Abstract
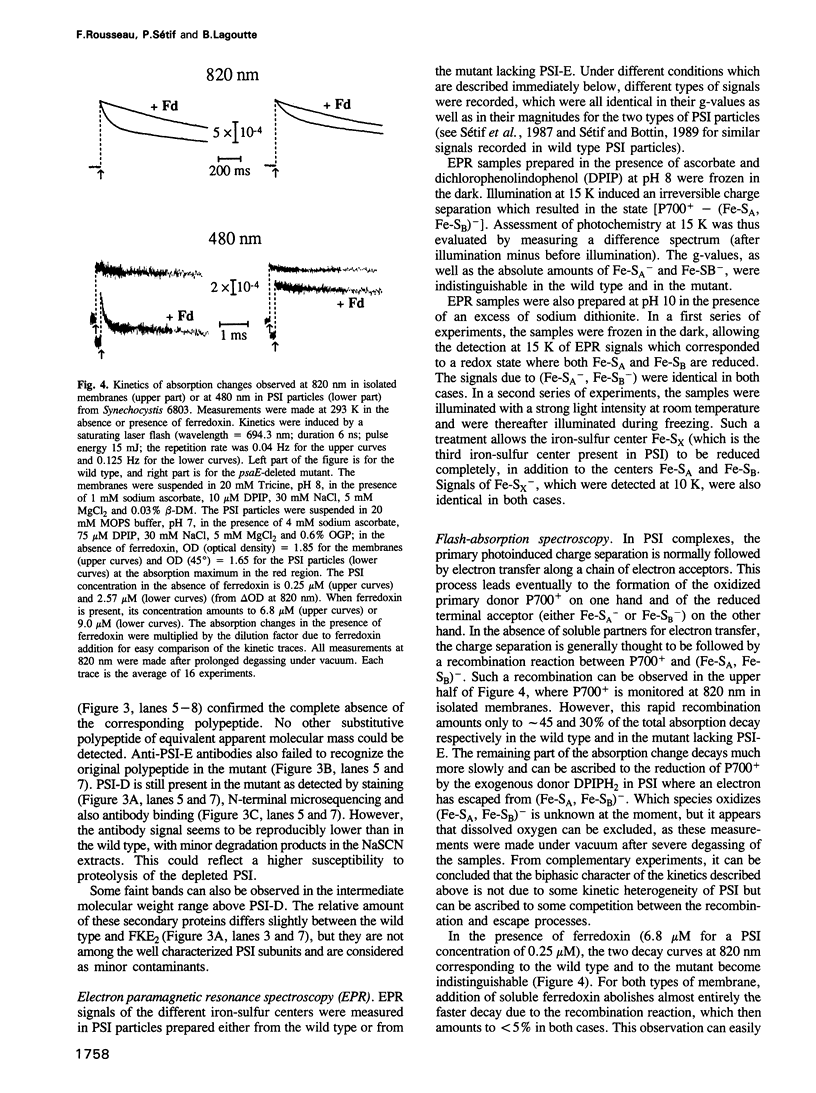
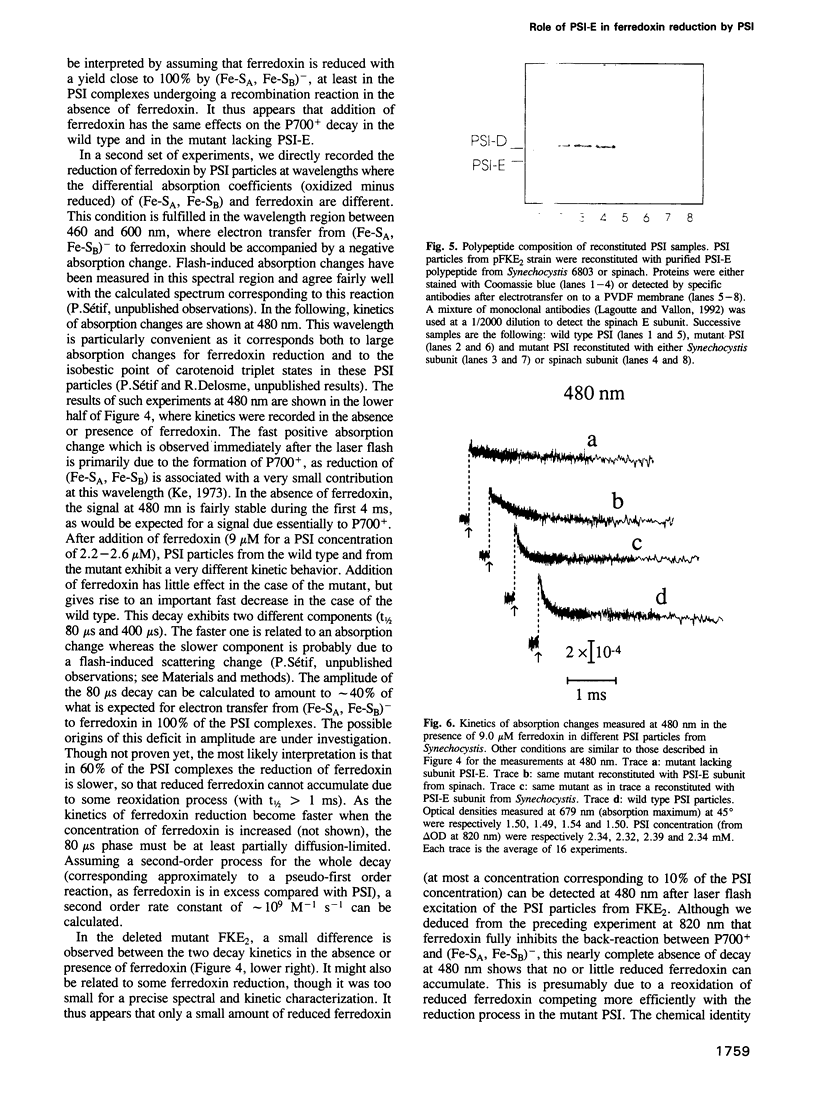
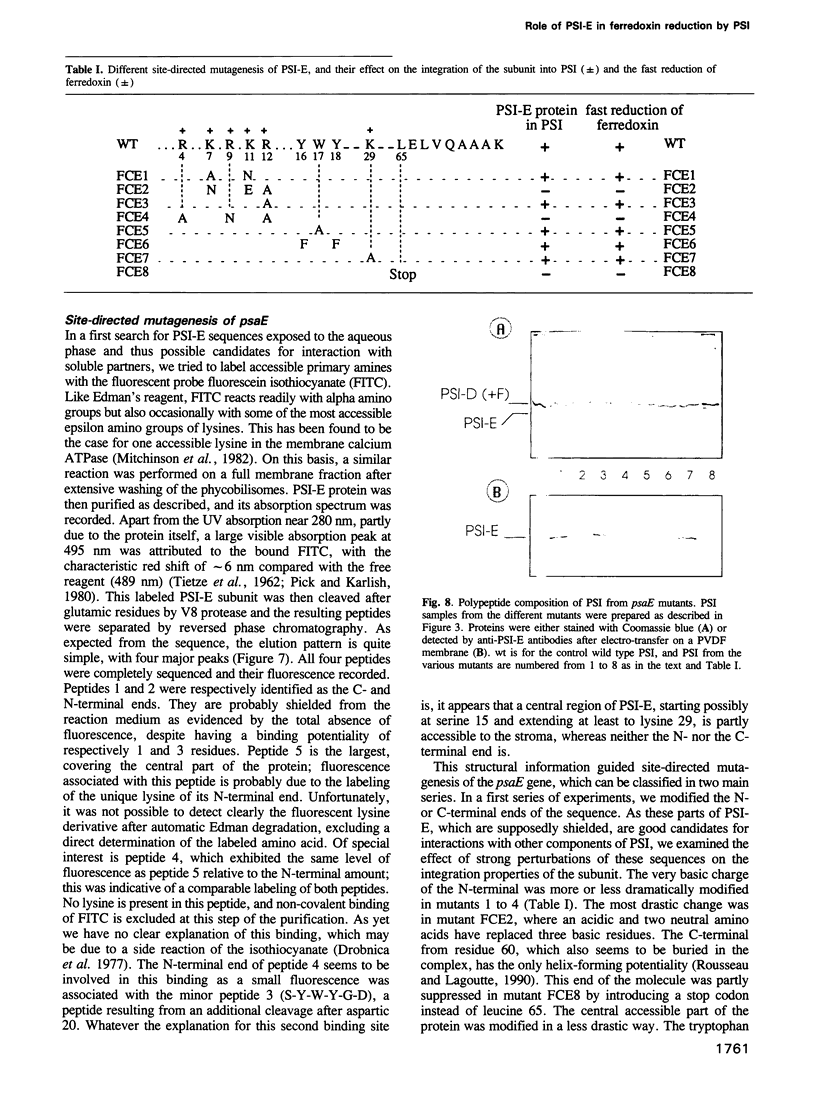
Of the stroma-accessible proteins of photosystem I (PSI) from Synechocystis sp. PCC 6803, the PSI-C, PSI-D and PSI-E subunits have already been characterized, and the corresponding genes isolated. PCR amplification and cassette mutagenesis were used in this work to delete the psaE gene. PSI particles were isolated from this mutant, which lacks subunit PSI-E, and the direct photoreduction of ferredoxin was investigated by flash absorption spectroscopy. The second order rate constant for reduction of ferredoxin by wild type PSI was estimated to be approximately 10(9) M-1s-1. Relative to the wild type, PSI lacking PSI-E exhibited a rate of ferredoxin reduction decreased by a factor of at least 25. After reassociation of the purified PSI-E polypeptide, the original rate of electron transfer was recovered. When a similar reconstitution was performed with a PSI-E polypeptide from spinach, an intermediate rate of reduction was observed. Membrane labeling of the native PSI with fluorescein isothiocyanate allowed the isolation of a fluorescent PSI-E subunit. Peptide analysis showed that some residues following the N-terminal sequence were labeled and thus probably accessible to the stroma, whereas both N- and C-terminal ends were probably buried in the photosystem I complex. Site-directed mutagenesis based on these observations confirmed that important changes in either of the two terminal sequences of the polypeptide impaired its correct integration in PSI, leading to phenotypes identical to the deleted mutant. Less drastic modifications in the predicted stroma exposed sequences did not impair PSI-E integration, and the ferredoxin photoreduction was not significantly affected. All these results lead us to propose a structural role for PSI-E in the correct organization of the site involved in ferredoxin photoreduction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B., Scheller H. V., Møller B. L. The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase. FEBS Lett. 1992 Oct 19;311(2):169–173. doi: 10.1016/0014-5793(92)81391-x. [DOI] [PubMed] [Google Scholar]
