Abstract
Surface disinfection of fertilized fish eggs is widely used in aquaculture to reduce extraovum pathogens that may be released from brood fish during spawning, and this is routinely used in zebrafish (Danio rerio) research laboratories. Most laboratories use approximately 25 – 50 ppm unbuffered chlorine solution for 5 – 10 min. Treatment of embryos with chlorine has significant germicidal effects for many Gram-negative bacteria, viruses, and trophozoite stages of protozoa, it has reduced efficacy against cyst or spore stages of protozoa and certain Mycobacterium spp. Therefore, we evaluated the toxicity of unbufferred and buffered chlorine solution to embryos exposed at 6 or 24 hours post-fertilization (hpf) to determine if higher concentrations can be used for treating zebrafish embryos. Most of our experiments entailed using an outbred line (5D), with both mortality and malformations as endpoints. We found that 6 hpf embryos consistently were more resistant than 24 hpf embryos to the toxic effects of chlorine. Chlorine is more toxic and germicidal at lower pHs, and chlorine causes elevated pH. Consistent with this, we found that unbufferred chlorine solutions (pH ca 8–9) were less toxic at corresponding concentrations than solutions buffered to pH 7. Based on our findings here, we recommend treating 6 hpf embryos for 10 min and 24 hpf for 5 min with unbuffered chlorine solution at 100 ppm. One trial indicated that AB fish, a popular outbred line, are more susceptible to toxicity than 5Ds. This suggests that variability between zebrafish lines occurs, and researchers should evaluate each line or strain under their particular laboratory conditions for selection of the optimum chlorine treatment procedure.
Keywords: Danio, rerio, zebrafish, chlorine, mortality, malformations
Introduction
Surface disinfection of fertilized fish eggs has been widely used in aquaculture to reduce or eliminate extraovum pathogens that may be released from brood fish during spawning. This is an integral step used by aquaculturists in attempt to avoid maternal transmission. Iodine is the disinfectant of choice for salmonids and other species whereas chlorine is most often used with zebrafish. Most zebrafish research laboratories disinfect the embryos with chlorine with new introductions to a facility, and some use this procedure on a routine basis with each spawn. Common concentrations and doses used by most zebrafish laboratories are between 25–50 ppm for about 10 min (Westerfield 2007; Harper and Lawrence 2011). These regimens were established without extensive evaluation of the lethality and toxicity of chlorine. The germicidal properties of chlorine are reduced at higher pH levels, and addition of chlorine to water raises the pH of water (Clarke et al. 1989). These standard protocols used in zebrafish laboratories do not include buffering the chlorine solutions.
These treatment regimens that are presently used should be effective at significantly reducing the viability of most Gram negative bacteria (Goñi-Urriza, et.al. 2000), and trophozoite stages of protozoa (Vaerewijck et al. 2012). However, these concentrations would be less effective for protozoan spores, cysts, and helminth eggs. For example Pseudoloma neurophilia (Microsporidia) is the most common infectious agent seen in zebrafish colonies, and Ferguson et al. (2007) showed that 25 and 50 ppm of chlorine in which the solution is not pH adjusted (as used by most laboratories) killed only 40 and 60 % of the spores of this microsporidium, respectively. There have been numerous studies evaluating the toxicity of chlorine to fish embryos and larvae (Johnson et al. 1977; Morgan et al. 1977). These studies involved longer exposure times (e.g. 24 – 48 h), and resulted in LC50 concentrations considerably lower than 25 to 50 ppm.
Here we evaluated the toxicity of chlorine to zebrafish embryos exposed to chlorine solution at levels and durations routinely used by zebrafish researchers. We also conducted experiments in which the solutions were adjusted to pH 7 or not adjusted, and used mortality and malformations in the fish as endpoints.
Materials and Methods
Fish
All exposure studies were conducted at the Sinnhuber Aquatic Research Laboratory (SARL), Oregon State University, using an outbred wild type zebrafish line referred to as 5D. Embryos were obtained from large group spawns from 3′ circular tanks holding 800–1000 adult fish, in which eggs are collected with a catch system.
