Abstract
Myeloma bone disease (MBD) is the most visible aspect of plasma cell myeloma (PCM), which is characterized by the displacement of hematopoiesis and the formation of osteolytic bone lesions. The secreted glycoprotein Dickkopf-1 (DKK1), an inhibitor of the Wnt signaling pathway, is broadly expressed in myeloma cells but highly restricted in normal tissues. DKK1 plays a critical role in several aspects of bone biology and actively participates in regulating MBD by inhibiting osteoblasts and by activating osteoclasts. Based on these findings, ongoing research has been targeting DKK1 to find novel therapeutic strategies for MBD, such as DKK1-neutralizing antibodies, proteasome inhibitors, and vaccines. All these strategies have produced encouraging clinical results and consequently, revealed the significance of DKK1 in MBD. This review discusses the recent advances in our understanding of the DKK1 pathway signaling and how DKK1 can be exploited in the therapeutic intervention of MBD.
Keywords: Myeloma bone disease, DKK1, antibody, proteasome inhibitor, vaccine
Introduction
Myeloma bone disease (MBD) is the most visible aspect of plasma cell myeloma (PCM, also known as multiple myeloma or Kahler’s disease), which is characterized by the displacement of hematopoiesis and the formation of osteolytic bone lesions. 1, 2 MBD results from increased osteoclastic activity and impaired osteoblastic bone formation, which cause severe pain and pathologic fractures in PCM patients. The complexity of the underlying mechanisms of MBD makes treatment very challenging. A central pathway for bone development and homeostasis is wingless-related integration site (Wnt) signaling. The Dickkopf (DKK) family of proteins (DKK1-4) is generally known to inhibit the Wnt signaling pathway and suppress tumor growth, 3 but secretion of DKK1 by myeloma cells is a major factor in inhibiting osteoblast precursors and thus bone regeneration. 4 DKK1 is produced by myeloma cells and is overexpressed in the myeloma microenvironment of patients with extensive bone disease. 2 Several animal studies and preclinical trials have confirmed that DKK1 can inhibit osteoblasts and activate osteoclasts, resulting in the imbalance of bone metabolism, and that DKK1 plays a vital role in the pathogenesis of MBD. 5–7
Owing to this severe imbalance of bone remodeling, MBD is the hallmark of PCM’s complications, particularly the destruction of bone and the high mortality rate. The basis for the increase in bone destruction and the decrease in bone formation in PCM has been extensively investigated during the last 10 years. Recently, the identification of a mechanistic link between bone mass in PCM patients and the Wnt signaling pathway has attracted attention in the field of bone metabolism research. It is evident that Wnt signaling plays a pivotal role not only in the development and morphogenesis of embryos but also in the pathogenesis of cancer. Importantly, the Wnt signaling pathway is a central pathway for bone development and homeostasis, as demonstrated by several genetic murine models that have underlined the importance of the Wnt signaling pathway in skeletal biology and disease. 8–10 Recent advances have been made in our understanding of DKK1’s role in MBD, and the development of emerging immunotherapeutic strategies targeting this pathway is ongoing. Several preclinical studies, such as those on developing a neutralizing antibody, have shown promising results. This review will focus on the biological functions of DKK1, the possible mechanisms of MBD, and novel strategies targeting DKK1.
The structure and biological function of DKK1
