beta cell killing, as MHC I is expressed on beta cells, and CD4 T cells likely impact pathogenesis via an indirect route.(22, 26) Both cell types secrete a number of proinflammatory cytokines, such as TNFα, IFNγ, IL-6 and IL-1, which not only recruit and activate accessory cells, thereby magnifying the inflammatory process, but also are directly toxic to beta cells.(26-28)
Although it is believed that beta-cell antigen-specific T cells are a necessary component of autoimmune diabetes, their very presence is not sufficient for disease because such cells are also found in healthy individuals; and, not all genetically predisposed mice develop diabetes (e.g. in NOD colonies only ∼50% of males and ∼80% of females develop disease despite harboring autoreactive T cells).(20, 29, 30) This strongly suggests that there are critical peripheral tolerance mechanisms that play a role in restraining self-reactive T cells that have escaped central (thymic) tolerance. Although there are a number of mechanisms of peripheral tolerance (including anergy and exhaustion), evidence now points to active regulation to be the primary mechanism of peripheral tolerance in T1D.
In the past decade, the concept of a T cell population that can suppress the activity effector T cells has reemerged. Most focus has been on a subset of CD4 T cells that express the transcription factor FoxP3.(31, 32) The role of FoxP3 and regulatory T cells (Tregs) is most obvious in conditions where there is genetic disruption of FoxP3, leading to multisystem autoimmunity in humans (the IPEX syndrome) and in mice (the Scurfy mouse), which lack Tregs.(33-37) NOD mice appear to have a loss of (functional) Tregs early in life concordant with the development of diabetes, and adoptive transfer of Tregs (either isolated directly from congenic mice or ex vivo expanded) can prevent and even reverse disease.(33, 35) Although the data from human studies has been more difficult to interpret, it now appears that although healthy individuals and those with T1D have similar circulating levels of Tregs, Tregs in T1D have functional deficits, i.e., reduced suppressive ability.(35, 38-41) Tregs exert suppression through secretion of certain immunomodulatory cytokines (e.g. IL-4, IL-10, and TGFβ) and via direct interaction with T cells or antigen presenting cells.(42-45)
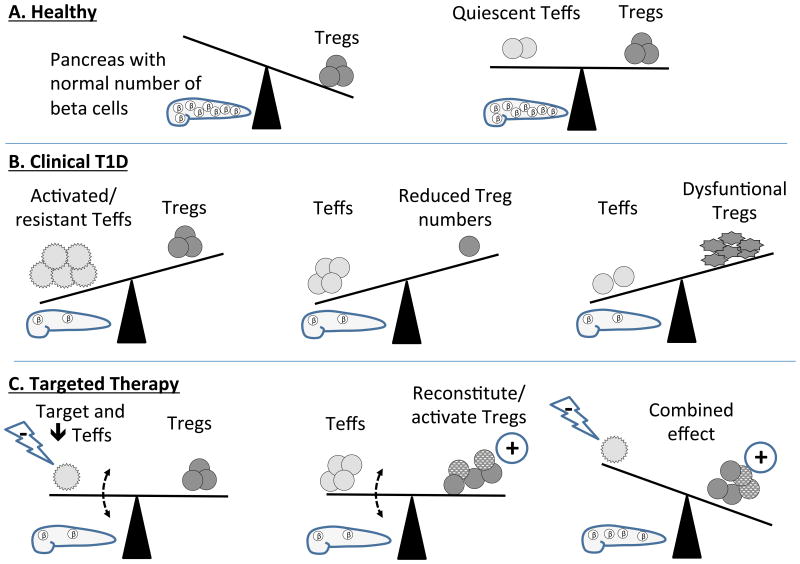
These observations have led to the concept that the development of autoimmunity (including T1D) is dependent on the “balance” of self-reactive effector T cells and Tregs (Figure 1). Although frequently thought of in terms of stoichiometric ratios, there are likely functional considerations of the T cells that must also be accounted for. For example, simply having sufficient numbers of Tregs may not be enough to prevent disease, and the functional state – either baseline (genetically determined) suppressive activity or impaired activity due to effects of the immunologic microenvironment – should be taken into consideration. Alternatively, it is well known that there are differences in activation requirements of naïve (Tn) and memory T (Tmem) cells, and that Tmems expand much more robustly than Tns (Figure 1B). Thus, depending on the maturation stage and time after antigen encounter, Tregs may have vastly different abilities to suppress the effector T (Teff) cell response.(46, 47) Indeed, recent studies have suggested that Teff resistance to suppression by Tregs may be a primary defect in T1D.(46) Thus the paradigm of “re-balancing” the Teff/Treg ratio to prevent, stabilize, or reverse diabetes autoimmunity may need to take into consideration both quantitative and qualitative factors (Figure 1c).
