Abstract
OBJECTIVES
Human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) may increase the risk of fatty liver disease. We determined the prevalence of and risk factors for fatty liver by comparing HIV-infected men with HIV-uninfected men who have sex with men in the Multicenter AIDS Cohort Study (MACS).
METHODS
In 719 MACS participants who consumed less than three alcoholic drinks daily, fatty liver was defined as a liver-to-spleen attenuation ratio < 1 on noncontrast computed tomography (CT). We genotyped single nucleotide polymorphisms in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene and in other genes previously associated with nonalcoholic fatty liver disease. Risk factors for fatty liver were determined using multivariable logistic regression.
RESULTS
Among 254 HIV-uninfected men and 465 HIV-infected men, 56 % were White with median age 53 years and median body mass index 25.8 kg/m 2. The vast majority of HIV-infected men (92 %) were on ART, and 87 % of the HIV-infected men were treated with a nucleoside reverse transcriptase inhibitor for a median duration of 8.5 years. Overall, 15 % of the cohort had fatty liver, which was more common in the HIV-uninfected compared with the HIV-infected men (19 vs. 13 %, P= 0.02). In multivariable analysis, HIV infection was associated with a lower prevalence of fatty liver (odds ratio (OR) = 0.44, P= 0.002), whereas a higher prevalence of fatty liver was seen in participants with PNPLA3 (rs738409) non-CC genotype (OR = 2.06, P= 0.005), more abdominal visceral adipose tissue (OR = 1.08 per 10 cm2, P< 0.001), and homeostatic model assessment of insulin resistance (HOMA-IR) ≥ 4.9 (OR = 2.50, P= 0.001). Among HIV-infected men, PNPLA3 (rs738409) non-CC genotype was associated with a higher prevalence of fatty liver (OR = 3.30, P= 0.001) and cumulative dideoxynucleoside exposure (OR = 1.44 per 5 years, P= 0.02).
CONCLUSIONS
CT-defined fatty liver is common among men at risk for HIV infection and is associated with greater visceral adiposity, HOMA-IR, and PNPLA3 (rs738409). Although treated HIV infection was associated with a lower prevalence of fatty liver, prolonged exposure to dideoxynucleo side analogs is associated with higher prevalence.
INTRODUCTION
Liver disease is one of the most common non-AIDS-related causes of death among human immunodeficiency virus (HIV)-infected individuals, accounting for 14 % of all deaths and 50 % of hospital deaths (1–3). Although hepatitis C virus (HCV) coinfection is a well-recognized cause of liver disease among HIV-infected persons, mounting evidence suggests that hepatic steatosis is common among HIV-infected individuals with or without HCV coinfection (4–6). Nonalcoholic fatty liver disease refers to hepatic steatosis in individuals with little or no alcohol use and affects ~ 30 % of the adult US population (7). Well-established risk factors for fatty liver disease include hyperglycemia, diabetes mellitus, hypertriglyceridemia, and obesity, particularly abdominal visceral adiposity (8,9). Metabolic abnormalities are common among antiretroviral therapy (ART)-treated HIV-infected persons and have previously been associated with hepatic steatosis in this group (6,10,11).
The impact of additional host and viral factors on liver steatosis and fibrosis risk in the setting of HIV infection is poorly understood. The rs738409 single nucleotide polymorphism (SNP) in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene is strongly associated with steatosis, steatohepatitis, and fibrosis in HIV-uninfected persons, including patients with chronic HCV infection (12–15). Polymorphisms in other genes such as NCAN (rs2228603), GCKR (rs780094), LYPLAL1 (rs12137855), and PPP1R3B (rs4240624) have also been associated with steatosis in HIV-uninfected individuals, but these have not been studied in HIV-infected persons (16). HIV infection itself may lead to hepatic steatosis by direct interaction with the sterol regulatory element-binding protein-1, which stimulates lipogenesis, and peroxisome proliferator-activated receptor gamma, which is involved in insulin signaling (17). In addition, antiretrovirals of the nucleoside reverse transcriptase inhibitor (NRTI) class may cause steatosis via inhibition of mitochondrial polymerase γ and protease inhibitors by inducing hepatic overexpression of sterol regulatory element-binding protein-1 (18,19).
It has been suggested that HIV-infected persons comprise a population at high risk for fatty liver disease (20). However, it is unclear whether HIV per se increases fatty liver risk as studies of hepatic steatosis and HIV infection lack HIV-uninfected controls. The objective of this study was to determine the prevalence of and risk factors for fatty liver by comparing HIV-infected participants with HIV-uninfected participants in the Multicenter AIDS Cohort Study (MACS).
METHODS
Study population
We conducted a cross-sectional study nested within the MACS, an ongoing prospective cohort study involving HIV-infected and HIV-uninfected men who have sex with men. Details of MACS participant recruitment and study design have been described elsewhere (21,22). In brief, men were enrolled from four US metropolitan areas (Baltimore, MD/Washington DC; Chicago, IL; Pittsburgh, PA; and Los Angeles, CA) during three recruitment periods (1984–1985, 1987–1990, and 2001–2003). Subjects were followed up every 6 months for interview, physical examination, laboratory testing, and collection of biological specimens for repository specimens.
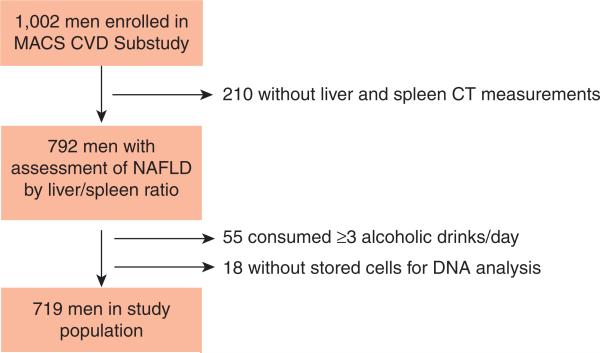
Eligibility for the fatty liver study was participation in the MACS cardiovascular substudy, as these men underwent computed tomography (CT) imaging studies. The cardiovascular substudy recruited 1,002 men aged 40–71 years to undergo noncontrast cardiac CT imaging, cuts of which were extended to include liver and spleen imaging, between 1 January 2010 and 19 August 2013. Exclusion criteria were a history of cardiac surgery, history of coronary angioplasty, or weight > 300 pounds. After excluding 210 men whose CT images lacked adequate visualization of both the liver and spleen, 55 men who consumed more than or equal to three alcoholic drinks daily, and 18 men without stored cells or cell pellets for DNA extraction, 719 men were evaluated for the fatty liver study (Figure 1). Comparison of the 719 men who were included in the analysis and the 283 excluded men is detailed in Supplementary Table S1 online. The study was approved by the Institutional Review Boards of all participating sites. All participants signed informed consent.