The DKK1 gene, located in the tenth chromosome, encodes a 266-amino acid protein that is a member of the Dickkopf family 11, 12. Dkk-1 is a secreted protein that contains a 31-residue N-terminal signal peptide, 2 cysteine rich domains, and a putative carboxy terminal N-glycosylation site. DKKs play an important role in vertebrate development. For example, whereas DKK1−/− knockout mice are embryonic lethal, mice with hypomorphic DKK1 doubleridge alleles that express low amounts of DKK1 are viable. 13 Glinka et al. 12 reported that radial microinjection of DKK1 mRNA caused blastomeres of four-cell Xenopus embryos to develop big heads, enlarged cement glands, and short trunks. Overall, these findings suggest that DKK1 can affect regionalization of neuroectoderm independently of the dorso-anterior mesendoderm. Moreover, DKK1 could nonautonomously induce cardiogenic differentiation cell through the action of the homeodomain transcription factor Hex. 14 Importantly, DKK1 has been proven to play a central role in bone biology. MacDonald et al. 15 indicate that the progressive DKK1 reduction increases trabecular and cortical bone mass and that even a 25% reduction in Dkk1 expression could produce significant increases in trabecular bone volume fraction in mice. In humans, DKKs are implicated in bone disease. 16, 17
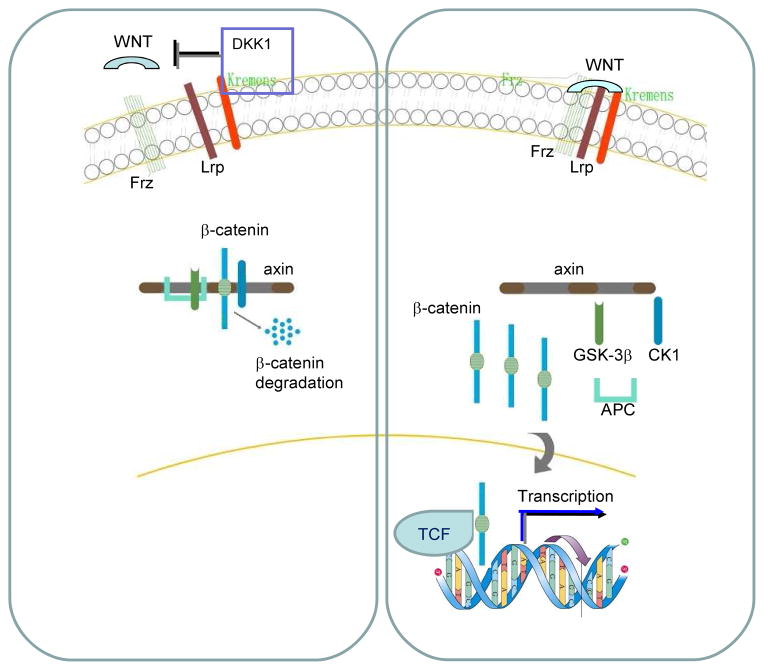
Wnt signaling plays an important role in embryonic development and tumorigenesis 6. The formation of the Wnt-Frizzled-low-density lipoprotein-related protein (LRP) complex activates Wnt/β-catenin signaling. Activating the β-catenin/T cell factor (TCF) transcription complex consequently regulates downstream target genes. In the absence of Wnt signaling, a multiprotein complex composed of axin, glycogen synthase kinase (GSK)-3β, and tumor suppressor adenomatous polyposis coli phosphorylates β-catenin, leading to β-catenin ubiquitin/proteasome-mediated degradation. The Dickkopf family of secreted inhibitors of Wnt signaling ensures proper morphological development by antagonizing different stages of the Wnt cascade. Recent new findings have demonstrated that the DKK1 protein is a classic inhibitor of the Wnt signaling pathway that typically antagonizes the Wnt/β-catenin signaling by binding to the Wnt LRP co-receptors and blocking their interactions with Wnt and the transmembrane co-receptor Frizzled. 18, 19 Moreover, Kremen proteins, which are single-pass transmembrane DKK1 receptors, synergize with DKK1 to inhibit Wnt signaling by promoting the endocytosis of LRP. 20, 21 Based on these current discoveries, a suggested loop is shown in Figure 1.
Figure 1. DKK1 inhibits Wnt signaling pathway in MBD.
DKK1 protein is a classical inhibitor of Wnt signaling pathway that typically antagonizes Wnt/beta-catenin signaling, by binding to the Wnt co-receptors Lrp and blocking their interaction with Wnt and transmembrane co-receptors Frizzled. Kremens are single-pass transmembrane DKK1 receptors that synergize with DKK1 to inhibit Wnt signaling by promoting the endocytosis of LRP. DKK1 protein act as natural Wnt antagonists by bridging LRP and Kremens, inducing the internalization of the complex. Activating the β-catenin/T cell factor (TCF) transcription complex consequently regulates downstream target genes. Thus, it is expected to negatively regulation bone formation in MBD.
Mechanisms of MBD
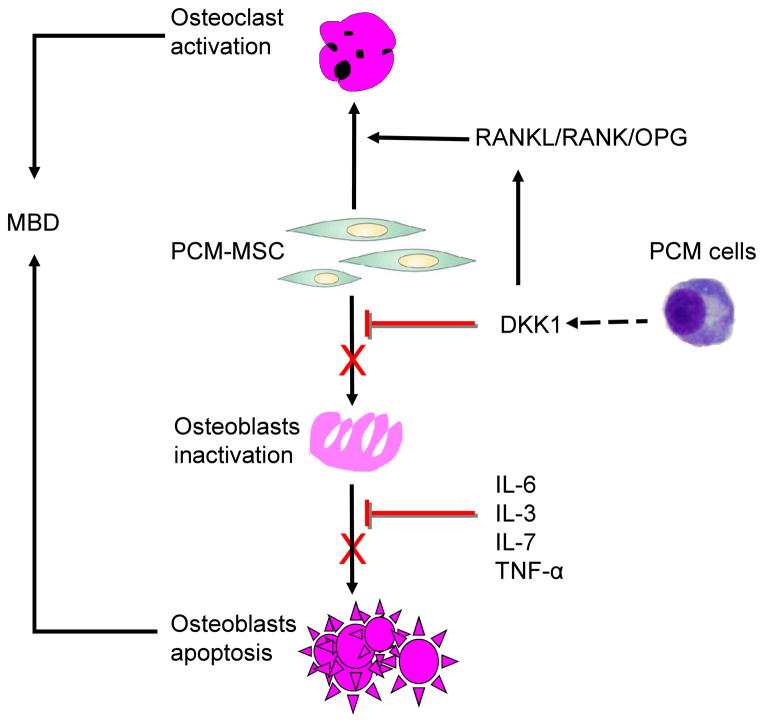
Improvement in diagnostic and therapeutic strategies in PCM requires better understanding of its underlying pathology. On the basis of current research, MBD is believed to be mediated through a mechanism related to Wnt signaling that can be inhibited by DKK1. Elevated levels of DKK1 in bone marrow, plasma, and peripheral blood are associated with the presence of osteolytic bone lesions in patients with PCM. 22 The proposed model of how the DKK1 pathway regulates MBD is illustrated in Figure 2.
