TO THE EDITOR
Insulin analogs are molecularly altered forms of insulin. Compared with human synthetic and animal insulin for type 2 diabetes, short-acting insulin analogs may offer flexible dosing and convenience, long-acting insulin analogs less nocturnal hypoglycemia,1 but both at 2–4 times the cost.2 As insulin analogs have become increasingly popular,3, 4 we examined trends in insulin utilization, out-of-pocket expenditures, and concurrent trends in severe hypoglycemic events among privately insured U.S. adults with type 2 diabetes, from 2000 through 2010.
METHODS
We conducted a retrospective analysis of data from Optum Labs Data Warehouse, an administrative claims database of privately insured enrollees from throughout the U.S., but with more representation from the South and Midwest. Because this study involved analysis of pre-existing, de-identified data, it was exempt from Institutional Review Board approval. Our sample included adults aged ≥18 years with type 2 diabetes mellitus with at least 2 years of continuous enrollment between January 2000 and September 2010. We defined diabetes according to Health Plan Employer Data and Information Set criteria and excluded patients with claims for type 1 diabetes in the absence of oral antihyperglycemic medications. First, we calculated the proportion of patients with type 2 diabetes who used any insulin in each year and characterized insulin users through descriptive analyses. Second, we calculated the proportion of patients who obtained each specific insulin type. We used the Cochran-Armitage test to assess for trends in insulin use. Third, we calculated median out-of-pocket costs of insulin associated with each insulin prescription per year (adjusted to 2010 U.S. dollars5). We compared median out-of-pocket costs across years using quantile regression. Finally, among insulin users, we examined age-sex adjusted rates of severe hypoglycemic events, defined as hospitalization or emergency department visit with a primary discharge diagnosis of hypoglycemia.6 For all analyses two-sided p-value <0.05 was considered significant. All analyses were performed using SAS® statistical software (version 9.2; SAS Institute Inc., Cary, North Carolina).