Figure 1.
Flow chart demonstrating inclusion in study sample.
Assessment of hepatic steatosis
Noncontrast CT is a reliable noninvasive imaging method to detect hepatic steatosis of moderate or greater severity (23). Although the liver normally has a higher CT-attenuation value compared with the spleen, the presence of steatosis in the liver leads to a reversal of the liver/spleen attenuation ratio (24). Multidetector row CT scan was performed once for each participant. A single reader who was blinded to demographic data and HIV serostatus measured each scan. Liver and spleen attenuation values were measured by obtaining minimum, maximum, and mean Hounsfield units (HU) for regions of interest (ROI) > 100 mm2. Two ROIs were placed in the right liver lobe, one ROI in the left hepatic lobe, and one ROI in the spleen. The mean hepatic HU was derived by averaging the mean HU across all liver ROIs. The liver/spleen ratio was calculated by dividing the mean hepatic HU measurements by the mean spleen HU measurements. Fatty liver was defined as a liver/spleen ratio < 1.0. For measurements of abdominal adipose tissue, one axial image was obtained in the space between the fourth and fifth lumbar vertebrae. Abdominal adipose tissue areas were calculated in cm2 for visceral adipose tissue (VAT) and subcutaneous adipose tissue. The reader was blinded to all clinical and demographic data.
Laboratory testing and definitions
For SNP genotyping, DNA was extracted from stored cells and cell pellets using the DNeasy Blood and Tissue kit (Qiagen, Germantown, MD). Genotyping of PNPLA3 (rs738049), NCAN (rs2228603), GCKR (rs780094), LYPLAL1 (rs12137855), and PPP1R3B (rs4240624) was performed via TaqMan SNP genotyping assays (Life Technologies, Carlsbad, CA), which use unique primers and probes for each SNP. DNA extraction and SNP testing were both accomplished using standard protocols. HIV antibody testing was performed using enzyme immunoassay and confirmed with western blot, as previously described (22). HCV infection was defined as serum HCV antibody and HCV RNA positivity. HCV antibody testing was performed with a third-generation enzyme immunoassay (ADVIA Centura HCV assay, Siemens Healthcare Diagnostics, Tarrytown, NY). If positive, HCV RNA testing was conducted from the same serum sample using Taqman HCV assay (lower limit of detection 43 IU/ml; COBAS AmpliPrep COBAS TaqMan HCV assay, Roche Molecular Systems, Pleasanton, CA). HCV antibody was tested in all participants within 2 years of entry into MACS and at most recent follow-up, with additional HCV antibody testing performed to identify the timing of all HCV seroconversions (25). Among HIV-infected subjects, CD4 T-cell counts and plasma HIV RNA levels were measured at each visit using standard assays.
Eight-hour fasting blood tests collected and assayed at the time of CT scanning or, if not available, within 6 months of CT scanning included the following: insulin, glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Impaired fasting glucose or diabetes was defined as a fasting glucose ≥ 100 mg/dl or use of diabetes medication. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using fasting insulin and glucose values. For analysis, the HOMA-IR was dichotomized as <4.9 or ≥4.9. The 4.9 cutoff was used because it defined the 75th percentile in our cohort. Body mass index at the time of CT or, if not available, within 6 months of CT scanning was calculated as (body weight (kg)/height (m))2 and was categorized by a modification of the World Health Organization's classification system: normal, <25 kg/m2 ; overweight, 25–30 kg/m2 ; or obese, ≥30 kg/m2. Hepatic fibrosis was estimated using the AST-to-platelet ratio index and the FIB-4, which were calculated from labs assayed from specimens at the time of CT scan or, if not available, within 6 months of the scan (26,27). AST-to-platelet ratio index was calculated using the formula ((AST/upper limit of normal AST)× 100)/platelet count (109/l), and FIB-4 was calculated using the formula (age (years) × AST)/(platelet count (109/l) × ALT 1/2). We used an upper limit of normal of 30 U/l for calculations.
Statistical analysis
Demographic and clinical factors were compared by HIV serostatus and the presence or absence of fatty liver using the χ2 -test for categorical variables and the Wilcoxon rank-sum test for continuous variables. For each SNP, Hardy–Weinberg equilibrium was determined by the χ2 -test using one degree of freedom. Multivariable logistic regression was used to evaluate the association between fatty liver and the covariates of interest, including HIVinfection, PNPLA3 (rs738049), other candidate genes, and metabolic factors. The final model included HIV serostatus, age, race (White, Black, or other), MACS site, abdominal VAT, and the PNPLA3 (rs738049), ALT >40 U/l, HOMA-IR ≥4.9, and HCV serostatus. Separate sensitivity analyses were performed excluding HCV-infected subjects, adjusting for AST-to-platelet ratio index and FIB-4, and using a fatty liver cutoff of liver/spleen ratio <0.9 (which is less sensitive but 97 % specific for fatty liver) (24). We repeated the multivariable logistic regression after stratification by HIV serostatus. Among the HIV-infected men, associations between HIV-specific variables and fatty liver were explored, including current and cumulative ART exposure. All statistical analyses were performed using STATA 12.1 (StataCorp, College Station, TX).