Exposure
Thirty-two eggs/treatment (2 replicates of 16) were exposed for each concentration of chlorine (J.T. Baker Analytical grade 5.8%), in which the solution was either buffered to pH 7 with acetic acid or unbuffered. The pH was recorded with an American Marine Pinpoint pH Monitor (Ridgefield, CT). The chlorine solutions were prepared within 1 hour of exposure, and the water source was from the reverse osmosis system at the SARL laboratory (calcium hardness 17 ppm, total alkalinity 28 ppm).
Embryos from the same clutch were exposed at either 6 or 24 hours post fertilization (hpf) for either 5 or 10 min. These two ages of embryos were chosen because embryos are usually treated within a few hours of fertilization, but sometimes treatments occur following shipment of embryos. Exposure was accomplished by first placing eggs in 50 ml plastic conical tubes in which the bottom had been replaced with 500 μm screen. Containers were then transferred to the appropriate solution, held for either 5 or 10 min then rinsed with chlorine free water. In the first trial, fish were exposed to buffered (pH 7) or unbuffered chlorine solutions ranging from 0 to 100 ppm at the following concentrations and pH values (for unbuffered solutions): 0 ppm (pH 5); 6.25 ppm (pH 5), 12.5 ppm (pH 5.9), 25 ppm, (pH 6.7); 50 ppm (pH 7.5); 100 ppm (pH 8.3).
Following the first experiment described above, we conducted a second trial with higher concentrations of chlorine, exposing embryos at 6 hpf for 5 min to the following concentrations of chlorine in unbuffered solutions: at 0 ppm (pH 5.7), 100 ppm (pH 8.5), 125 ppm (pH 9.0), 150 ppm (pH 9.4), 175 ppm (pH 9.5) or 200 ppm (pH 9.8). A total of 64 embryos (4 plates at 16/plate) were exposed at each concentration. A third experiment using 225 ppm (pH 9.5) and 250 ppm (pH 9.7) ppm unbuffered chlorine solution was conducted as there was high survival at 200 ppm, with 64 embryos/concentration (2 replicates of 32).
A larger scale exposure study using unbuffered chlorine was then conducted using two selected exposure regimes based on findings of the trials above. A total of 400 6 hpf embryos were exposed for 5 min at 175 ppm (pH 8.9), and the same numbers of 24 hpf embryos of both lines were exposed at 125 (pH 8.4) ppm for 5 min. Embryos were exposed by placing them in 400 ml tri-pours with mesh bottoms as described above, rinsed following exposure, then divided into four groups of 100 embryos, and incubated in 150 mm Petri dishes with 150 ml water.
Toxicity Evaluation
Following exposure, embryos were placed individually in 100 μl E2 embryo medium (Westerfield 2007) solution in 340 μl wells (96 wells/plate) (BD Biosciences, San Jose, CA). This medium had total alkalinity of 76 ppm and calcium hardness of 29 ppm. With 6 hpf exposures, embryos were evaluated at 24 hpf for mortality, developmental delay, spontaneous movement, and notochord malformations. For 24 hpf exposures, embryos were evaluated 30 minutes post exposure for immediate mortality. The survivors from both time period exposures were also evaluated at 5 dpf for the following endpoints: mortality, natural hatch, any aberrations of the yolk sac, axis, eye, snout, jaw, otolith, pericardial edema, brain, somites, pectoral fin, caudal fin, pigmentation, circulatory system, trunk, swim bladder, notochord, and touch response (head and tail) following protocols routinely used in the Tanguay laboratory for zebrafish embryo toxicity assays. (Kim et al. 2013; Truong et al. 2011). Death was determined by lack of heartbeat.
Statistics
Statistical difference between each exposure concentration were compared to controls (0 ppm chlorine) in each trial using SigmaPlot 11 (Systat Software Inc., San Jose, CA). Fisher’s Exact T was used for groups of 32 or less as a separate control was used with each exposure group. Experiments with greater numbers (e.g., n=64) were evaluated with Chi Square as the Sigma Plot program automatically decides which test it is qualified to execute, and the former test was rejected as appropriate for the larger data sets. Differences between a given concentration and 0 ppm were considered statistically different at P < 0.05.
Results
The various trials consistently showed correlations with higher incidence of mortality and malformations associated with higher concentrations. Overall, mortality and malformations in the three experiments were statistically greater than controls compared to controls only at the levels > 100 ppm (Figs. 1, 2). Solutions with chlorine adjusted to a pH 7 were consistently more toxic than unbuffered solutions, the latter which ranged up to over pH 9 at the higher concentrations. The 24 hpf embryos were more susceptible to chlorine exposure than 6 hpf embryos, and exposure for 10 min was associated with greater mortality than 5 min exposure (Figs. 1, 2).