Figure 2. DKK1 is involved in the mechanisms of MBD.
PCM cells inhibit osteoblasts differentiation by deregulating the WNT signaling pathway via the secretion of inhibitory cytokines DKK1. DKK1 appears to actively participate in the regulation of MBD by inhibiting osteoblasts and activating osteoclasts, which are potentially involved in myeloma-induced inhibition of osteoblast formation and differentiation.
(a) Osteogenesis
Osteoblasts are bone-forming cells in osteogenesis. Osteoblasts at various stages of maturation are essential for skeletal development, growth, and maintenance. Elucidation of the mechanisms that control osteoblast numbers is of major interest for the treatment of skeletal disorders characterized by abnormal bone formation. Osteoclasts, another type of cell involved in bone formation, remove bone tissue, a process called resorption. Recent studies have begun to elucidate the biological mechanisms involved in the osteoblast inhibition induced by PCM cells, a process crucial to the development of osteolytic lesions. Forced overexpression of Dkk1 in osteoblasts causes osteopenia, disruption of the hematopoietic stem cell niche, defects in HSC function. and also inhibits fracture repair. 23 In MBD, osteoblasts are systematically defeated by a number of inhibiting factors induced by the malignant clone within the bone marrow microenvironment. Thus, MBD primarily affects the osteoblast lineage, particularly in overt PCM, where serum markers of osteoblast genesis, such as osteocalcin and osteoprotegerin (OPG), are extremely low in contrast to their slight increase in active PCM. 24
Bone marrow mesenchymal stem cells (BM-MSCs) from PCM patients could support the survival and proliferation of myeloma stem cells and, to some extent, inhibit the formation of new bone. Gregory et al. 25 demonstrated that DKK1 is secreted by BM-MSCs and allows the cells to proliferate by inhibiting the canonical Wnt/β-catenin signaling pathway. As well, Gunn et al. 26 showed that DKK1 could enhance interleukin (IL)-6 secretion by the osteoblasts. Corre et al. 27 further confirmed that BM-MSCs are abnormal in the microenvironment of PCM and that DKK1 is secreted by PCM and normal BM-MSCs. For example, A stromal cell-dependent myeloma cell line MOLP-6 lacks the capacity to differentiate into an osteoblastic lineage. Another study found that PCM-MSCs displayed deficient growth that could be explained in part by the reduced expression of several growth factor receptors such as platelet derived growth factor on the surface of PCM-MSCs compared with these receptors’ expression on normal donors (ND)-MSCs 28. The underlying mechanism by which DKK1 interacts with osteoblasts is under intensive exploration. Li et al. 29 have evaluated the potential role of DKK1 in bone formation by examining the normal expression pattern of DKK1 in normal young mice and by assessing the consequences of osteoblast overexpression of DKK1 in transgenic mice. These investigators found that DKK1 expression restricted to osteoblasts induced severe osteopenia. The decrease in bone mass in vivo resulted from 49% fewer osteoblasts and was reflected in a 45% reduction in serum osteocalcin concentration. Thus, BM-MSCs perform an act on the cells of the osteoblast lineage.
(b) Osteoclastogenesis
Wnt/β-catenin signaling is active in osteoclasts in PCM and is involved in osteoclastogenesis in bone marrow, where it negatively regulates osteoclast formation in an osteoblast-dependent manner. Wnt/β-catenin signaling in the bone marrow microenvironment or in PCM cells clearly suppresses osteoclastogenesis and DKK1 attenuated the increases in β-catenin induced by Wnt. Hyperactivation of osteoclasts presents a major obstacle to bone repair in erosion sites owing to osteoblast impairment. An increase in the receptor activator of nuclear factor-kappa B ligand (RANKL) and a decrease in OPG in the bone marrow microenvironment stimulate osteoclast formation and activity in PCM. 30 Bone resorption, however, is promoted by early osteoblasts, namely, stromal cells that respond to chronic stimulation by myeloma cells by enhancing marrow levels of RANKL and other osteoclastogenic factors and thus accelerating the maturation of osteoclast progenitors. Because osteoblasts are derived from MSCs, they coexist in proximity to MSCs in bone during development and remodeling. 29 In addition, two factors produced by stromal-osteoblastic cells seem critical to regulating bone resorption: OPG and its osteoprotegerin ligand (OPGL). OPGL stimulates osteoclast differentiation and activity, whereas OPG inhibits these processes. Human myeloma cells upregulate OPGL expression but strongly downregulate OPG production in preosteoblastic or stromal cells of primary human bone marrow. 31
Roles of DKK1 in MBD
The Wnt signaling pathway is a network of proteins that transduce the signals from the cell-surface receptors through the cytoplasm and ultimately to the nucleus, where the signaling cascade leads to the expression of specific target genes. Wnt signaling controls cell-cell communication including cell proliferation and differentiation during development and healing. 6, 27 The activation of Wnt signaling has been implicated in the multistep tumorigenesis of myeloma.