RESULTS
Study population
Among the 719 men included in the analysis, 465 were HIV infected and 254 were HIV uninfected. The HIV-infected men were younger (median age, 53 vs. 55 years, P < 0.001) and more likely to be non-White (Table 1). Although they had a lower median body mass index (25.5 vs. 26.3 kg/m2, P=0.002), there was no difference in abdominal VAT between the HIV-infected vs. HIV-uninfected men (median, 143 cm2 vs. 139 cm2, P=0.91). The HIV-infected men had higher median HOMA-IR (3.3 vs. 2.9, P=0.007), lower median high-density lipoprotein cholesterol (46 vs. 50 mg/dl, P < 0.001), lower median low-density lipoprotein cholesterol (103 vs. 108 mg/dl, P=0.02), and higher median triglyceride levels (135 vs. 106 mg/dl, P < 0.001). HIV-infected subjects were more likely to be HCV infected (12 vs. 4 %, P < 0.001), and all of the HCV-infected men had HCV genotype 1 or 2. Thirtyfive percent of the entire cohort was receiving a lipid-lowering medication at the time of CT scanning; this percentage did not differ significantly by HIV serostatus. Similarly, there was no difference in use of diabetes medications at the time of CT scanning by HIV serostatus. Among the HIV-infected subjects, 95 % were ART exposed, 92 % were on ART at the time of the CT scan, and 90 % had an undetectable plasma HIV RNA level within 6 months of the CT scan. The median cumulative ART duration was 9 years (interquartile range=6.5, 13). Eighty-seven percent of the HIV-infected men were treated with an NRTI for a median cumulative duration of 8.5 years (interquartile range=6, 12.5).
Table 1.
Characteristics of the study populationa
| HIV– (N=254) | HIV + (N=465) | P value | |
|---|---|---|---|
| Race | |||
| White non-Hispanic, N (%) | 162 (64) | 242 (52) | 0.008 |
| Black non-Hispanic | 69 (27) | 168 (36) | |
| Otherb | 22 (9) | 55 (12) | |
| Age (years), median (IQR) | 55 (51, 62) | 53 (48, 58) | <0.001 |
| BMI (kg/m2), median (IQR) | 26.3 (24.2, 29.9) | 25.5 (23.3, 28.6) | 0.002 |
| Abdominal VAT (cm2), median (IQR) | 139 (88, 210) | 143 (82, 211) | 0.91 |
| Abdominal SAT (cm2), median (IQR) | 231 (168, 304) | 181 (114, 278) | 0.007 |
| IFG or diabetesc, N (%) | 100 (43) | 194 (45) | 0.61 |
| HOMA-IR, median (IQR) | 2.9 (2.2, 4.5) | 3.3 (2.3, 5.1) | 0.007 |
| HOMA-IR ≥4.9, N (%) | 48 (21) | 114 (27) | 0.08 |
| On lipid-lowering agent, N (%) | 76 (31) | 167 (37) | 0.11 |
| Total cholesterol (mg/dl), median (IQR) | 187 (162, 209) | 182 (157, 212) | 0.41 |
| HDL (mg/dl), median (IQR) | 50 (41, 60) | 46 (37, 54) | < 0.001 |
| LDL (mg/dl), median (IQR) | 108 (89, 132) | 103 (81, 130) | 0.02 |
| Triglycerides (mg/dl), median (IQR) | 106 (80, 146) | 135 (97, 208) | < 0.001 |
| HTNd, N (%) | 115 (47) | 226 (50) | 0.41 |
| ALT (U/l), median (IQR) | 21 (17, 28) | 26 (19, 39) | < 0.001 |
| ALT > 40 U/l | 23 (9) | 104 (23) | < 0.001 |
| AST (U/l), median (IQR) | 21 (18, 26) | 24 (20, 34) | < 0.001 |
| AST > 40 U/l | 9 (4) | 67 (15) | < 0.001 |
| APRI > 1.5 | 1 (0.4)e | 19 (4.2)f | 0.004 |
| FIB-4 > 3.25 | 3 (1.2)g | 23 (5.1)h | 0.01 |
| HCV, N (%)i | 9 (4) | 56 (12) | < 0.001 |
| PNPLA3 (rs738409) | |||
| CC, N (%) | 145 (57) | 288 (62) | 0.46 |
| GC | 96 (38) | 157 (34) | |
| GG | 12 (5) | 19 (4) | |
| Detectable HIV VL, N (%) | 47 (10) | ||
| Nadir CD4 < 200 cells/ml, N (%) | 147 (33) | ||
| Current ART, N (%) | 420 (92) | ||
| Cumulative ART (years), median (IQR) | 8.5 (6.0, 12.5) | ||
| Current NRTI, N (%) | 400 (87) | ||
| Cumulative NRTI (years), median (IQR) | 8.5 (6.0, 12.5) | ||
| Current PI, N (%) | 223 (49) | ||
| Cumulative PI (years), median (IQR) | 4.0 (0.5, 8.0) | ||
| Current NNRTI, N (%) | 205 (45) | ||
| Cumulative NNRTI (years), median (IQR) | 3.0 (0.5, 6.5) |
ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; HCV, hepatitis C virus; HOMA-IR, homeostatic model assessment of insulin resistance; HTN, hypertension; IFG, impaired fasting glucose; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; VL, viral load.
Missing values: race=1, BMI=39, abdominal VAT=2, abdominal SAT=2, IFG or diabetes=52, HOMA-IR=63, lipid-lowering agent=15, total cholesterol=66, HDL=63, LDL=71, trigylcerides=63, HTN=24, ALT=14, AST=14, HCV=12, PNPLA3 (rs738409)=2, HIV viral load=8, nadir CD4=16, ART medication use=6.
70/77 Self-identified as Hispanic.
Defined by fasting glucose or use of diabetes medication.
Defined by blood pressure or use of anti-hypertensive medication.
0 HCV infected.
13 HCV infected.
2 HCV infected.
12 HCV infected.
58 Genotype 1, 7 Genotype 2.
Of the men without HIV or HCV, only 1 (0.4 %) had advanced fibrosis as estimated by FIB-4 > 3.25 compared with 11 (2.7 %) of the HIV-infected and HCV-uninfected men (P=0.04). Two HCV-monoinfected men (22 %) and 12 HIV/HCV-coinfected men (23 %) had advanced fibrosis. In the HIV-infected men without HCV coinfection, advanced fibrosis was associated with older age (median, 59 vs. 52, P=0.002), higher HOMA-IR (median, 6.3 vs. 3.1, P=0.04), and receipt of lipid-lowering agents (73 vs. 39 %, P=0.02; Supplemental Table S2). They also had longer cumulative ART (median years, 14 vs. 9, P=0.002), NRTI (median years, 13.5 vs. 8.5, P=0.002), non-NRTI (median years, 7 vs. 3, P=0.01), and dideoxynucleoside exposure (median years, 13.1 vs. 7.5, P=0.01). In the HIV/HCV-coinfected men, those with advanced fibrosis were more likely to have fatty liver (17 vs. 0, P=0.01) and to have had a nadir CD4 count <200 (P=0.048) compared with those without advanced fibrosis.