Figure 1.
Balancing effector and regulatory T cells in health, T1D, and with therapies. (A) Individuals free from diabetes may have no circulating beta-cell specific effector T cells (Teff; left) or have sufficient, functional peripheral regulatory T cells (Tregs) to counterbalance Teffs (right) and keep beta cells free from autoimmune damage. (B) In subjects who develop T1D, Teffs may become resistant to Tregs (left), Treg numbers may diminish (center), or, despite sufficient numbers, Tregs may become dysfunctional (right) resulting in T cell-mediated destruction of beta cells. (C) Therapies which temporarily suppress Teffs (left) or bolster Treg number or function (center) may be able to temporarily slow beta cell decline, but it may take therapies that both target Teffs and increase beta-cell-specific Tregs (right) to have a substantive and long-lasting effect.
It is now apparent that there are a number of cells and soluble factors that are involved in the immune dysregulation responsible for beta cell loss in T1D. Most of the information on contributions of immune-system components to diabetes pathogenesis has come from preclinical models of T1D, most frequently the NOD mouse and related strains. Based on these findings, approaches to modify the course of diabetes in these models have been developed and have provided the rationale for a number of clinical trials. However, as described in more detail below, although several interventions have been successful in preclinical models, to date none has translated into similar success in humans. An examination of these trials may provide important insights into human T1D and contribute to the development of future intervention trials.
Immune therapies in T1D
In the 1980s and early 1990s, several small-scale clinical intervention trials investigated the use of general immune-suppressive agents on the course of newly diagnosed T1D. For example, in 40 children with new-onset T1D, almost 2/3 were insulin-free about 6 weeks after starting cyclosporine, and this effect persisted for over a year while on therapy.(48) Yet once stopped, diabetes returned. Factors associated with response included shorter time from diagnosis, less weight loss, lower HbA1c, and less DKA.(48, 49) In another study, children treated with azathioprine and prednisone had evidence of improved glycemic control compared to controls, and in some cases achieved insulin independence, but again these benefits waned following discontinuation of therapy.(50) These and other examples provided the “proof-of-principal” that the diabetes autoimmune response, in some cases, could be slowed or even reversed using non-specific immune-suppressive agents.(51, 52) Concern for immune and non-immune side effects associated with what would likely need to be indefinite therapy precluded consideration of these as a viable approach. These studies also suggested that there was a variable response to therapy, suggesting heterogeneity in T1D, even in the pediatric population.
As diabetes-associated autoantigens were identified, the concept of disrupting specific autoimmune processes by administering these antigens – in essence overwhelming and dampening the autoimmune response by presenting autoantigens in a tolerogenic context – was examined in preclinical models. In some cases autoantigen therapy prevented or reversed diabetes and thus provided the justification for clinical trials.(53-55) A number of medium- to large-scale trials have been conducted evaluating insulin, Hsp60, and GAD on the progression of T1D. In the case of insulin, this has been tested by the oral, intranasal, and parenteral routes with no significant effect.(56-59) In some studies, Hsp60 peptide (also known as DiaPep277) given SQ has slowed beta cell loss, but minimally.(60-62) In phase II studies in children and adolescents, GAD65 bound to the adjuvant Alum given SQ appeared to slow beta cell loss, but this could not be confirmed in larger Phase III trials.(63, 64) Some studies evaluated immune responses in participants. Patients receiving the Hsp60 peptide did have increases in IL-10 and dampened T cell responses to antigen, and those receiving GAD-alum had increases in GAD antibodies and increases in proinflammatory cytokines, T cell proliferation, as well as Tregs (65, 66) in response to GAD. Taken together, although autoantigen treatment was successful in preclinical diabetes and may modulate specific aspects of the T1D autoimmune response, after much study there is little evidence that given as a monotherapy this approach can modulate the course of disease in humans. Further, in no other autoimmune disease has antigen therapy been shown to slow, prevent or reverse disease. These and other data presented below would strongly suggest that diabetes autoantigens alone are not able to significantly modify the course of T1D.