- Anderson S. L., McIntosh L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J Bacteriol. 1991 May;173(9):2761–2767. doi: 10.1128/jb.173.9.2761-2767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Bottin H., Lagoutte B. Ferredoxin and flavodoxin from the cyanobacterium Synechocystis sp PCC 6803. Biochim Biophys Acta. 1992 Jul 6;1101(1):48–56. doi: 10.1016/0167-4838(92)90465-p. [DOI] [PubMed] [Google Scholar]
- Chauvat F., Rouet P., Bottin H., Boussac A. Mutagenesis by random cloning of an Escherichia coli kanamycin resistance gene into the genome of the cyanobacterium Synechocystis PCC 6803: selection of mutants defective in photosynthesis. Mol Gen Genet. 1989 Mar;216(1):51–59. doi: 10.1007/BF00332230. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Miedel M. C., Nelson N. Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18374–18380. [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Nelson N. Insertional inactivation of the gene encoding subunit II of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18381–18385. [PubMed] [Google Scholar]
- Haselkorn R. Genetic systems in cyanobacteria. Methods Enzymol. 1991;204:418–430. doi: 10.1016/0076-6879(91)04022-g. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Labarre J., Chauvat F., Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989 Jun;171(6):3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagoutte B., Vallon O. Purification and membrane topology of PSI-D and PSI-E, two subunits of the photosystem I reaction center. Eur J Biochem. 1992 May 1;205(3):1175–1185. doi: 10.1111/j.1432-1033.1992.tb16888.x. [DOI] [PubMed] [Google Scholar]
- Li N., Warren P. V., Golbeck J. H., Frank G., Zuber H., Bryant D. A. Polypeptide composition of the Photosystem I complex and the Photosystem I core protein from Synechococcus sp. PCC 6301. Biochim Biophys Acta. 1991 Aug 23;1059(2):215–225. doi: 10.1016/s0005-2728(05)80206-3. [DOI] [PubMed] [Google Scholar]
- Li N., Zhao J. D., Warren P. V., Warden J. T., Bryant D. A., Golbeck J. H. PsaD is required for the stable binding of PsaC to the photosystem I core protein of Synechococcus sp. PCC 6301. Biochemistry. 1991 Aug 6;30(31):7863–7872. doi: 10.1021/bi00245a028. [DOI] [PubMed] [Google Scholar]