Factors associated with fatty liver in the entire cohort
The prevalence of fatty liver was 15 % in the entire cohort and was higher in HIV-uninfected compared with HIV-infected men (19 vs. 13 %, P=0.02). In univariate analysis, subjects with fatty liver were less likely to be Black (15 vs. 36 %, P <0.001) or HCV infected (4 vs. 10 %, P=0.04) and were more likely to have impaired fasting glucose or diabetes (63 vs. 41 %, P<0.001) or hypertension (62 vs. 47 %, P=0.006; Table 2). They also had significantly higher median body mass index (28.4 vs. 25.4 kg/m2, P<0.001), abdominal VAT volume (median, 223 cm2 vs. 134 cm2, P<0.001), abdominal sub-cutaneous adipose tissue (median, 224 cm2 vs. 195 cm2, P=0.006), HOMA-IR (4.8 vs. 3.0, P<0.001), triglycerides (160 vs. 119 mg/dl, P<0.001), and lower median high-density lipoprotein cholesterol (43 vs. 48 mg/dl, P<0.001). Median ALT was higher among the men with fatty liver (32 vs. 23 mg/dl, P<0.001), as was median AST (25 vs. 23 mg/dl, P=0.03).
Table 2.
Characteristics of participants without and with fatty livera
| No fatty liver (N=611) | Fatty liver (N=108) | P value | |
|---|---|---|---|
| HIV-infected, N (%) | 406 (66) | 59 (55) | 0.02 |
| Race | |||
| White non-Hispanic, N (%) | 325 (54) | 79 (73) | < 0.001 |
| Black non-Hispanic | 221 (36) | 16 (15) | |
| Other | 64 (10) | 13 (12) | |
| Age (years), median (IQR) | 53 (49, 59) | 54 (49, 59) | 0.48 |
| BMI (kg/m2), median (IQR) | 25.4 (23.4, 28.4) | 28.4 (25.6, 31.8) | < 0.001 |
| Abdominal VAT (cm2), median (IQR) | 134 (79, 197) | 223 (134, 304) | < 0.001 |
| Abdominal SAT (cm2), median (IQR) | 195 (125, 280) | 224 (161, 339) | 0.006 |
| IFG or diabetesb, N (%) | 228 (41) | 66 (63) | < 0.001 |
| HOMA-IR, median (IQR) | 3.0 (2.2, 4.4) | 4.8 (3.1, 6.8) | < 0.001 |
| HOMA-IR≥4.9, N (%) | 112 (20) | 50 (50) | < 0.001 |
| On lipid-lowering agent, N (%) | 198 (33) | 45 (43) | 0.05 |
| Total cholesterol (mg/dl), median (IQR) | 184 (159, 212) | 187 (158, 213) | 0.82 |
| HDL (mg/dl), median (IQR) | 48 (40, 58) | 43 (35, 51) | < 0.001 |
| LDL (mg/dl), median (IQR) | 105 (83, 131) | 105 (81, 132) | 0.85 |
| Triglycerides (mg/dl), median (IQR) | 119 (85, 178) | 160 (110, 225) | < 0.001 |
| HTNc, N (%) | 277 (47) | 64 (62) | 0.006 |
| ALT (U/I), median (IQR) | 23 (17, 33) | 32 (22, 47) | < 0.001 |
| ALT > 40 U/I | 93 (16) | 34 (32) | < 0.001 |
| AST (U/I), median (IQR) | 23 (19, 30) | 25 (20, 35) | 0.010 |
| AST > 40 U/I | 58 (10) | 18 (17) | 0.03 |
| HCV, N (%) | 61 (10) | 4 (4) | 0.04 |
| APRI > 1.5 | 17 (3) | 3 (3) | 0.99 |
| FIB-4 > 3.25 | 23 (4) | 3 (3) | 0.62 |
| PNPLA3 (rs738409) | |||
| CC, N (%) | 385 (63) | 48 (44) | 0.001 |
| GC | 198 (33) | 55 (51) | |
| GG | 26 (4) | 5 (5) | |
| NCAN (rs2228603) | |||
| CC, N (%) | 554 (86) | 92 (14) | 0.01 |
| TC | 54 (82) | 12 (18) | |
| TT | 2 (40) | 3 (60) | |
| GCKR (rs780094) | |||
| CC, N (%) | 263 (86) | 43 (14) | 0.78 |
| TC | 276 (84) | 51 (16) | |
| TT | 70 (83) | 14 (17) | |
| LYPLAL1 (rs12137855) | |||
| TT, N (%) | 26 (93) | 2 (7) | 0.41 |
| CT | 177 (83) | 35 (17) | |
| CC | 406 (85) | 70 (15) | |
| PPP1R3B (rs4240624) | |||
| GG, N (%) | 13 (100) | 0 | 0.18 |
| AG | 138 (87) | 20 (13) | |
| AA | 459 (84) | 88 (16) | |
| Detectable HIV VLd, N (%) | 42 (11) | 5 (9) | 0.66 |
| Nadir CD4 < 200d, N (%) | 130 (33) | 17 (30) | 0.69 |
| Current ARTd, N (%) | 367 (92) | 53 (92) | 0.97 |
| Current NRTId, N (%) | 349 (87) | 51 (88) | 0.85 |
| Current PId, N (%) | 197 (49) | 26 (45) | 0.54 |
| Current NNRTId, N (%) | 174 (43) | 31 (54) | 0.15 |
| Cumulative ART (years)d, median (IQR) | 8.5 (6.0, 12.5) | 9.8 (8.0, 13.5) | 0.006 |
| Cumulative NRTI (years)d, median (IQR) | 8.5 (5.5, 12.5) | 9.5 (8.0, 13.0) | 0.006 |
| Cumulative PI (years)d, median (IQR) | 4.0 (0.5, 7.5) | 5.0 (1.0, 8.0) | 0.23 |
| Cumulative NNRTI (years)d, median (IQR) | 2.5 (0.5, 6.5) | 5.0 (1.0, 8.0) | 0.02 |
| Cumulative Lamivudine (years)d, median (IQR) | 5.4 (1.2, 9.0) | 7.8 (3.7, 11.0) | 0.01 |
| Cumulative Efavirenz (years)d, median (IQR) | 1.0 (0, 4.7) | 2.5 (0, 7.1) | 0.25 |
| Cumulative dideoxy (years)d, median (IQR) | 1.3 (0, 5.8) | 4.1 (0, 8.0) | 0.04 |
ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HTN, hypertension; IFG, impaired fasting glucose; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Missing values: race=1, BMI=39, abdominal VAT=2, abdominal SAT=2, IFG or diabetes=52, HOMA-IR=63, lipid-lowering agent=15, total cholesterol=66, HDL=63, LDL=71, trigylcerides=63, HTN=24, ALT=14, AST=14, HCV=12, PNPLA3 (rs738409)=2, HIV viral load=8, nadir CD4=16, ART medication use=6.