Although it appears that diabetes autoantibodies have little role in the pathogenesis or progression of T1D, murine studies demonstrated that agents that deplete B cells can prevent diabetes.(67) Rituximab is a monoclonal antibody to CD20, specifically depletes B cells and is used clinically to treat B cell lineage malignancies, autoimmune disease and organ transplant rejection. This agent was tested in those 8-40 years old (yo) diagnosed with T1D within the past 100 days.(68) A 4-dose course was associated with what appeared to be a pause in beta cell loss, which resumed at 3 months post enrollment. At 12 months, the rituximab group had higher endogenous insulin production than placebo patients, but still lower than baseline. Rituximab caused significant B cell depletion, but the effect on autoantibodies was not reported. It is unclear if the B cell depletion mediated by rituximab produced clinical efficacy due to their function as antigen presenting cells, producers of antibodies, or another mechanism.(68, 69)
Neutralizing proinflammatory and Th1 cytokines has been a successful approach to prevent diabetes in preclinical models and has been one of the most successful approaches to manage other human autoimmune diseases. Both TNFα and IL-1β are secreted by immunocytes infiltrating inflamed islets, and both not only assist in propagating inflammation but are toxic to beta cells, and thus may be both directly and indirectly involved in T1D.(27, 28, 70, 71) Some studies have shown elevations in these cytokines in humans with T1D, and treating mice with neutralizing antibodies prevents, and in some cases reverses, disease. In 2009 a small-scale trial of 18 children 7-18 yo studied the effect of etanercept (a recombinant TNFα receptor fusion protein) on disease progress.(72) After 6 months of treatment, those treated with etanercept showed lower HbA1c levels with lower insulin needs and a rise (versus a drop) in C-peptide compared to placebo-treated participants. A larger, confirmatory study has not been conducted. Last year, a publication reported results of two trials using different agents to antagonize IL-1β.(73) One conducted in Europe used anakinra (an IL-1 receptor antagonist) and enrolled adolescents and adults 18-35 yo, 35 in the treatment arm and 34 in the placebo arm. The other was conducted in North America and studied canakinumab (an anti-IL-1β MAb) in those 6-45 yo, 47 in the treatment arm. Neither trial showed any metabolic effect of IL-1β antagonism on T1D course within one year. Again, as exemplified for IL-1β blockade, not all agents that can successfully interfere with autoimmunity in murine or other human autoimmune diseases are effective in human T1D; but, in the case of TNFα antagonism there are opportunities for future study.
To date, agents that selectively target T cells have comprised the most numerous T1D intervention trials. In the 1980s and ′90s, monoclonal antibodies against T cells were developed and proved successful to treat organ allograft rejection. In rodent models, antibodies to CD3 can prevent and reverse diabetes.(53, 74) The first trials using a biologic agent in T1D used monoclonal antibodies to the CD3. In 2002 a modified form of OKT3 with a mutated (non-Fc receptor binding) Fc region called hOKT3 γ1(Ala-Ala) (teplizumab) was tested in 12 new-onset patients 7-27 yo.(75) Compared to placebo-treated patients, drug-treated participants had better maintenance of C-peptide secretion, lower insulin requirements, and lower HbA1c at 12 months. A follow-up study repeating dosing at 12 months (the AbATE trial) showed lasting metabolic improvement at 24 months, and post hoc analysis was able to identify responders from non-responders by lower HbA1c and insulin requirements, lower levels of some types of memory and naïve T cells, and lower IFNγ-producing CD8 T cells at baseline.(76) A large (n=516), industry-sponsored, multinational Phase III RCT (Protégé) of teplizumab that tested multiple treatment regimens did not meet its primary endpoint (which was the percent of patients with both insulin use of <0.5 U/kg/day and HbA1c <6.5%) at 12 months.(77) Post-hoc analysis identified factors at baseline associated with C-peptide preservation, including better metabolic control, higher C-peptide response, and time from diagnosis to enrollment.