Figure 1. Danio rerio.
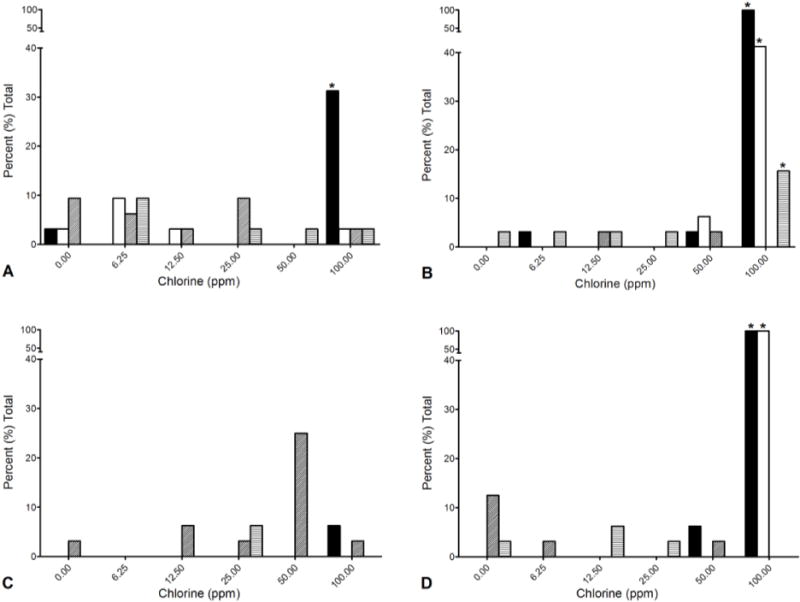
Mortality and malformations in 6 and 24 hour post fertilization (hpf) zebrafish embryos exposed to chlorine solution buffered to pH 7 or unbuffered solution. Solid black = buffered mortality; solid white = unbuffered mortality; diagonal lines = buffered malformations; horizontal lines = unbuffered malformations. Asterisk = statistically different from 0 ppm, (Fishers Exact, p < 0.05). A = 6 hpf, 5 min exposure. B = 6 hpf, 10 min exposure, C = 24 hpf, 5 min exposure, D = 24 hpf, 10 min exposure.
Figure 2. Danio rerio.
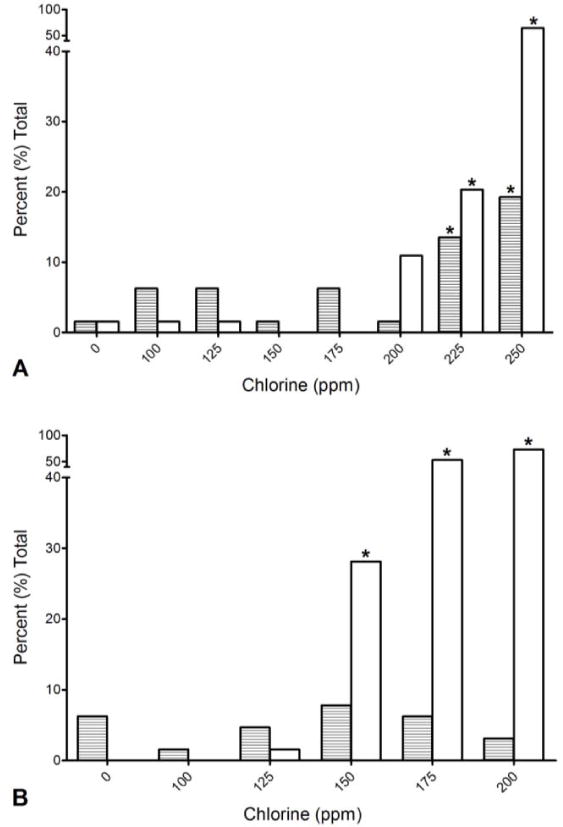
Mortality and malformations in 6 and 24 hour post fertilization (hpf) zebrafish embryos exposed to high concentration unbufferred chlorine solution for 5 min. Solid white = unbuffered mortality, horizontal lines = unbuffered malformations. Asterisk indicates statistically different from 0 ppm (Chi Square, p < 0.05). A = 6 hpf, B = 24 hpf.