- Mannan R. M., Whitmarsh J., Nyman P., Pakrasi H. B. Directed mutagenesis of an iron-sulfur protein of the photosystem I complex in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10168–10172. doi: 10.1073/pnas.88.22.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson C., Wilderspin A. F., Trinnaman B. J., Green N. M. Identification of a labelled peptide after stoicheiometric reaction of fluorescein isothiocyanate with the Ca2+ -dependent adenosine triphosphatase of sarcoplasmic reticulum. FEBS Lett. 1982 Sep 6;146(1):87–92. doi: 10.1016/0014-5793(82)80710-2. [DOI] [PubMed] [Google Scholar]
- Münch S., Ljungberg U., Steppuhn J., Schneiderbauer A., Nechushtai R., Beyreuther K., Herrmann R. G. Nucleotide sequences of cDNAs encoding the entire precursor polypeptides for subunits II and III of the photosystem I reaction center from spinach. Curr Genet. 1988 Nov;14(5):511–518. doi: 10.1007/BF00521277. [DOI] [PubMed] [Google Scholar]
- Okkels J. S., Jepsen L. B., Hønberg L. S., Lehmbeck J., Scheller H. V., Brandt P., Høyer-Hansen G., Stummann B., Henningsen K. W., von Wettstein D. A cDNA clone encoding a 10.8 kDa photosystem I polypeptide of barley. FEBS Lett. 1988 Sep 12;237(1-2):108–112. doi: 10.1016/0014-5793(88)80181-9. [DOI] [PubMed] [Google Scholar]
- Ortiz W., Lam E., Chollar S., Munt D., Malkin R. Topography of the protein complexes of the chloroplast thylakoid membrane : studies of photosystem I using a chemical probe and proteolytic digestion. Plant Physiol. 1985 Feb;77(2):389–397. doi: 10.1104/pp.77.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Williams J. G., Arntzen C. J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988 Feb;7(2):325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Indications for an oligomeric structure and for conformational changes in sarcoplasmic reticulum Ca2+-ATPase labelled selectively with fluorescein. Biochim Biophys Acta. 1980 Nov 20;626(1):255–261. doi: 10.1016/0005-2795(80)90216-0. [DOI] [PubMed] [Google Scholar]