A nonglycosylated form of anti-CD3 (ChAglyCD3; otelixizumab) was tested in the early 2000s in 40 patients 12-39 yo, and it was found to partially preserve beta cell function, resulting in less insulin requirements at 6, 12, and 18 months after treatment. Two follow-up Phase III industry-sponsored RCTs (DEFEND-1 and -2) enrolled participants 12-45 yo, yet these too failed to meet their primary endpoints, the change in C-peptide levels at 12 months, perhaps because the studied dose was too low.(78, 79)
In order for T cells to become fully activated, they require both antigen-specific signals (i.e. binding of MHC:peptide from antigen presenting cells (APC) to the T cell receptor) and antigen non-specific, costimulatory signals (i.e. binding of CD40 and B7 molecules on the APC to CD154 and CD28 on T cells).(80-84) Blocking T cell costimulation can prevent or dampen T cell responses and is an effective means to modify or prevent diabetes in rodent models. CTLA4-Ig (abatacept) is a fusion protein that binds to B7 molecules and interrupts CD28 signaling in T cells. This agent is a component of therapies in organ transplantation and is approved for a number of human autoimmune diseases.(85) Abatacept was given for two years to 77 patients 6-45 yo and produced a delay in C-peptide decline and lower HbA1c levels with similar insulin use at 2 years compared to placebo subjects.(86) Statistical modeling suggested a number of factors, including younger (6-12) or older (18-45) age, lower baseline C-peptide, and white race, were associated with a more robust response. However, despite continuous therapy for 2 years, the C-peptide decline resumed in the abatacept group at 6 months.
During the 2000s, technical improvements and advances in immunomodulation resulted in major strides in human islet cell transplantation (ICT).(87) A variation of the ICT immune protocol from the Edmonton group was assessed in reversing diabetes in new-onset T1D.(88, 89) This trial used anti-CD25 (daclizumab) to target CD25-expressing (activated) T cells and mycophenolate mofetil (MMF) as a non-specific immunosuppressant. In patients 8-45 yo, neither MMF alone nor MMF+daclizumab had any effect on beta cell loss or metabolic parameters over 24 months.(90) This was surprising as this regimen met with some success in ICT (which comprises both allo- and auto-immune responses (87)), and anti-CD25 and MMF in alone and in combination can delay or prevent autoimmune diabetes in the BioBreeding (BB) rat.(91) One possibility is that this regimen inhibited of Tregs, which are strongly dependent on signaling through the high-affinity IL-2 receptor that includes the α subunit (CD25).(92)
A number of recent trials have provided insight on how therapies may modulate Tregs. A phase I trial of interleukin 2 (IL-2) and rapamycin was tested in 9 adults specifically to evaluate if this could increase Tregs, and its effect on beta cell function.(93) IL-2 is known to be involved in Treg survival and function (they express high levels of CD25), while rapamycin inhibits activation and function of Th1 and Th17 effector T cells, and is effective in preventing diabetes in mouse models.(94) This approach transiently increased the numbers of Tregs in the first month after therapy, but concomitantly metabolic parameters were worsened, likely due to unintended Teff activation. A trial of anti-thymocyte globulin (ATG; the START trial) was based on the concept that significant T cell depletion might eliminate diabetogenic T cells and “reset” the autoimmune response and the effector to regulatory T cell balance, resulting in long-term remission.(95) ATG is a rabbit antiserum that depletes human T cells, and is used in organ transplantation and some autoimmune diseases, and analogous therapies can prevent and reverse diabetes in preclinical models. Multiple doses of ATG were given to 38 participants 12-35 yo with new-onset T1D over one week. Most recipients acutely developed cytokine release syndrome (CRS) and serum sickness 7-10 days later. ATG had no effect on C-peptide preservation or metabolic control. In mechanistic evaluations, recipients had acute serum elevations in a number of proinflammatory cytokines during therapy and, interestingly, a preferential depletion of Tregs over effector CD4 and CD8 T cells. In a post-hoc analysis, it appears that older participants may have had beta cell sparing, while the younger subjects treated with ATG had an acute loss of beta cells in the first 6 months.[76] A clinical trial of ATG and GCSF is being planned.
An ongoing trial evaluating specific depletion of effector and memory T cells (the T1DAL trial) recently published its 12-month results.(96) Alefacept is a fusion protein consisting of an LFA3 head and an IgG tail. The drug preferentially targets memory and effector CD4 and CD8 T cells (which express high levels of CD2, the cognate receptor for LFA3), the cells that appear to be most involved in beta cell destruction. The trial randomized 49 participants 12-40 yo (33 to alefacept, 16 to placebo) and found that treated subjects had lower insulin requirements, fewer hypoglycemic episodes and, in some analyses, preservation of C-peptide at 12 months. In the mechanistic evaluation it was shown that alefacept significantly depleted CD4 and CD8 effector and memory cells, while sparing Tregs, leading to a favorable Treg:Teff ratio.[77] Additional data from this trial will be forthcoming.