The Wnt pathway is critical for differentiating progenitor cell lines into osteoblasts. Wnt inhibitors secreted by PCM cells inhibit osteogenesis and promote osteoclastogenesis; therefore, rapidly targeting Wnt inhibitors is essential to prevent potentially irreversible effects on the stroma. 32 DKK1 prevents MSCs from differentiating into osteoblasts and is a key player in MBD. Blocking DKK1 activity in myelomatous bones reduces osteolytic bone resorption, increases bone formation, and helps control PCM growth. Gregory et al. 33 found that the human homolog of DKK1 drives MSCs to enter the cell cycle. Importantly, an approximately five-times higher in expression of osteoblast inhibitor DKK1 is shown at both the transcript and the protein levels in PCM-MSCs compare to those in normal donors (ND-MSCs). 28
Morvan et al. 34 reported that the DKK1 protein negatively regulates osteoblasts in vitro and in vivo. Retroviral expression of DKK1 in rat primary calvarial cells resulted in a complete inhibition of osteoblast differentiation and formation of mineralized nodules, with a marked decrease in the expression of alkaline phosphatase. Structural, dynamic, and cellular analysis of bone remodeling in DKK1+/− mice showed an increase in all bone-formation parameters, with no change in bone resorption, leading to a marked increase in bone mass. Importantly, the number of osteoblasts, mineral apposition, and bone formation rate were all increased several fold. Wnt/β-catenin signaling and DKK1 have distinct functions in the stages of osteoblast differentiation 35. Several molecular genetic studies in mice have consistently confirmed the critical importance of the Wnt signaling pathway in skeletal biology and disease. Fujita et al. 36 found that DKK1 and DKK2 increased the expression of the osteoclast differentiation factors, receptor activator of RANKL, and macrophage colony-stimulating factor while downregulating the decoy receptor of RANKL OPG, all of which indicate that DKK1 facilitates osteoclastogenesis by enhancing RANKL/RANK and macrophage colony-stimulating factor/c-fms interactions. DKK1 produced by myeloma cells blocks the differentiation of osteoblasts, promotes the early proliferation of MSCs, and, subsequently, reduces the viability of MSCs. Therefore, increased DKK1 production by tumor cells in the bone may lead to focal bone loss.
BM-MSCs can promote myeloma growth and survival and osteolytic bone disease. Fowler et al. showed that BM-MSC inoculation alone induced osteoblast suppression an increase serum concentrations of DKK1. Moreover, knockdown of DKK1 expression in BM-MSCs decreased their ability to promote myeloma and the associated bone disease in mice. 37 PCM cells secrete DKK1, which prevents the MSCs from differentiating into osteoblasts, and the undifferentiated MSCs produce IL-6, which stimulates further proliferation of DKK1-secreting PCM cells. 26 DKK1 and soluble Frizzled protein 2 were identified as factors that directly stimulate osteoclastic bone destruction in myeloma, and two cytokines IL-3 and IL-7 have also been reported to directly or indirectly inhibit osteoblast differentiation in patients with myeloma. 38
Short-term exposure to low levels of DKK1 induces moderate proliferation of MSCs in vitro, whereas long-term exposure to high levels of DKK1 causes a loss of cell viability. Thus, in addition to blocking the terminal differentiation of osteoblasts, the sustained high levels of DKK1 in the bone marrow of patients with PCM may also cause a loss in viability of osteoblast stem cells. 33 Gregory et al. confirmed the secretion of DKK1 in ANBL and XG1 PCM cell lines and found that the presence of MSC-conditioned medium enhanced the production of DKK1. Using an in vitro osteogenesis assay, Gregory et al. showed that treating MSCs with DKK1 inhibited the osteogenic differentiation of the cells. Recombination DKK1 and plasma from PCM patients containing high levels of DKK1 blocked Wnt3a-induced β-catenin accumulation. Importantly, DKK1 abrogated bone morphogenic protein-2 mediated osteoblast differentiation. 30 Autocrine Wnt signaling in osteoblasts is required to promote bone morphogenic protein-2-mediated differentiation of preosteoblast cells. DKK1 inhibits this process and may be a key factor in regulating preosteoblast differentiation and MBD. 39
In addition to its direct inhibitory effect on osteoblasts, DKK1 disrupts the expression of Wnt3a-regulated OPG and of the receptor activator of RANKL in osteoblasts, and thus it indirectly enhances osteoclast function in PCM. 40 Several clinical studies have shown the relationship between DKK1 and MBD. Tian et al. 7 first reported the high level of expression of the Wnt inhibitor DKK1 in PCM cells as compared with plasma cells from patients with monoclonal gammopathy of undetermined significance (MGUS)or with normal plasma cells and suggested that it may affect osteogenesis. Gunn et al. 26 confirmed the secretion of DKK1 in two PCM cell lines. Similar results were described by Terpos et al. 41