Defined by fasting glucose or use of diabetes medication.
Defined by blood pressure or use of anti-hypertensive medication.
Among HIV + only.
Finally, we examined the distribution of potential genetic associations with fatty liver status. The PNPLA3 (rs738409) CC genotype was most common in the cohort (60 %), followed by GC (35 %) and GG (4.4 %). This distribution was in Hardy–Weinberg equilibrium and did not differ by HIV serostatus (P=0.46). The genotypes varied by race, with Blacks having the highest frequency of the CC genotype (P<0.001 across groups; Table 3). Men with fatty liver were more likely to have the non-CC genotype compared with men without fatty liver (56 vs. 37 %, P<0.001). The NCAN (rs2228603) TT genotype was also more frequent among the men with fatty liver but was not included in the multivariable model owing to its low frequency (0.7 %). The additional SNPs, GCKR (rs780094), LYPLAL1 (rs12137855), and PPP1R3B (rs4240624), were not associated with fatty liver in our cohort.
Table 3.
Distribution of PNPLA3 (rs738409) genotype by race
| PNPLA3 (rs738409) genotypea,b |
|||
|---|---|---|---|
| CC | GC | GG | |
| White, non-Hispanic, N (%) | 222 (55) | 158 (39) | 23 (6) |
| Black, non-Hispanic, N (%) | 180 (76) | 54 (23) | 3 (1) |
| Otherc, N (%) | 30 (39) | 41 (54) | 5 (7) |
| Total, N (%) | 432 (60) | 253 (35) | 31 (4) |
Missing=3.
P value across groups < 0.001.
70/77 (91%) Identified as Hispanic.
In multivariable analysis, HIV serostatus remained associated with a lower odds of fatty liver (odds ratio (OR)=0.44; 95 % confidence interval (CI)=0.26, 0.74; P=0.002; Table 4). Factors independently associated with increased odds of fatty liver were abdominal VAT (OR=1.08 per 10 cm2 ; 95 % CI=1.04, 1.11; P<0.001), PNPLA3 (rs738409) non-CC genotype (OR=2.06; 95 % CI=1.24, 3.33; P=0.005), ALT > 40 U/l (OR=2.99; 95 % CI=1.65, 5.42; P<0.001), and HOMA-IR ≥ 4.9 (OR=2.50; 95 % CI=1.44, 4.36; P=0.001). Black race was not significantly associated with decreased odds of fatty liver after adjusting for the other variables in the model. Using a liver/spleen ratio < 0.9 as a cutoff for fatty liver, as expected, the overall prevalence of fatty liver was lower (60 subjects, 8 %). However, the factors associated with fatty liver on multivariable analysis remained the same.
Table 4.
Multivariable analysis of factors associated with fatty liver among the entire cohort and among the HIV-infected men only
| Entire cohort (n=652)a |
HIV uninfected (n=230)a |
HIV infected (n=421)a |
||||
|---|---|---|---|---|---|---|
| OR | P value | OR | P value | OR | P value | |
| HIV | 0.44 (0.26, 0.74) | 0.002 | ||||
| Abdominal VAT (per 10 cm2) | 1.08 (1.04, 1.11) | < 0.001 | 1.08 (1.03, 1.14) | 0.002 | 1.07 (1.03, 1.12) | < 0.001 |
| PNPLA3 (rs738409) non-CC | 2.03 (1.24, 3.33) | 0.005 | 1.14 (0.51, 2.54) | 0.746 | 3.30 (1.66, 6.57) | 0.001 |
| ALT > 40 U/l | 2.99 (1.65, 5.42) | < 0.001 | 7.21 (2.13, 24.41) | 0.002 | 2.54 (1.20, 5.36) | 0.015 |
| HOMA-IR≥4.9b | 2.50 (1.44, 4.36) | 0.001 | 2.27 (0.89, 5.76) | 0.084 | 2.54 (1.24, 5.19) | 0.011 |
| Race (ref=white) | ||||||
| Black | 0.49 (0.24, 1.03) | 0.059 | 0.48 (0.13, 1.74) | 0.266 | 0.54 (0.21, 1.39) | 0.200 |
| Other | 0.95 (0.41, 2.24) | 0.915 | 1.11 (0.27, 4.59) | 0.883 | 0.61 (0.19, 2.02) | 0.423 |
| HCV | 0.55 (0.17, 1.82) | 0.329 | 0.40 (0.03, 5.95) | 0.503 | 0.54 (0.14, 2.18) | 0.394 |
| Cumulative dideoxynucleoside (per 5 year exposure) | 1.44 (1.06, 1.97) | 0.021 | ||||
ALT, alanine aminotransferase; HOMA-IR, homeostatic model assessment of insulin resistance; VAT, visceral adipose tissue.
Also adjusted for age and MACS site.
75% Percentile for HOMA in the cohort.
The finding of lower odds of fatty liver among HIV-infected men was unexpected. To exclude confounding by HCV infection, we performed a sensitivity analysis limited to HCV-uninfected participants, and the results of the multivariable analysis were qualitatively unchanged. We also considered that, even in the absence of HCV coinfection, the HIV-monoinfected subjects might have increased hepatic fibrosis, which might alter the liver attenuation on noncontrast CT scan (28). However, the results were unchanged after adjusting for AST-to-platelet ratio index or FIB-4.