Conclusions
Armed with the knowledge of the immune basis for T1D, the observations that the course of T1D could be modified with non-specific immune suppressants, and the advent of novel agents to target specific immune processes, the past decade was filled with promise that an approach to reverse and stabilize T1D would be discovered. Unfortunately, despite tremendous effort with nearly a dozen trials enrolling many hundreds of participants, none has been found. In many cases, despite well-founded preclinical data and/or experiences from other human auto- or allo-immune conditions, there was no apparent impact on the course of T1D. Even in those trials showing some impact on disease course, no approach to induce true clinical remission (i.e. insulin independence) has been found.
Although no approach has been able to achieve frank remission or prolonged beta cell preservation, there are a number of lessons from these trials that may help guide the next phase of studies (Table 1). The human T1D immune response has proven resistant to a number of potent immune interventions that are effective in other human conditions and preclinical models. It thus appears that on a relative scale, T1D autoimmunity is more intractable than a number of other autoimmune diseases or the alloimmune response to organ transplantation, including that to islet allografts. It is also clear that what is shown to be effective in preclinical (rodent) models does not necessarily correlate with efficacy in humans – further putting into question the utility of these models as a litmus test for clinical trials.(97) Certainly, rodent studies have provided critical information for a general understanding of the pathogenesis of T1D, but more translational studies in the clinic are urgently needed.
Table 1. Lessons Learned to Date from Targeted Immunotherapy Trials in T1D.
| 1. Treatments that are effective in other human auto- or allo-immune conditions have marginal or little efficacy in T1D. Possible explanations include: T1D has a unique immunopathogenesis compared to other autoimmune conditions; short-term immune-modulation does not restore tolerance and autoimmunity resumes after a variable interval once treatment ends; the residual beta cell mass has fallen below a critical threshold and cannot recover even after successful ablation of the autoimmune attack. |
| 2. As powerful immune modulatory agents have little or no effect in changing the course of T1D, the immune process in T1D appears to be extremely robust, and thus agents with minimal impact on immune responses are unlikely to alter the progression of T1D. |
| 3. Many interventions that are effective in rodent (primarily NOD) models of T1D are not similarly effective in humans, and therefore the use of rodent models as the prerequisite rationale for human trials may not be appropriate. |
| 4. In some cases, different subpopulations of patients with T1D appear to respond differently to immune interventions, suggesting significant heterogeneity in human T1D. |
Taken together, more in depth evaluation of existing studies is warranted and further fundamental study of human T1D is needed to guide the next phases of intervention trials in this area.
Because the immune response in T1D is more robust and complex than previously considered, trials which interfere with a number of pathways (i.e. through the use of combination therapies) are warranted, and the use of therapies that are likely to have minimal immunological effect (e.g. dietary modification or vitamin supplementation) may be futile. In-depth mechanistic evaluation from some studies has suggested that therapies may differentially impact effector and regulatory cells. There is an emerging consensus that an effective therapy must combine inhibition of Teff cells (by depletion, enhanced suppressibility, or both) with stimulation of Tregs (by increased frequency or function, including ablation of the proinflammatory milieu). Further, if possible, such changes in effector and regulatory cells should be antigen-specific. Such an outcome may require combinations comprising a Teff-depleting agent, a Treg-boosting agent, and an antigen.(45, 98) While such combinations will present substantial practical and regulatory challenges, they will likely be our best shot at inducing a durable remission of autoimmunity in this disease.
Key Points.
Type 1 diabetes (T1D) is the result of a multifaceted immune attack on pancreatic beta cells.
Agents that directly affect the immune system (immune suppressants and modulators) have had the most and most reliable success in modifying the course of T1D.
Although well founded and successful in rodent models of T1D, recent trials using agent targeting T cells, B cells or cytokines have had less then the expected effect on the course of T1D.
Future trials incorporating data from more detail studies in human T1D and combining targeted therapies, specifically those which combine targeting effector cells and enhancing regulation, may hold the most promise for inducing durable remission in T1D.
Footnotes
Conflict of interest: The authors declare no scientific, financial or personal conflicts of interest.