- Porter R. D. DNA transformation. Methods Enzymol. 1988;167:703–712. doi: 10.1016/0076-6879(88)67081-9. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Lagoutte B. Amino acid sequence of photosystem I subunit IV from the cyanobacterium Synechocystis PCC 6803. FEBS Lett. 1990 Jan 29;260(2):241–244. doi: 10.1016/0014-5793(90)80113-w. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shestakov S. V., Khyen N. T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. Mol Gen Genet. 1970;107(4):372–375. doi: 10.1007/BF00441199. [DOI] [PubMed] [Google Scholar]
- Smart L. B., Anderson S. L., McIntosh L. Targeted genetic inactivation of the photosystem I reaction center in the cyanobacterium Synechocystis sp. PCC 6803. EMBO J. 1991 Nov;10(11):3289–3296. doi: 10.1002/j.1460-2075.1991.tb04893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sétif P., Ikegami I., Biggins J. Light-induced charge separation in Photosystem I at low temperature is not influenced by vitamin K-1. Biochim Biophys Acta. 1987 Nov 19;894(2):146–156. doi: 10.1016/0005-2728(87)90184-8. [DOI] [PubMed] [Google Scholar]
- TIETZE F., MORTIMORE G. E., LOMAX N. R. Preparation and properties of fluorescent insulin derivatives. Biochim Biophys Acta. 1962 May 21;59:336–346. doi: 10.1016/0006-3002(62)90182-8. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Borrias W. E., Kuhlemeier C. J., Castets A. M., van Arkel G. A., van den Hondel C. A. A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene. 1982 Nov;20(1):111–119. doi: 10.1016/0378-1119(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Williams J. G., Rutherford A. W., Mathis P., Arntzen C. J. Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9474–9477. doi: 10.1073/pnas.83.24.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G., Merati G. Interaction between photosystem I and ferredoxin. Identification by chemical cross-linking of the polypeptide which binds ferredoxin. Eur J Biochem. 1987 Nov 16;169(1):143–146. doi: 10.1111/j.1432-1033.1987.tb13591.x. [DOI] [PubMed] [Google Scholar]
- Zilber A. L., Malkin R. Ferredoxin Cross-Links to a 22 kD Subunit of Photosystem I. Plant Physiol. 1988 Nov;88(3):810–814. doi: 10.1104/pp.88.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber A. L., Malkin R. Organization and topology of photosystem I subunits. Plant Physiol. 1992 Jul;99(3):901–911. doi: 10.1104/pp.99.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]