RESULTS
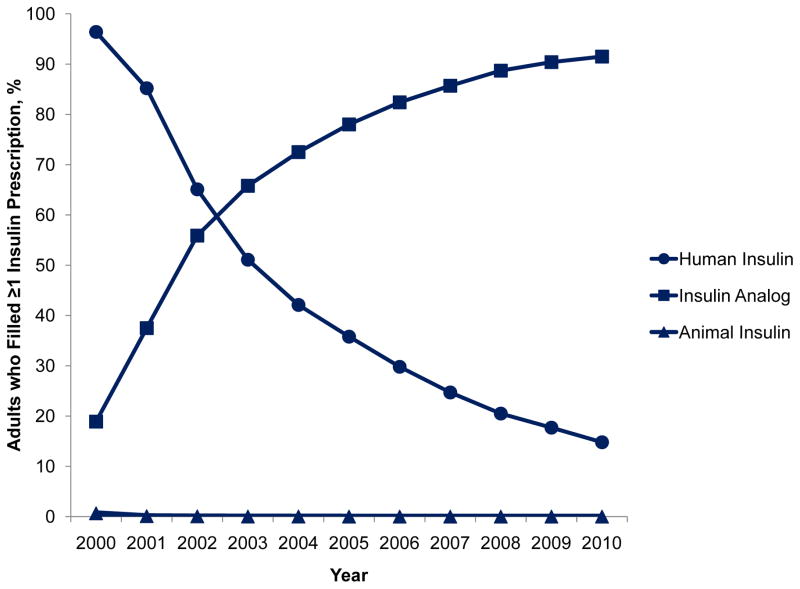
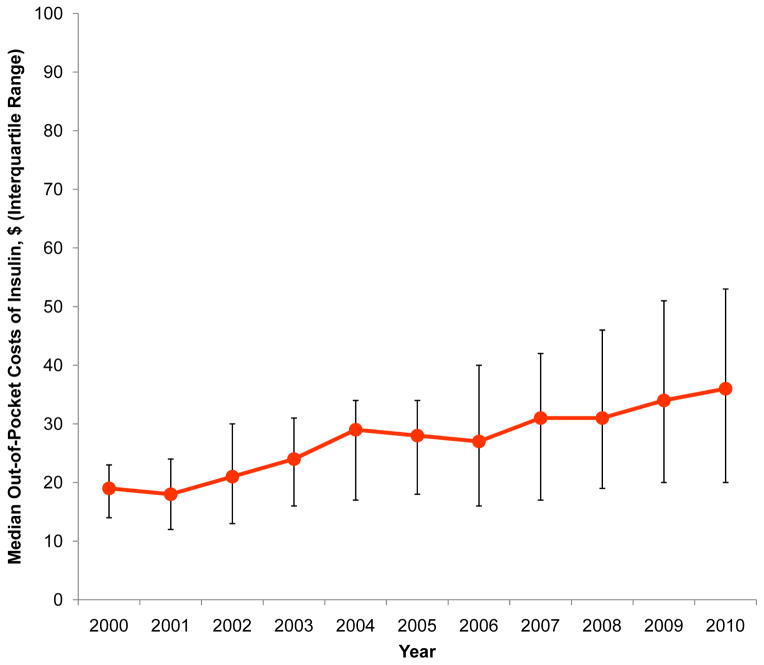
Between 2000 and 2010, 123,486 unique patients filled at least 1 prescription for insulin, comprising 9.7% (95% CI, 9.5–9.8%) of adults with type 2 diabetes in 2000 and 15.1% (95% CI, 15.0–15.3%) in 2010 (p=0.001). Characteristics of the study sample are presented in the Table. Among adults who used insulin, 96.4% (95% CI, 96.0–96.8%) filled prescriptions for human synthetic insulin in 2000 but only 14.8% (95% CI, 14.5–15.2%) did so in 2010 (p<0.0001). In contrast, 18.9% (95% CI, 18.2–19.7%) filled prescriptions for insulin analogs in 2000 but 91.5% (95% CI, 91.2–91.8%) did so in 2010 (p<0.0001). Use of animal insulin was <1% in all years. Median out of pocket costs per prescription for all insulins increased from $19 (IQR, $14–23) in 2000 to $36 (IQR, $20–53) in 2010 (p<0.0001). These trends were accompanied by a small decline in the rate of severe hypoglycemic events among insulin users that was not statistically significant (21.1 and 17.7 events per 1,000 person-years in 2000 and 2010, respectively, p=0.054).
Table.
Characteristics of patients with type 2 diabetes mellitus who were dispensed insulin, 2000–2010.
| 2000–2001 | 2002–2003 | 2004–2005 | 2006–2007 | 2008–2010 | |
|---|---|---|---|---|---|
| Number of Patients | 21,475 | 35,457 | 48,625 | 58,370 | 63,962 |
| Demographics | |||||
| Age, years mean (SD) | 64.4 (11.7) | 63.2 (12.1) | 62.8 (12.4) | 61.8 (12.5) | 61.2 (12.4) |
| Sex, % female | 48.2 | 47.5 | 47.0 | 45.9 | 45.1 |
| Race, % | |||||
| Asian | 1.1 | 1.5 | 1.7 | 1.7 | 1.9 |
| Black/African American | 8.2 | 10.1 | 10.4 | 9.9 | 9.6 |
| Hispanic | 4.6 | 7.0 | 8.0 | 8.6 | 9.1 |
| White/Caucasian | 48.3 | 61.3 | 63.5 | 62.0 | 61.4 |
| Missing/Other | 37.7 | 20.1 | 16.4 | 17.8 | 18.0 |
| Geographic Region, % | |||||
| Northeast | 5.7 | 7.2 | 8.0 | 8.1 | 8.3 |
| Midwest | 38.6 | 34.5 | 32.2 | 29.5 | 27.7 |
| South | 47.8 | 49.0 | 49.4 | 50.8 | 52.3 |
| West | 7.5 | 9.0 | 10.2 | 11.4 | 11.5 |
| Missing/Other | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 |
| Comorbidities, % | |||||