References
- 1.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–20. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 3.JDRF. Type 1 diabetes facts. 2014 http://jdrf.org/about-jdrf/fact-sheets/type-1-diabetes-facts/ cited 2013.
- 4.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson SI, Willis J, Florkowski CM, Scott RS. All-cause mortality in insulin-treated diabetic patients: a 20-year follow-up. Diabetes Res Clin Pract. 2008;80(1):e6–9. doi: 10.1016/j.diabres.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279–83. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 7.Haskins K, Wegmann D. Diabetogenic T-cell clones. Diabetes. 1996;45(10):1299–305. doi: 10.2337/diab.45.10.1299. [DOI] [PubMed] [Google Scholar]
- 8.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313(6):353–60. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 9.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5(9):1026–31. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 10.Eisenbarth GS. Type 1 diabetes: molecular, cellular and clinical immunology. Adv Exp Med Biol. 2004;552:306–10. [PubMed] [Google Scholar]
- 11.La Torre D, Lernmark A. Immunology of beta-cell destruction. Adv Exp Med Biol. 2010;654:537–83. doi: 10.1007/978-90-481-3271-3_24. [DOI] [PubMed] [Google Scholar]
- 12.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, et al. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60(5):1370–9. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Evans-Molina C, Hatanaka M, Mirmira RG. Lost in translation: endoplasmic reticulum stress and the decline of beta-cell health in diabetes mellitus. Diabetes, obesity & metabolism. 2013;15(Suppl 3):159–69. doi: 10.1111/dom.12163. Excellcent review of how ER stress may contribute not only to Type 2, but Type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360(16):1646–54. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 16.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J Clin Endocrinol Metab. 2004;89(8):3896–902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 17.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 18.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes. 2000;49(10):1621–6. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 19.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32(12):2269–74. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–63. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 21.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406(6797):739–42. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 22.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17(6):624–31. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Peterson LD, van der Keur M, de Vries RR, Roep BO. Autoreactive and immunoregulatory T-cell subsets in insulin-dependent diabetes mellitus. Diabetologia. 1999;42(4):443–9. doi: 10.1007/s001250051177. [DOI] [PubMed] [Google Scholar]
- 24.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113(3):451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortis F, Pirot P, Naamane N, Kreins AY, Rasschaert J, Moore F, et al. Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia. 2008;51(7):1213–25. doi: 10.1007/s00125-008-0999-7. [DOI] [PubMed] [Google Scholar]
- 27.Argiles JM, Lopez-Soriano J, Lopez-Soriano FJ. Cytokines and diabetes: the final step? Involvement of TNF-alpha in both type I and II diabetes mellitus. Horm Metab Res. 1994;26(10):4–9. doi: 10.1055/s-2007-1001730. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovitch A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1998;14(2):129–51. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25(4):303–11. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Viglietta V, Kent SC, Orban T, Hafler DA. GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes. J Clin Invest. 2002;109(7):895–903. doi: 10.1172/JCI14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307–8. doi: 10.1038/ni.2554. Letter to the editor and breif review of consensus from Third International Conference on Regulatory T Cells and Th Subsets and Clinical Application in Human Diseases (Shanghai, China, 2012) suggesting most appropriate ways to define and document regulatory T cells. [DOI] [PubMed] [Google Scholar]
- 32.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229(1):41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyara M, Sakaguchi S. Human FoxP3(+)CD4(+) regulatory T cells: their knowns and unknowns. Immunol Cell Biol. 2011;89(3):346–51. doi: 10.1038/icb.2010.137. [DOI] [PubMed] [Google Scholar]
- 34.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 35.D'Alise AM, Auyeung V, Feuerer M, Nishio J, Fontenot J, Benoist C, et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc Natl Acad Sci U S A. 2008;105(50):19857–62. doi: 10.1073/pnas.0810713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171(3):1216–23. doi: 10.4049/jimmunol.171.3.1216. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 38.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sgouroudis E, Albanese A, Piccirillo CA. Impact of protective IL-2 allelic variants on CD4+ Foxp3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice. J Immunol. 2008;181(9):6283–92. doi: 10.4049/jimmunol.181.9.6283. [DOI] [PubMed] [Google Scholar]