For clinical research, more persuasive evidence elucidates the relationship between DKK1 and MBD. Tian et al. 7 demonstrated that DKK1 is produced at high levels by myeloma cells from patients with osteolytic lesions, indicating that DKK1 inhibits differentiation of bone marrow stromal cells into osteoblasts. Giuliani et al. 42 confirmed that fresh purified multiple myeloma cells secrete DKK-1 and the bone marrow plasma DKK1 levels in PCM patients are much higher than levels in patients with MGUS or in healthy donors. Moreover, bone marrow plasma DKK1 levels were correlated with myeloma staging according to the Durie-Salmon system. One study analyzed a large series of 184 previously untreated PCM patients and showed for the first time that DKK1 serum concentration significantly correlates with the number of osteolytic bone lesions 22. These results were confirmed by Politou et al. 43, who showed that DKK1 protein is detectable in serum samples and that DKK1 serum concentrations are higher in myeloma patients than in patients with monoclonal gammopathy of undetermined significance (MGUS) patients and controls. Interestingly, Haaber et al. 44 showed that overexpression of DKK1 was significantly correlated to the degree of osteolytic bone disease in PCM patients. DKK1 serum and bone marrow plasma levels are increased in PCM patients and correlated with advanced International Staging System stage and with the presence of osteolytic lesions. 39 In addition, a DNA single nucleotide polymorphism (SNP) chip containing 3404 SNPs was used to test genomic DNA from myeloma patients classified by the extent of bone disease, and multivariate regression analysis displayed that DKK1 expression correlates with bone disease. 45 Bisphosphonates are a well-established treatment of myeloma-related skeletal disease and are the current standard of care. However, even with effective bisphosphonate treatment, a significant proportion of patients with high level expression of DDK1 still developing MBD. 46
Recent advances in targeting DKK1 for treating MBD
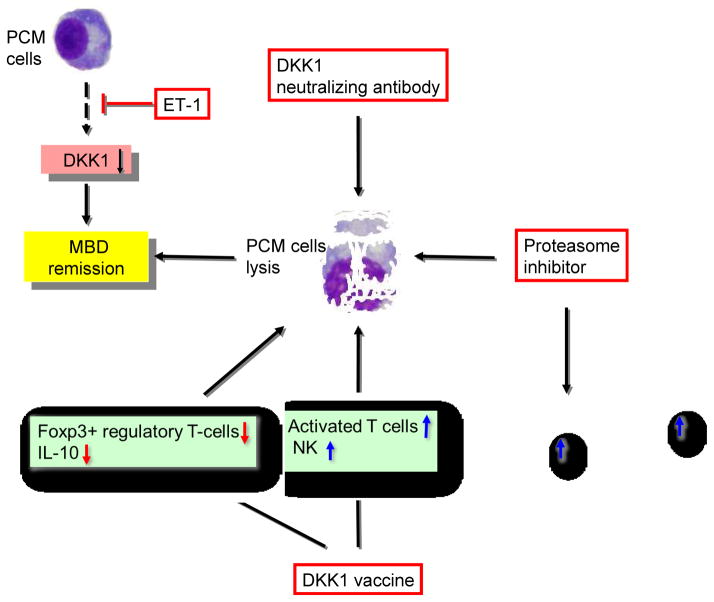
Many studies have confirmed that MBD results from complex interactions between myeloma cells and the bone marrow microenvironment, characterized by osteoclast activation and osteoblast inhibition. Therefore, DKK1 provides a potential therapeutic target to stimulate bone formation. Several effective therapies targeting DKK1 to treat MBD and to control myeloma development and progression have been developed, including neutralizing inhibitors of Wnt signaling, proteasome inhibitors, DKK1 peptide vaccines, and tumor-produced endothelin-1 (ET-1). Recently, several observations derived from the trials suggested a potential role for these agents as direct anti-myeloma drugs (Figure 3).
Figure 3. DKK1-targeted therapies in MBD.
DKK1 secreted by myeloma cells provides us a potentially therapeutic means of intervention in drug discovery. DKK1 targeted therapies, including DKK1 neutralizing antibodies, proteasome inhibitor, vaccine and regulatory factor (e.g. ET-1), have been carried out and all of them have shown many curative clinical benefits on MBD.
(a) DKK1-neutralizing antibodies
DKK1 serum and bone marrow plasma levels are increased in PCM patients and are correlated with advanced International Staging System stage and the presence of osteolytic lesions. Anti-DKK1 strategies are clinically relevant since high serum levels of DKK1 are thought to contribute to osteolytic lesion formation in PCM. 38 Heider et al. 4 found that DKK1 levels significantly decreased only in patients who had complete remission or partial remission, suggesting that myeloma cells are the main source of circulating DKK1 protein; this finding provides a framework for clinical trials of anti-DKK1 therapies. A study carried out by Yaccoby et al. 47 found that bone mineral density of the implanted myelomatous bone decreased in control mice but increased in mice treated with DKK1-neutralizing antibodies for 4–6 weeks. Moreover, myelomatous bones of anti-DKK1-treated mice had more osteocalcin-expressing osteoblasts and fewer multinucleated tartrate-resistant acid phosphatase-expressing osteoclasts than did controls. Antibodies that block DKK1 and glycogen synthase kinase-3β inhibitors also provide novel opportunities for the enhancement of bone repair in PCM. 7 The bone anabolic effect of anti-DKK1 is associated with reduced myeloma burden, showing that DKK1 plays a pivotal role in bone health and the management of MBD.