Factors associated with fatty liver among HIV-infected subjects
To determine whether HIV-specific factors or medications were associated with fatty liver, we restricted the remaining analysis to HIV-infected subjects. In univariate analysis, HIV-specific factors associated with fatty liver included longer cumulative ART exposure (median, 9.8 years vs. 8.5 years, P=0.006), NRTI exposure duration (median, 9.5 years vs. 8.5 years, P=0.006), NNRTI exposure duration (median, 5 years vs. 2.5 years, P=0.02), lamivudine exposure (median, 7.8 years vs. 5.4 years, P=0.01), and dideoxynucleoside exposure (median, 4.1 years vs. 1.3 years, P=0.04; Table 2). Nadir CD4 cell count, presence of detectable plasma HIV RNA, current ART, and current antiretroviral use by medication class were not associated with fatty liver.
In multivariable analysis, more abdominal VAT (OR=1.07 per cm2 ; 95 % CI=1.03, 1.12; P<0.001), ALT > 40 U/l (OR=2.54; 95 % CI=1.20, 5.36; P=0.02), HOMA-IR ≥ 4.9 (OR=2.54; 95 % CI=1.24, 5.19; P=0.01), greater cumulative dideoxynucleoside exposure (OR=1.44 per 5 years; 95 % CI=1.06, 1.97; P=0.02), and PNPLA3 non-CC genotype (rs738409) (OR=3.30; 95 % CI=1.66, 6.57; P=0.001) were independently associated with increased odds of fatty liver (Table 4). Cumulative ART, NRTI, and NNRTI exposure was no longer significantly associated with fatty liver after adjusting for other covariates.
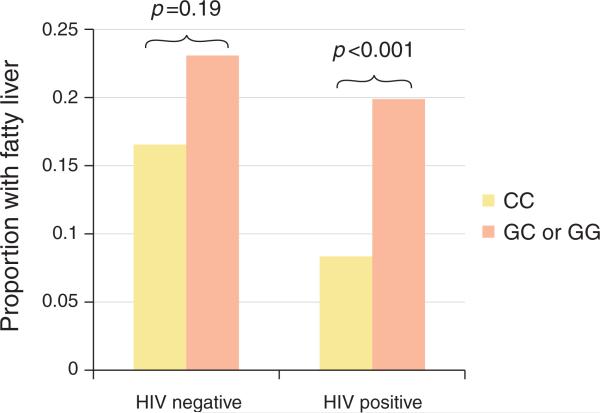
Interestingly, PNPLA3 (rs738409) was not significantly associated with fatty liver in analysis limited to the HIV-uninfected men (OR=1.14; 95 % CI=0.51, 2.54; P=0.75). This was also noted in univariate analysis, comparing the prevalence of fatty liver by PNPLA3 (rs738409) non-CC genotype after stratifying by HIV serostatus (Figure 2). To test whether PNPLA3 (rs738409) geno-type modified the association of HIV and fatty liver (or vice versa), we constructed an HIV and PNPLA3 (rs738409) non-CC genotype interaction term. The interaction term trended toward a higher impact of PNPLA3 (rs738409) among the HIV-infected men, but it was not statistically significant in multivariable analysis (OR=2.56; 95 % CI=0.94, 6.98; P=0.07).
Figure 2.
Proportion of participants with fatty liver by human immunodeficiency virus (HIV) and phospholipase domain-containing 3 (PNPLA3 ; rs738409) gene status.
DISCUSSION
In this well-characterized cohort of HIV-infected men who were extensively ART experienced, we found that HIV-infected men had a higher frequency of factors commonly associated with fatty liver, but, unexpectedly, they had a lower prevalence of CT-defined fatty liver compared with the HIV-uninfected men from the same cohort. This lower risk of fatty liver among HIV-infected men persisted after excluding HIV/HCV-coinfected men, adjusting for possible fibrosis (which may alter liver attenuation on CT), and using a more specific cutoff for CT-measured fatty liver. The only ART medications independently associated with fatty liver were dideoxynucleoside analogs. In both HIV-uninfected and -infected men, abdominal visceral adiposity and ALT > 40 IU/ml were associated with fatty liver. Notably, on stratified analysis, the PNPLA3 (rs738409) non-CC genotype was associated with fatty liver only in HIV-infected men. Thus, in the current ART era, fatty liver prevalence is not increased in HIV-infected men in the MACS, and traditional metabolic and genetic factors are correlates of fatty liver in this setting.
The overall prevalence of fatty liver was 15 % in our cohort, a finding that was consistent with a report from another US-based cohort study involving HIV-uninfected persons that demonstrated a 17 % prevalence of nonalcoholic fatty liver disease using the same CT definition (29), and similar to the National Health and Nutrition Examination Survey data indicating a prevalence of 19 % using ultrasonography (30). Although noncontrast CT has high sensitivity and specificity for steatosis affecting ≥ 30 % of hepatic parenchyma, it is less sensitive for detecting mild (5–30 %) hepatic steatosis (24). In addition, our study excluded men with a history of cardiac surgery or coronary angioplasty as well as men weighing >300 pounds; therefore, out sample is likely biased toward a lower prevalence of fatty liver. For these reasons, our estimate of fatty liver is likely to be conservative in both the HIV-infected and -uninfected groups.
Thirteen percent of the HIV-infected men in our cohort had CT evidence of fatty liver. Other studies of HIV-infected individuals have estimated a prevalence of hepatic steatosis ranging from 13 to 53 % (4,5,31,32). Three of these studies used ultrasound to diagnose hepatic steatosis (4,31,32), a modality that tends to yield a higher prevalence, and two of these studies did not exclude participants with a history of heavy alcohol use (31,32). A study of 225 HIV-monoinfected patients at a metabolic clinic in Italy found a 37 % prevalence of fatty liver, as defined by noncontrast CT liver/ spleen ratio < 1.1 (5). In addition to using a different liver/spleen ratio cutoff for fatty liver than our study, this cohort had a different racial distribution and a very high prevalence of lipodystrophy. Unlike our study, these studies did not include HIV-uninfected controls and therefore could not address whether fatty liver is more common in the setting of HIV infection.