- 40.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54(1):92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 41.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54(5):1407–14. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 42.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 45.Cabrera SM, Rigby MR, Mirmira RG. Targeting regulatory T cells in the treatment of type 1 diabetes mellitus. Curr Mol Med. 2012;12(10):1261–72. doi: 10.2174/156652412803833634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181(10):7350–5. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Haseda F, Imagawa A, Murase-Mishiba Y, Terasaki J, Hanafusa T. CD4(+) CD45RA(-) FoxP3high activated regulatory T cells are functionally impaired and related to residual insulin-secreting capacity in patients with type 1 diabetes. Clin Exp Immunol. 2013;173(2):207–16. doi: 10.1111/cei.12116. This report strongly suggests of a functional impairment of regulatory T cells in patients with T1D and that severity may be related to the level of dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bougneres PF, Carel JC, Castano L, Boitard C, Gardin JP, Landais P, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318(11):663–70. doi: 10.1056/NEJM198803173181103. [DOI] [PubMed] [Google Scholar]
- 49.Carel JC, Boitard C, Eisenbarth G, Bach JF, Bougneres PF. Cyclosporine delays but does not prevent clinical onset in glucose intolerant pre-type 1 diabetic children. J Autoimmun. 1996;9(6):739–45. doi: 10.1006/jaut.1996.0096. [DOI] [PubMed] [Google Scholar]
- 50.Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319(10):599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 51.Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39(10):1264–72. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- 52.Harrison LC, Colman PG, Dean B, Baxter R, Martin FI. Increase in remission rate in newly diagnosed type I diabetic subjects treated with azathioprine. Diabetes. 1985;34(12):1306–8. doi: 10.2337/diab.34.12.1306. [DOI] [PubMed] [Google Scholar]
- 53.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116(5):1371–81. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peakman M, von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes. 2010;59(9):2087–93. doi: 10.2337/db10-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarikonda G, Sachithanantham S, Manenkova Y, Kupfer T, Posgai A, Wasserfall C, et al. Transient B-cell depletion with anti-CD20 in combination with proinsulin DNA vaccine or oral insulin: immunologic effects and efficacy in NOD mice. PLoS One. 2013;8(2):e54712. doi: 10.1371/journal.pone.0054712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28(5):1068–76. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 57.Diabetes Prevention Trial--Type 1 Diabetes Study G. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–91. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 58.Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372(9651):1746–55. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 59.Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol. 2009;155(2):156–65. doi: 10.1111/j.1365-2249.2008.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev. 2007;23(4):269–75. doi: 10.1002/dmrr.691. [DOI] [PubMed] [Google Scholar]
- 61.Huurman VA, van der Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, et al. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol. 2008;152(3):488–97. doi: 10.1111/j.1365-2249.2008.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358(9295):1749–53. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 63.Axelsson S, Hjorth M, Akerman L, Ludvigsson J, Casas R. Early induction of GAD(65)-reactive Th2 response in type 1 diabetic children treated with alum-formulated GAD(65) Diabetes Metab Res Rev. 2010;26(7):559–68. doi: 10.1002/dmrr.1126. [DOI] [PubMed] [Google Scholar]
- 64.Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366(5):433–42. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 65.Hjorth M, Axelsson S, Ryden A, Faresjo M, Ludvigsson J, Casas R. GAD-alum treatment induces GAD65-specific CD4+CD25highFOXP3+ cells in type 1 diabetic patients. Clin Immunol. 2011;138(1):117–26. doi: 10.1016/j.clim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 66**.Axelsson S, Cheramy M, Akerman L, Pihl M, Ludvigsson J, Casas R. Cellular and Humoral Immune Responses in Type 1 Diabetic Patients Participating in a Phase III GAD-alum Intervention Trial. Diabetes Care. 2013 doi: 10.2337/dc12-2251. This study shows that even in the absence of a clinical response, antigen therapy mayhave important effects on the diabetes immune response, providing hope that ifappropriatley modified, the may be a role for autoantigens in T1D therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857–67. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, et al. Effect of rituximab on human in vivo antibody immune responses. The Journal of allergy and clinical immunology. 2011;128(6):1295–302. doi: 10.1016/j.jaci.2011.08.008. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunger A, Schroder D, Augstein P, Witstruck T, Wachlin G, Vogt L, et al. Impact of metabolic activity of beta cells on cytokine-induced damage and recovery of rat pancreatic islets. Acta Diabetol. 1995;32(4):217–24. doi: 10.1007/BF00576253. [DOI] [PubMed] [Google Scholar]