Many DKK1 antibodies have emerged, and most of them show encouraging results in PCM cell lines, in both preclinical and clinical trials. Fulciniti et al. 48 showed hat DKK1-neutralizing antibody BHQ880 increased osteoblast differentiation, neutralized the negative effect of PCM cells on osteoblastogenesis, and reduced IL-6 secretion. Furthermore, BHQ880 significantly inhibited growth of PCM cells, upregulated β-catenin levels and downregulated NF-κB activity in the presence of bone marrow stromal cells. In addition, Heath et al. 49 reported that injecting murine 5T2MM myeloma cell lines that expressed DKK1 into C57BL/KaLwRij mice resulted in the development of osteolytic bone lesions, fewer osteoblasts, and less mineralizing surface. However, BHQ880 reduced trabecular bone loss and prevented the development of MBD. Furthermore, Pozzi et al. 50 found that anti-DKK1 monoclonal antibodies reduced tumor growth and induced a bone anti-catabolic effect in vivo and in vitro. Similarly, Zhao et al. 51 concluded that anti-DKK1 antibodies effectively treat MBD. All these results confirm DKK1 as an important therapeutic target in myeloma and provide the rationale for clinical evaluation of DKK1 antibodies to treat bone disease.
(b) Bortezomib
Proteasome-inhibition therapy in PCM may function by subverting tumor-induced suppression of canonical Wnt signaling in the bone microenvironment. Bortezomib is a novel proteasome inhibitor with anti-tumor efficacy in PCM. Bortezomib promotes matrix mineralization and calcium deposition by osteoprogenitor cells and primary MSCs via Wnt signaling. Since bortezomib was approved by the United States Food and Drug Administration, many clinical therapeutic strategies have used it as one of the most important components in treatment regimens 52. Recent studies have shown that bortezomib not only effectively kills PCM cells but also significantly induces osteoblast differentiation and inhibits the progression of osteolytic lesions in patients with MBD. 53 However, the underlying mechanism by which bortezomib repairs bone damage remains unclear. Bortezomib has been shown to enhance new bone formation in mouse calvarial cultures, an effect that could be blocked by DKK1. Bortezomib inhibited DKK1 expression in calvarial cells and BMSCs, suggesting a novel mechanism by which bortezomib exerts its effects in bone. 54 In addition, bortezomib administration significantly reduced serum DKK1 levels and dramatically increased bone alkaline phosphatase and osteocalcin in relapsed myeloma patients. Moreover, remission rates in refractory and relapse patients were significantly improved. 55
(c) DKK1 vaccines
In immunotherapeutic strategies, active immunity still remains the most important and effective mechanism to control PCM progression. DKK1 is a good candidate for developing an PCM peptide vaccine. 56 Qian et al. 57 identified and synthesized DKK1 peptides for human leukocyte antigen (HLA)-A*0201 and confirmed their immunogenicity by in vivo immunization of HLA-A*0201 transgenic mice. DKK1 peptide-specific CD8-positive (+) T cells efficiently lysed DKK1+/HLA-A*0201+ myeloma cell lines U266 and IM-9 and, more importantly, HLA-A*0201+ primary myeloma cells from patients. A further study proved that the DKK1 DNA vaccine not only protected mice in the MOPC-21 myeloma model from developing myeloma but also had a therapeutic effect against established myeloma. These investigators found that the DKK1 vaccine elicited strong DKK1- and tumor-specific CD4+ and CD8+ immune responses and that treatment with B7H1 or OX40 antibodies significantly reduced the numbers of IL-10-expressing and Foxp3+ regulatory T cells in vaccinated mice. 5
(d) Tumor-produced ET-1
Tumor-produced endothelin-1 (ET-1), a 21-amino acid peptide, was first identified as a potent vasoconstrictor.58, 59 ET-1 stimulates osteoblasts to form new bone and is an important mediator of osteoblastic bone metastasis. Clines et al 60 found that ET-1 directed nuclear translocation of beta-catenin in osteoblasts, and ET-1 stimulates osteoblast activity by reducing autocrine production of DKK1.