Regarding 6 hpf embryos, in the first trial (Fig 1 a,b), at 5 and 10 min, no statistically significant sublethal or lethal effects above background (0 ppm) for either solution (buffered or unbuffered) was observed at 50 ppm or below. At 100 ppm, high mortality occurred at both exposure time points with chlorine at pH 7. With unbuffered chlorine at 100 ppm for 10 min, the 6 hpf fish showed 40% mortality (Fig 1 b). About 15 % of the surviving embryos exposed to 100 ppm unbuffered chlorine for 10 min exhibited malformations, including yolk sac edema, pericardial edema, body axis curvature, snout and jaw malformations.
The 24 hpf exposed embryos showed a similar pattern, except no significant mortality was observed with a 5 min exposure, including 100 ppm. However, significant (P < .05) malformations were observed at 50 ppm (Fig 1) in buffered chlorine. All the embryos died when exposed for 10 min at 100 ppm chlorine, with both unbuffered and buffered solutions.
The second experiment, using higher concentrations of only unbuffered chlorine at 5 min was then conducted (Fig 2). The 6 hpf embryos showed minimal mortality and malformations (Fig 2). In contrast, 24 hpf embryos showed significantly increased malformations at 150 ppm and above, but mortality was still less than 30% at these high levels. Exposure of 6 hpf embryos to 200, 225 or 250 ppm unbuffered chlorine solution in another trial resulted in mortalities and malformations above 10% only at the highest concentration.
A large scale exposure, using several embryos exposed to unbuffered chlorine at 6 hpf and 24 hpf showed again that the embryos could tolerate high levels of unbuffered chlorine. The 6 hpf embryos exposed to 175 pmm chlorine showed no mortality and 2.0 % malformations, compared to 0.5% and 2.0% in the controls, respectively. The 24 hpf embryos exposed to 125 ppm showed 3.0% mortality and 1.8% malformations, compared to 1.0% and 3.0 % in the controls, respectively. There were no statistical differences between the exposure concentrations in controls with both exposure regimens.
Discussion
Our study demonstrated that zebrafish embryos, particularly those at 6 hpf, can tolerate higher chlorine concentrations than the routine protocol of 25 or 50 ppm for 10 min that is used by most zebrafish laboratories (Westerfield 2007; Harper and Lawrence 2011). Buffering of the chlorine solutions to pH 7 was consistently associated with higher corresponding levels of mortality and malformations. Addition of chlorine to water increases pH, and as pH increases significantly above pH 7, the germicidal properties and toxic activity of chlorine is reduced. (Clark et al. 1989). For example, using the formula developed by Clark et al. (1989), adjustment of pH from 9 to 7 should double the toxicity to microorganisms (based on results with Giardia) (Health Canada 2004). The most germicidal form of chlorine is HOCl, and above pH 7.5 very little of chlorine exists in this form, while most becomes the less active form, –OC (Clark et al. 1989). In our previous study with Pseudoloma neurophilia (Ferguson et al. 2007), 100 ppm buffered chlorine (pH 7) killed almost 100% of the spores, while the unbuffered chlorine (pH 9.5) killed about 80%.
Embryos at 6 hpf usually tolerated chlorine better than 24 hpf embryos exposed under similar conditions. Therefore, the general recommendation would be to conduct chlorine treatments at close to 6 hpf. This is not always possible, as often laboratories treat embryos after receiving them from other facilities. In this case, the duration or concentration of chlorine should be reduced. Based on our findings here, our present general recommendation is to treat embryos for 5 min with unbuffered chlorine at 100 ppm to obtain minimal mortalities and malformations. Researchers may also consider using higher concentrations or time exposures when importing new fish for the first time into the facility. This would likely result a higher level of toxic changes with the first generation, but provide better protection for introduction of exotic pathogens to a facility. For example, our data indicate that exposing 6 hpf embryos for 10 min in unbuffered chlorine solution should result in mortalities and malformations well below 50%. Once a line is established, low concentration treatments could be employed as the goal in this situation is mostly pathogen reduction, rather than complete avoidance within a facility. Conversely, one might consider not treating embryos with chlorine at all for specific toxicology or behavior experiments as we still have not elucidated all the potential long term effects of these chlorine treatments. The SARL laboratory’s rapid through put testing facility indeed does not bleach embryos prior to testing, largely because bleaching interferes with pronase-mediated chorion removal (Mandrell et al. 2012).