| Cardiovascular Disease | 26.8 | 28.4 | 28.7 | 28.8 | 28.0 |
| Chronic Lung Disease | 11.3 | 12.1 | 12.6 | 13.0 | 13.0 |
| Chronic Kidney Disease | 8.7 | 10.7 | 12.4 | 12.9 | 13.7 |
| Cancer | 6.8 | 7.4 | 8.1 | 8.7 | 9.5 |
| Depression | 9.5 | 10.4 | 10.2 | 10.7 | 11.2 |
| Hypertension | 57.0 | 63.2 | 68.3 | 72.3 | 76.2 |
| Hyperlipidemia | 48.3 | 58.5 | 66.48 | 71.7 | 75.1 |
| Type of Insulin,* % (95% CI) | |||||
| Human Insulin | 88.2 (87.8, 88.7) | 59.4 (58.9, 59.9) | 41.0 (40.6, 41.5) | 29.4 (29.0, 29.8) | 21.5 (21.2, 21.8) |
| Insulin Analog | 35.6 (35.0, 36.3) | 64.8 (64.3, 65.3) | 77.1 (76.7, 77.5) | 85.1 (84.8, 85.4) | 91.0 (90.8, 91.3) |
| Animal Insulin | 0.39 (0.30, 0.47) | 0.05 (0.02, 0.07) | 0.02 (0.01, 0.03) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) |
| Type of Insulin Agent, % (95% CI) | |||||
| NPH | 81.9 (81.4, 82.5) | 52.8 (52.2, 53.3) | 34.5 (34.1, 35.0) | 23.1 (22.8, 23.5) | 16.2 (16.0, 16.5) |
| Regular | 58.5 (57.9, 59.2) | 40.0 (39.5, 40.5) | 28.6 (28.2, 29.0) | 21.2 (20.9, 21.6) | 15.5 (15.3, 15.8) |
| Lente | 2.1 (1.9, 2.3) | 1.1 (1.0, 1.2) | 0.57 (0.50, 0.63) | 0.11 (0.09, 0.14) | 0.0 (0.0, 0.0) |
| Ultralente | 2.7 (2.5, 2.9) | 1.1 (1.0, 1.3) | 0.48 (0.41, 0.54) | 0.07 (0.05, 0.09) | 0.0 (0.0, 0.0) |
| Glargine | 11.3 (10.8, 11.7) | 44.1 (43.6, 44.6) | 58.4 (58.0, 58.9) | 63.2 (62.8, 63.6) | 64.3 (63.9, 64.6) |
| Detemir | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 7.3 (7.1, 7.5) | 16.4 (16.1, 16.7) |
| Aspart | 0.36 (0.28, 0.44) | 8.6 (8.3, 8.9) | 21.4 (21.0, 21.8) | 28.4 (28.0, 28.7) | 32.4 (32.1, 32.8) |
| Lispro | 29.5 (28.9, 30.1) | 34.3 (33.8, 34.8) | 27.8 (27.4, 28.2) | 21.0 (20.7, 21.3) | 20.1 (19.8, 20.4) |
| Glulisine | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.00 (0.0, 0.0) | 1.7 (1.6, 1.8) | 2.5 (2.3, 2.6) |
Human insulins include: neutral protamine Hagedorn (NPH), regular, insulin zinc human (lente), insulin zinc human extended (ultralente); insulin analogs include: aspart, detemir, glargine, glulisine, lispro; and animal insulins include: insulin isophane beef-pork, insulin isophane pork pure, insulin regular beef-pork, insulin zinc beef-pork, insulin zinc pork-purified.