Conclusion
To date, PCM is still considered a fatal disease, and relapse rates in patients remain high regardless of the intensity of treatment. MBD, a common complication of PCM, results in a significant morbidity and an impaired quality of life. Increased osteoclastic bone resorption and decreased osteoblastic bone formation result in impaired bone formation, which contributes to the difficulty in bone healing in PCM. In recent years, DKK1 has become a promising area in research in the mechanisms and molecular biology of MBD (Table 1). The advancements of DKK1 pave the way for further exciting discoveries that lie ahead. DKK1 may play a key role in the development of MBD by directly interrupting Wnt-regulated differentiation of osteoblasts, whereas blocking DKK1 activity in myelomatous bones reduces osteolytic bone resorption, increases bone formation, and helps control PCM growth. Regulating DKK1 function or expression may have therapeutic significance in the management of low bone mass disorders. Ongoing, targeted therapies based on this mechanism have achieved some encouraging results in MBD.
Table 1.
DKK1 features in MBD.
| Features | Details | References |
|---|---|---|
| Similarity | Belongs to the Dickkopf family. | 12 |
| Location | 10q21.1 | 16 |
| Protein | Size 266aa Signal peptide 1 to 24 aa, Low complexity region 45 to 57 aa, Dickkopf domain 84 to 140 aa |
11, 12 |
| Derivation | DKK1 is secreted by PCM and normal BM-MSCs. |
25 27 42 |
| Elevated levels | Bone marrow Plasma PCM cell lines Peripheral blood |
7 16, 17 22 39 42 43 44 45 |
| Protein binding | DKK1 interacts with LRP5/6; Antagonizes canonical Wnt signaling by inhibiting LRP5/6 interaction with Wnt and by forming a ternary complex with the transmembrane protein Kremens that promotes internalization of LRP5/6. Transmembrane proteins Kremen1 and Kremen2 are high-affinity Dkk1 receptors that functionally cooperate with Dkk1 to block Wnt/beta-catenin signaling. |
2 18, 19 20, 21 |
| Signal transduction | Dkk-1 directly interrups Wnt-regulated differentiation of osteoblasts and indirectly enhances osteoclast function via a DKK1-mediated increase in RANKL-to-OPG ratios in PCM. |
30 39 40 |
| Function | Forced overexpression of Dkk1 in osteoblasts causes osteopenia, disruption of the hematopoietic stem cell niche, defects in HSC function. and also inhibits fracture repair. Knockdown of DKK1 expression in BM-MSCs decreased their ability to promote myeloma and the associated bone disease in mice. |
5–7 23 37 |
Practice points.
The mechanisms and molecular biology of MBD should be considered to determine the choice of regimen.
Targeting DKK1 is likely to trigger anti-myeloma effects.
A series of works is still needed to clarify the mechanism underlying this observed effect
Ongoing clinical studies will help elucidate the efficacy of anti-DKK1 therapy.
New Strategies will be cost-effective in patients with MBD by targeting DKK1.
Research agenda.
DKK1 is involved in the development of MBD through its inhibition of the Wnt signaling pathway.
DKK1 participates in regulating MBD by inhibiting osteoblasts and by activating osteoclasts.
Relationship between over-expression of DKK1 and clinical outcomes in patients with MBD.
Dkk1 is a suitable target for the management of MBD.
Acknowledgments
This project was supported by a grant from the National Natural Science Foundation of China (No. 81172257 to FZ); the Xi’an Fundamental Research Funds of China (HM1117-4 to FZ); the National Cancer Institute (RO1-CA90853 to FXC); and the National Institutes of Health through The University of Texas MD Anderson Cancer Center Support Grant (CA016672). We thank Markeda L. Wade and Ann Sutton for editing this manuscript.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Robbiani DF, Chesi M, Bergsagel PL. Bone lesions in molecular subtypes of multiple myeloma. N Engl J Med. 2004;351:197–8. doi: 10.1056/NEJM200407083510223. [DOI] [PubMed] [Google Scholar]
- 3.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–81. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 4.Heider U, Kaiser M, Mieth M, Lamottke B, Rademacher J, Jakob C, et al. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur J Haematol. 2009;82:31–8. doi: 10.1111/j.1600-0609.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 5.Qian J, Zheng Y, Zheng C, Wang L, Qin H, Hong S, et al. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood. 2012;119:161–9. doi: 10.1182/blood-2011-07-368472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 7.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 8.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–5. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 9.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–42. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cianferotti L, Demay MB. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem. 2007;101:80–8. doi: 10.1002/jcb.21151. [DOI] [PubMed] [Google Scholar]
- 11.Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–72. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 12.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–34. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 14.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–96. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–9. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckland J. Rheumatoid arthritis: Genetic variants in DKK1 linked to severity of joint damage in RA. Nat Rev Rheumatol. 2012;8:694. doi: 10.1038/nrrheum.2012.189. [DOI] [PubMed] [Google Scholar]