A protocol using buffered chlorine would be more precise, but this is not practical with large scale and frequent treatments as used in most zebrafish laboratories. Whereas the germicidal capability of chlorine is profoundly affected by pH, we realize that buffering chlorine for egg treatments is not employed in zebrafish laboratories. Considering that water hardness and alkalinity directly influence the buffering capacity of water, chlorine at a given concentration will have different germicidal and toxic effects between laboratories. Hence, pH of egg disinfectant media should be monitored, at least periodically.
The recommended dosages we provide here will not be 100% effective for the spores of the microspordium Pseudoloma neururophilia, the most common zebrafish pathogen in research laboratories. However, increasing the concentration from 50 to 100 ppm unbuffered chlorine solution increased killing of P. neurophilia spores from about about 61 to 82 % (Ferguson et al. 2007). These levels would likely be effective for marked reduction of bacterial pathogens associated with zebrafish. For example, 50 ppm chlorine is very effective against Aeromonas species. (Goñi-Urriza et.al. 2000). This is particularly important as the highly pathogenic Gram negative bacterium Edwardsiella ictaluri has recently been detected in zebrafish held in quarantine at several zebrafish laboratories (Hawk et al. 2013). Mycobacteriosis is also very common in zebrafish facilities caused most often by M. chelonae, M. marinum and M. haemophilum (Whipps et al. 2013). Mycobacteria are generally more resistant to chlorine than Gram negative bacteria, and within the genus, there is great variability. For example, fast-growing environmental species (e.g., Mycobacterium chelonae and M. fortuitum) are more resistant than M. marinum (Bardouniotis et al. 2003; Mainous and Smith 2005). Studies are underway in Dr. Christopher Whipps’ laboratory (SUNY-Syracuse, NY) evaluating the germicidal properties of chlorine using M. chelonae and M. marinum isolates from zebrafish, including our recommended protocol of 5 or 10 min unbuffered chlorine.
It should be noted that all of our experiments were conducted with the 5D line, a very robust outbred line derived from pet store fish and used extensively for toxicology studies at the SARL. Variations in quality and viability of eggs and embryos has been documented in several fish species in aquaculture, including zebrafish (Lawrence 2007), and has been attributed to several factors, including genetics, contaminants in the water and parents, nutritional and disease status of parents, and variations in temperature (Schreck et al. 2001; Brooks et al. 1997; Bobe and Labbé 2010). The zebrafish research community uses a wide variety of fish lines (Trevarrow and Robison (2004). Certain inbred lines are less fit than others (Meyer et al. 2013; Monson and Sadler 2010), whereas we are not aware of documentation of variability in chlorine toxicity of embryos amongst different lines, caution should be applied when applying the treatment protocols that we recommend here to other, perhaps more fragile populations.
Given the instability of chlorine, alternative disinfectants should be investigated. For example, iodine is routinely used in food fish aquaculture to disinfect eggs, and a few zebrafish laboratories are now using this disinfectant to treat eggs (Milligan-Myhre et al. 2011). Regardless of the efficacy of disinfectants to destroy microorganisms, these will not prevent the transmission of intraovum pathogens, such as Pseudoloma neurophilia (Sanders and Kent 2013).
Acknowledgments
This study was supported by grants from the National Institutes of Health NIH NCRR 5R24RR017386-02, P30ES000210 and RC4ES019764.