A major strength of our study is that both the HIV-infected and HIV-uninfected men came from the same cohort, which eliminates cohort differences that can confound comparisons. To our knowledge, this is the first study to directly compare the prevalence of fatty liver between these groups in a predominantly HCV-uninfected cohort. One novel finding is the lower fatty liver prevalence in HIV-infected men compared with HIV-uninfected men despite adjusting for metabolic risk factors. The reason for this is unclear, but it is possible that the HIV-infected men had more frequent physician encounters, thus allowing for better control of risk factors for fatty liver disease. Our findings should also be interpreted with caution, as the correlation between noncontrast CT and histologic steatosis has been validated primarily among healthy HIV-uninfected individuals (24,33). CT may be less accurate in detecting steatosis in the setting of HIV infection and may miss both microvesicular steatosis and milder degrees of macrovesicular steatosis. Nevertheless, our findings suggest that in the current ART era, moderate or greater steatosis is common but is not increased in HIV-infected individuals on ART compared with HIV-uninfected persons. Prior studies evaluating the role of HIV infection on liver steatosis have been limited to HCV-infected individuals; results have been conflicting, and a meta-analysis failed to demonstrate an increased risk of histologic steatosis associated with HIV-coinfection (11,34–36).
Our study is the first to examine the relationship between the rs738409 SNP in the PNPLA3 gene and fatty liver in the setting of HIV infection. This SNP, which has been noted in previous studies of HIV-uninfected persons (12–15), was associated with an increased risk of fatty liver in our cohort. PNPLA3 encodes a protein with triglyceride hydrolase activity. The rs738409 mutation leads to markedly reduced enzymatic activity, but susceptibility for steatosis is increased via a gain-of-function mechanism that appears to be independent of lipid abnormalities or insulin resistance (16,37). What was unexpected in our findings was that stratification by HIV serostatus revealed an association only in HIV-infected subjects. It has been postulated that this SNP is important as a secondary risk when an environmental stressor such as obesity or alcohol is present (38,42,43). Among HIV-infected subjects, HIV may constitute the environmental stressor, as we have shown that HIV monoinfection affects ALT levels (28). The effects of PNPLA3 may also be modified by gender, with a stronger association of PNPLA3 and steatosis among women (13,39). These findings may also explain, in part, why we did not see an association of PNPLA3 rs738409 and fatty liver in the HIV-uninfected men in this relatively lean cohort.
In the whole cohort as well as in analytic models limited to HIV-infected men, we found a strong positive association between metabolic factors and fatty liver, with greater amounts of abdominal visceral adiposity and HOMA-IR, each independently associated with fatty liver. These findings are consistent with well-established literature correlating metabolic risk factors and hepatic steatosis in the general population as well as in HCV-infected individuals (with or without HIV coinfection) (8,9,11,40). Other studies of HIV-monoinfected individuals have also demonstrated an increased risk of hepatic steatosis with greater waist circumference, higher serum triglyceride level, and lower high-density lipoprotein levels (4,5). We did not find an association between HCV infection and fatty liver risk. All of the HCV-infected men in our cohort had HCV genotype 1 or 2. Although it is postulated that HCV geno-type 3 causes steatosis via viral-mediated dysregulation of lipoprotein metabolism (41), in non-genotype 3, HCV infection steatosis appears to be primarily mediated by metabolic factors (40,42).
Among HIV-infected men, cumulative exposure to dideoxynucleoside analogs was the only ART factor that was significantly associated with an increased risk of fatty liver in multivariable analysis. The dideoxynucleoside analogs inhibit mitochondrial polymerase γ ; subsequent accumulation of dysfunctional mitochondria leads to increased lipid accumulation within hepatocytes (43). Dideoxynucleoside analog use has previously been associated with increased risk of hepatic steatosis and steatosis progression in a cross-sectional and prospective study, respectively, of HIV/HCV-coinfected individuals (44,45). In an HIV-monoinfected cohort, cumulative NRTI exposure was independently associated with increased risk of nonalcoholic fatty liver disease, although no individual agents increased the risk of nonalcoholic fatty liver disease (5). It is encouraging that the newer antiretroviral agents which comprise the majority of current use were not associated with fatty liver.
One limitation of our study was the inability to assess whether HIV infection itself directly affects steatosis, as 92 % of the men were receiving ART. Another limitation is that, although we adjusted for numerous factors in our multivariable model, residual confounding is likely. Finally, our findings may not be applicable to the general HIV-infected population as the MACS is a male cohort, includes few injection drug users, and mostly comprises people with relatively high socioeconomic status. Further studies in other cohorts that have HIV-infected and -uninfected persons are needed.
In summary, in this large cohort of well-characterized HIV-infected and HIV-uninfected men, we found that CT-defined fatty liver was common and was positively associated with abdominal visceral adiposity volume, HOMA-IR, and PNPLA3 rs738409, whereas ART-treated HIV infection was associated with a lower risk of fatty liver. Among the HIV-infected men, prolonged exposure to dideoxynucleoside analog drugs was associated with a higher risk of fatty liver but none of the newer agents, which are used commonly today, were associated with it. These findings may help clinicians identify HIV-infected patients who are at increased risk of developing fatty liver so that potentially modifiable fatty liver risk factors can be targeted for intervention. Future studies are needed to determine whether HIV infection modifies the risk for development of hepatic fibrosis and adverse liver-related outcomes in persons with fatty liver disease.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
✓ Nonalcoholic fatty liver disease is common in the general population.
✓ Metabolic abnormalities and abdominal visceral adiposity are associated with an increased risk of fatty liver and are also common in antiretroviral therapy (ART)-treated HIV-infected individuals.
✓ Whether individuals with HIV on ART are at increased risk of fatty liver is unclear.
WHAT IS NEW HERE
✓ HIV-infected men (92 % on ART) in the Multicenter AIDS Cohort Study (MACS) had a lower prevalence of CT-defined fatty liver compared with HIV-uninfected controls, even after adjusting for metabolic and genetic risk factors for fatty liver.
✓ This suggests that, in the current ART era, HIV-infected individuals are not at increased risk of fatty liver and that fatty liver is predominantly mediated by metabolic and genetic factors in the setting of HIV.
✓ Further research is needed to confirm these findings and to determine whether HIV modifies the risk of steatohepatitis and fibrosis in persons with fatty liver.
ACKNOWLEDGMENTS
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA).
Financial support: The MACS CVD study is funded by NHLBI: RO1 HL095129 (Post). The MACS is funded by NIAID, with additional supplemental funding from NCI, UO1-AI-35042, UL1-RR025005 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041. This work was supported by a Clinical Research Award from the American College of Gastroenterology.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantor of the article: Jennifer C. Price, MD.