- 18.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L, et al. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol. 2011;18:1204–10. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 21.Cselenyi CS, Lee E. Context-dependent activation or inhibition of Wnt-beta-catenin signaling by Kremen. Sci Signal. 2008;1:pe10. doi: 10.1126/stke.18pe10. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80:490–4. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 23.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–25. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvestris F, Lombardi L, De Matteo M, Bruno A, Dammacco F. Myeloma bone disease: pathogenetic mechanisms and clinical assessment. Leuk Res. 2007;31:129–38. doi: 10.1016/j.leukres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–78. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- 26.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–91. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 27.Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–88. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garderet L, Mazurier C, Chapel A, Ernou I, Boutin L, Holy X, et al. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leuk Lymphoma. 2007;48:2032–41. doi: 10.1080/10428190701593644. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–33. doi: 10.1182/blood.v98.13.3527. [DOI] [PubMed] [Google Scholar]
- 32.Gunn WG, Krause U, Lee N, Gregory CA. Pharmaceutical inhibition of glycogen synthetase kinase-3beta reduces multiple myeloma-induced bone disease in a novel murine plasmacytoma xenograft model. Blood. 2011;117:1641–51. doi: 10.1182/blood-2010-09-308171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory CA, Gunn WG, Reyes E, Smolarz AJ, Munoz J, Spees JL, et al. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005;1049:97–106. doi: 10.1196/annals.1334.010. [DOI] [PubMed] [Google Scholar]
- 34.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 35.van der Horst G, van der Werf SM, Farih-Sips H, van Bezooijen RL, Lowik CW, Karperien M. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res. 2005;20:1867–77. doi: 10.1359/JBMR.050614. [DOI] [PubMed] [Google Scholar]
- 36.Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowler JA, Mundy GR, Lwin ST, Edwards CM. Bone marrow stromal cells create a permissive microenvironment for myeloma development: a new stromal role for Wnt inhibitor Dkk1. Cancer Res. 2012;72:2183–9. doi: 10.1158/0008-5472.CAN-11-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roodman GD. New potential targets for treating myeloma bone disease. Clin Cancer Res. 2006;12:6270s–3s. doi: 10.1158/1078-0432.CCR-06-0845. [DOI] [PubMed] [Google Scholar]
- 39.Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD., Jr Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42:669–80. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Gavriatopoulou M, Dimopoulos MA, Christoulas D, Migkou M, Iakovaki M, Gkotzamanidou M, et al. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets. 2009;13:839–48. doi: 10.1517/14728220903025770. [DOI] [PubMed] [Google Scholar]
- 41.Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–92. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 42.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, et al. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67:7665–74. doi: 10.1158/0008-5472.CAN-06-4666. [DOI] [PubMed] [Google Scholar]
- 43.Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119:1728–31. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 44.Haaber J, Abildgaard N, Knudsen LM, Dahl IM, Lodahl M, Thomassen M, et al. Myeloma cell expression of 10 candidate genes for osteolytic bone disease. Only overexpression of DKK1 correlates with clinical bone involvement at diagnosis. Br J Haematol. 2008;140:25–35. doi: 10.1111/j.1365-2141.2007.06871.x. [DOI] [PubMed] [Google Scholar]
- 45.Durie BG, Van Ness B, Ramos C, Stephens O, Haznadar M, Hoering A, et al. Genetic polymorphisms of EPHX1, Gsk3beta, TNFSF8 and myeloma cell DKK-1 expression linked to bone disease in myeloma. Leukemia. 2009;23:1913–9. doi: 10.1038/leu.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu P, Walker BA, Brewer D, Gregory WM, Ashcroft J, Ross FM, et al. A gene expression-based predictor for myeloma patients at high risk of developing bone disease on bisphosphonate treatment. Clin Cancer Res. 2011;17:6347–55. doi: 10.1158/1078-0432.CCR-11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–9. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Jr, Evans HR, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24:425–36. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 50.Pozzi S, Fulciniti M, Yan H, Vallet S, Eda H, Patel K, et al. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone. 2013;53:487–96. doi: 10.1016/j.bone.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Kim KA, Abo A. Tipping the balance: modulating the Wnt pathway for tissue repair. Trends Biotechnol. 2009;27:131–6. doi: 10.1016/j.tibtech.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Qiang YW, Heuck CJ, Shaughnessy JD, Jr, Barlogie B, Epstein J. Proteasome inhibitors and bone disease. Semin Hematol. 2012;49:243–8. doi: 10.1053/j.seminhematol.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, et al. Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–30. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Munoz S, et al. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol. 2007;139:434–8. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patriarca F, Prosdocimo S, Tomadini V, Vasciaveo A, Bruno B, Fanin R. Efficacy of bortezomib therapy for extramedullary relapse of myeloma after autologous and non-myeloablative allogeneic transplantation. Haematologica. 2005;90:278–9. [PubMed] [Google Scholar]
- 56.Zhou FL, Meng S, Zhang WG, Wei YC, Cao XM, Bai GG, et al. Peptide-based immunotherapy for multiple myeloma: current approaches. Vaccine. 2010;28:5939–46. doi: 10.1016/j.vaccine.2010.06.088. [DOI] [PubMed] [Google Scholar]
- 57.Qian J, Xie J, Hong S, Yang J, Zhang L, Han X, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110:1587–94. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin ER. Endothelins. N Engl J Med. 1995;333:356–63. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 59.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 60.Clines GA, Mohammad KS, Bao Y, Stephens OW, Suva LJ, Shaughnessy JD, Jr, et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol. 2007;21:486–98. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]