Literature Cited
- Bardouniotis E, Ceri H, Olson ME. Biofilm formation and biocide susceptibility testing of Mycobacterium fortuitum and Mycobacterium marinum. Curr Microbiol. 2003;46:28–32. doi: 10.1007/s00284-002-3796-4. [DOI] [PubMed] [Google Scholar]
- Bobe J, Labbé C. Egg and sperm quality in fish. Gen Comp Endocrinol. 2010;165:535–548. doi: 10.1016/j.ygcen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Brooks S, Tyler CR, Sumpter JP. Egg quality in fish: what makes a good egg. Rev Fish Biol Fish. 1997;7:387–416. [Google Scholar]
- Clark RM, Eleanor RJ, Hoff J. Analysis of inactivation of Giardia lamblia by chlorine. J Environ Engineer. 1989;115:80–90. [Google Scholar]
- Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish Microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Dis Aquat Org. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Goñi-Urriza M, Pineau L, Capdepuy M, Roques C, Caumette P, Quentin C. Antimicrobial resistance of mesophilic Aeromonas spp. isolated from two European rivers. J Antimicrobial Chemotherp. 2000;46:297–301. doi: 10.1093/jac/46.2.297. [DOI] [PubMed] [Google Scholar]
- Harper C, Lawrence . The laboratory zebrafish. CRC Press; Boca Raton, Florida: 2011. [Google Scholar]
- Hawke JP, Kent ML, Rogge M, Baumgartner W, Wiles J, Shelly J, Savolainen LC, Wagner R, Murray K, Peterson TS. Edwardsiellosis caused by Edwardsiella ictaluri in laboratory populations of zebrafish Danio rerio. J Aquat Animal Health. 2013;25:171–183. doi: 10.1080/08997659.2013.782226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Supporting Documentation — Protozoa: Giardia and Cryptosporidium. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada; Ottawa, Ontario: 2004. [Google Scholar]
- Kim KT, Tanguay RL. Integrating zebrafish toxicology and nanoscience for safer product development. Green Chem. 2013;15:872–880. doi: 10.1039/C3GC36806H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): A review Aquaculture. 2007;269:1–20. [Google Scholar]
- Meyer BM, Froehlich JM, Galt NJ, Biga PR. Inbred strains of zebrafish exhibit variation in growth performance and myostatin expression following fasting. Comp Biochem Physiol A Mol Integr Physiol. 2013;164:1–9. doi: 10.1016/j.cbpa.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous ME, Smith SA. Efficacy of common disinfectants against Mycobacterium marinum. J Aquat Animal Health. 2005;17:284–288. [Google Scholar]
- Milligan-Myhre K, Charettey JR, Phenniciey RT, Stephens WZ, Rawlsz JF, Guillemin K, Kimy CH. Study of host–microbe interactions in zebrafish. Meth Cell Biol. 2011;107:87–116. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CA, Sadler KC. Inbreeding depression and outbreeding depression are evident in wild-type zebrafish lines. Zebrafish. 2010;7:189–197. doi: 10.1089/zeb.2009.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan-Myhre K, Charette JR, Phennicie RT, Stephens WZ, Rawls JF, Guillemin K, Kim CH. Study of host-microbe interactions. Meth Cell Biol. 2011;105:87–115. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier PA, Moulin GC, Stottmeier KD. Mycobacteria in public water supplies: comparative resistance to chlorine. Microbiol Sci. 1988;5:147–148. [PubMed] [Google Scholar]
- Peters M, Müller C, Rüsch-Gerdes S, Seidel C, Göbel HD, Ruf B. Isolation of atypical mycobacteria from tap water in hospitals and homes: is this a possible source of disseminated MAC infection in AIDS patients? J Infect. 1995;31:39–44. doi: 10.1016/s0163-4453(95)91333-5. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Kent ML. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish, Danio rerio. PLOS one. 2013;8(9):e76064. doi: 10.1371/journal.pone.0076064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck CB, Contreras-Sanchez Wilfrido, Fitzpatrick Martin S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 2001;19:3–24. [Google Scholar]
- Trevarrow B, Robison B. Genetic backgrounds, standard lines, and husbandry of zebrafish. The Zebrafish: Genetics, Genomics and Informatics. Meth Cell Biol. 2004;77:599–616. doi: 10.1016/s0091-679x(04)77032-6. [DOI] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Meth Molecular Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerewijck MJM, Sabbe K, Baré J, Spengler HP, Favoreel HW, Houf K. Assessment of the efficacy of benzalkonium chloride and sodium hypochlorite against Acanthamoeba polyphaga and Tetrahymena spp. J Food Protect. 2012;75:541–546. doi: 10.4315/0362-028X.JFP-11-359. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book, 5th Edition; A guide for the laboratory use of zebrafish (Danio rerio) University of Oregon Press; Eugene, Oregon: 2007. [Google Scholar]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in zebrafish colonies. ILAR J. 2012;53:95–105. doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