DISCUSSION
In our study of privately insured adults in the U.S., use of insulin among patients with type 2 diabetes mellitus increased by approximately 50% -- from 10% in 2000 to 15% in 2010 – which occurred in the context of widespread adoption of insulin analogs. Out-of-pocket expenditures increased significantly by 89%. Concurrently, severe hypoglycemic events declined slightly but this was not statistically significant.
Our study has some limitations. First, we examined insulin use only among privately insured patients; public healthcare systems with strong formularies may utilize insulin analogs to a lesser extent. Second, we had no information on total expenditures on insulin and may have underestimated the total cost to the healthcare system. Additionally, we could not account for the use or cost of insulin delivery devices (except for prefilled pens). Finally, we could not identify hypoglycemia that did not require medical assistance. Although we found a non-significant decline in severe hypoglycemia, our analyses may be underpowered and we cannot exclude changes in less severe hypoglycemic events.
In conclusion, we found a dramatic increase in the use of insulin analogs among privately insured patients with type 2 diabetes mellitus. The value of the nearly universal transition to this more expensive type of insulin is unclear.
Figure.
Utilization and median out-of-pocket expenditures on human, animal, and analog insulin among type 2 diabetes patients who filled at least 1 prescription for insulin, 2000–2010.
Acknowledgments
The authors would like to thank Harlan Krumholz, MD, SM in the Department of Medicine, Yale University School of Medicine, New Haven, Connecticut and Victor Montori, MD, MSc in the Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, Rochester, Minnesota for their valuable comments on an earlier draft. No compensation was received.
Funding/Support: This project was not supported by any external grants or funds. Dr. Lipska receives support from the Centers of Medicare and Medicaid Services (CMS) to develop ambulatory care performance measures and she is supported by the Pepper Center Career Development Award (P30 AG21342), Grants for Early Medical/Surgical Specialists’ Transition to Aging Research (R03 AG045086) from the National Institute on Aging, and the Yale Center for Clinical Investigation Scholar Award (CTSA Grant Number UL1 TR000142). Dr. Ross receives support from Medtronic, Inc. and Johnson and Johnson, Inc. to develop methods of clinical trial data sharing, from the CMS to develop and maintain performance measures that are used for public reporting, and from the Food and Drug Administration (FDA) to develop methods for post-market surveillance of medical devices. Dr. Ross is supported by the National Institute on Aging (K08 AG032886) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Ross reports that he is a member of a scientific advisory board for FAIR Health, Inc.
Author Contributions: Dr. Shah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lipska, Shah, Ross, Yudkin.
Acquisition of data: Shah.
Analysis and interpretation of data: Lipska, Ross, Van Houten, Shah.
Drafting of the manuscript: Lipska.
Critical revision of the manuscript for important intellectual content: Lipska, Ross, Van Houten, Beran, Yudkin, Shah.
Statistical analysis: Van Houten.
Obtained funding: Not applicable.
References
- 1.Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A. Long-acting insulin analogues versus nph insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane database of systematic reviews (Online) 2007:CD005613. doi: 10.1002/14651858.CD005613.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Gale EA. Newer insulins in type 2 diabetes. BMJ (Clinical research ed) 2012;345:e4611. doi: 10.1136/bmj.e4611. [DOI] [PubMed] [Google Scholar]
- 3.Gill GV, Yudkin JS, Keen H, Beran D. The insulin dilemma in resource-limited countries. A way forward? Diabetologia. 2011;54:19–24. doi: 10.1007/s00125-010-1897-3. [DOI] [PubMed] [Google Scholar]
- 4.Holden SE, Poole CD, Morgan CL, Currie CJ. Evaluation of the incremental cost to the national health service of prescribing analogue insulin. BMJ open. 2011;1:e000258. doi: 10.1136/bmjopen-2011-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consumer price indexes for major expenditure classes 1965–2009. 2012 [Google Scholar]
- 6.Ginde AA, Blanc PG, Lieberman RM, Camargo CA., Jr Validation of icd-9-cm coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]