- 71.Kawahara DJ, Kenney JS. Species differences in human and rat islet sensitivity to human cytokines.Monoclonal anti-interleukin-1 (IL-1) influences on direct and indirect IL-1-mediated islet effects. Cytokine. 1991;3(2):117–24. doi: 10.1016/1043-4666(91)90031-8. [DOI] [PubMed] [Google Scholar]
- 72.Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32(7):1244–9. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73**.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15. doi: 10.1016/S0140-6736(13)60023-9. Although a report of 2 “negative” T1D clinical trials, is an important addition to translating potential therapies from proclincal models to humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glandt M, Hagopian W, Herold KC. Treatment of type 1 diabetes with anti-CD3 monoclonal antibody. Reviews in endocrine & metabolic disorders. 2003;4(4):361–8. doi: 10.1023/a:1027354129493. [DOI] [PubMed] [Google Scholar]
- 75.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 76**.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: Metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013 doi: 10.2337/db13-0345. Important study suggesting that there may be differential responsiveness to immune therapu in subpopulations with new onset T1D Encouraging need for more studies of human T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tolerx Ga. GlaxoSmithKline and Tolerx announce phase III DEFEND-1 study of otelixizumab in type 1 diabetes did not meet its primary endpoint 2011. cited 2011 March 11. [Google Scholar]
- 79**.Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, Dayan CM. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2013 doi: 10.1111/dme.12361. Important large scale pase III clincal trial scaling up what were thought to be postive phase II trials. [DOI] [PubMed] [Google Scholar]
- 80.Green JM, Noel PJ, Sperling AI, Walunas TL, Lenschow DJ, Stack R, et al. T cell costimulation through the CD28 receptor. Proc Assoc Am Physicians. 1995;107(1):41–6. [PubMed] [Google Scholar]
- 81.Herold KG, Lenschow DJ, Bluestone JA. CD28/B7 regulation of autoimmune diabetes. Immunol Res. 1997;16(1):71–84. doi: 10.1007/BF02786324. [DOI] [PubMed] [Google Scholar]
- 82.Lenschow DJ, Herold KC, Rhee L, Patel B, Koons A, Qin HY, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5(3):285–93. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 83.Rigby MR, Trexler AM, Pearson TC, Larsen CP. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector t-cell inhibition and regulatory T-cell expansion. Diabetes. 2008;57(10):2672–83. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6(5 Pt 1):876–83. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 85.Lundquist L. Abatacept: a novel therapy approved for the treatment of patients with rheumatoid arthritis. Advances in therapy. 2007;24(2):333–45. doi: 10.1007/BF02849902. [DOI] [PubMed] [Google Scholar]
- 86.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2(7):a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. New England Journal of Medicine. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 89.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care. 2010;33(4):826–32. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care. 2010;33(4):826–32. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ugrasbul F, Moore WV, Tong PY, Kover KL. Prevention of diabetes: effect of mycophenolate mofetil and anti-CD25 on onset of diabetes in the DRBB rat. Pediatr Diabetes. 2008;9(6):596–601. doi: 10.1111/j.1399-5448.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 92.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev. 2011;241(1):63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61(9):2340–8. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes. 2002;51(3):638–45. doi: 10.2337/diabetes.51.3.638. [DOI] [PubMed] [Google Scholar]
- 95**.Gitelman SE, Gottlieb PA, Rigby MR, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. The Lancet Diabetes & Endocrinology. 2013;1(4):306–16. doi: 10.1016/S2213-8587(13)70065-2. A well designed and supported phase II T1D clinical trial. Although findings were negative, gives pause to agressiveness of human disease with this strong immune modulating agent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96**.Rigby MR, DiMeglio LA, Rendell MS. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. The Lancet Diabetes & Endocrinology. 2013;1(4):284–94. doi: 10.1016/S2213-8587(13)70111-6. A promising phase II trial that suggests that selective elimination of effector T cells while sparing regulatory T cells may preserve beta cell function in humans with T1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4(12):989–97. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- 98.Ehlers MR, Nepom GT. Immune-directed therapy for type 1 diabetes at the clinical level: the Immune Tolerance Network (ITN) experience. Rev Diabet Stud. 2012;9(4):359–71. doi: 10.1900/RDS.2012.9.359. [DOI] [PMC free article] [PubMed] [Google Scholar]