Specific author contributions: Jennifer C. Price was involved in study concept and design, analysis and interpretation of data, drafting of the manuscript, and obtaining funding for the study. Eric C. Seaberg was involved in analysis and interpretation of data, and in critical revision of the manuscript for important intellectual content. Rachel Latanich was involved in acquisition of data and technical support. Matthew J. Budoff was involved in study concept and design, acquisition of data, technical support, and critical revision of the manuscript for important intellectual content. Lawrence A. Kingsley was involved in critical revision of the manuscript for important intellectual content. Frank J. Palella was involved in study concept and design, and in critical revision of the manuscript for important intellectual content. Mallory D. Witt was involved in critical revision of the manuscript for important intellectual content. Wendy S. Post was involved in study concept and design, critical revision of the manuscript for important intellectual content, and obtaining funding for the study. Chloe L. Thio was involved in study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, acquisition of data, and study supervision.
Potential competing interests: None.
DISCLAIMER
The contents of this publication are solely the responsibility of the authors and do not represent the offi cial views of the National Institutes of Health (NIH).
REFERENCES
- 1.Smith C. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2011;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 2.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–73. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–7. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 6.Hadigan C, Liebau J, Andersen R, et al. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–7. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 7.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 9.Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–7. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 11.Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: a meta-analysis of the risk factors. Hepatology. 2010;52:71–8. doi: 10.1002/hep.23619. [DOI] [PubMed] [Google Scholar]
- 12.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–94. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 14.Trepo E, Pradat P, Potthoff A, et al. Impact of patatin-like phospholipase-3 (rs738409 C > G) polymorphism on fibrosis progression and steatosis in chronic hepatitis C. Hepatology. 2011;54:60–9. doi: 10.1002/hep.24350. [DOI] [PubMed] [Google Scholar]
- 15.Valenti L, Rumi M, Galmozzi E, et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791–9. doi: 10.1002/hep.24123. [DOI] [PubMed] [Google Scholar]
- 16.Speliotes EK, Yerges-Armstrong LM, Wu J, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemoine M, Barbu V, Girard PM, et al. Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS. 2006;20:387–95. doi: 10.1097/01.aids.0000206503.01536.11. [DOI] [PubMed] [Google Scholar]
- 18.Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:329–37. doi: 10.1016/s1473-3099(03)00654-6. [DOI] [PubMed] [Google Scholar]
- 19.Lemoine M, Ingiliz P. Liver injury in HIV monoinfected patients: should we turn a blind eye to it? Clin Res Hepatol Gastroenterol. 2012;36:441–7. doi: 10.1016/j.clinre.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Rivero-Juarez A, Camacho A, Merchante N, et al. Incidence of liver damage of uncertain origin in HIV patients not co-infected with HCV/HBV. PLoS One. 2013;8:e68953. doi: 10.1371/journal.pone.0068953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 22.Dudley J, Jin S, Hoover D, et al. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–30. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 23.Lee SS, Park SH, Kim HJ, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–85. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 25.Witt MD, Seaberg EC, Darilay A, et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis 2013. 57:77–84. doi: 10.1093/cid/cit197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 27.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 28.Price JC, Seaberg EC, Badri S, et al. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis. 2012;205:1005–13. doi: 10.1093/infdis/jir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFilippis AP, Blaha MJ, Martin SS, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2013;227:429–36. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013. 178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Vecchi V, Soresi M, Giannitrapani L, et al. Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int J Infect Dis. 2012;16:e397–402. doi: 10.1016/j.ijid.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Ryan P, Blanco F, Garcia-Gasco P, et al. Predictors of severe hepatic steatosis using abdominal ultrasound in HIV-infected patients. HIV Med. 2009;10:53–9. doi: 10.1111/j.1468-1293.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Park SH, Hahn L, et al. Specificity of unenhanced CT for noninvasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol. 2012;22:1075–82. doi: 10.1007/s00330-011-2349-2. [DOI] [PubMed] [Google Scholar]
- 34.Monto A, Dove LM, Bostrom A, et al. Hepatic steatosis in HIV/hepatitis C coinfection: prevalence and significance compared with hepatitis C monoinfection. Hepatology. 2005;42:310–6. doi: 10.1002/hep.20805. [DOI] [PubMed] [Google Scholar]
- 35.Gaslightwala I, Bini EJ. Impact of human immunodeficiency virus infection on the prevalence and severity of steatosis in patients with chronic hepatitis C virus infection. J Hepatol. 2006;44:1026–32. doi: 10.1016/j.jhep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Castera L, Loko MA, Le Bail B, et al. Hepatic steatosis in HIV-HCV coin-fected patients in France: comparison with HCV monoinfected patients matched for body mass index and HCV genotype. Aliment Pharmacol Ther. 2007;26:1489–98. doi: 10.1111/j.1365-2036.2007.03533.x. [DOI] [PubMed] [Google Scholar]
- 37.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sookoian S, Pirola CJ. PNPLA3, the history of an orphan gene of the potate tuber PROTEIN family that found an organ: the liver. Hepatology. 2014 doi: 10.1002/hep.26895. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 39.Graff M, North KE, Franceschini N, et al. PNPLA3 gene-by-visceral adipose tissue volume interaction and the pathogenesis of fatty liver disease: the NHLBI family heart study. Int J Obes (Lond) 2013;37:432–8. doi: 10.1038/ijo.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 41.Felmlee DJ, Hafirassou ML, Lefevre M, et al. Hepatitis C virus, cholesterol and lipoproteins--impact for the viral life cycle and pathogenesis of liver disease. Viruses. 2013;5:1292–324. doi: 10.3390/v5051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonardo A, Loria P, Adinolfi LE, et al. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73–80. doi: 10.1111/j.1365-2893.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 43.Stankov MV, Panayotova-Dimitrova D, Leverkus M, et al. Autophagy inhibition due to thymidine analogues as novel mechanism leading to hepatocyte dysfunction and lipid accumulation. AIDS. 2012;26:1995–2006. doi: 10.1097/QAD.0b013e32835804f9. [DOI] [PubMed] [Google Scholar]
- 44.McGovern BH, Ditelberg JS, Taylor LE, et al. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006;43:365–72. doi: 10.1086/505495. [DOI] [PubMed] [Google Scholar]
- 45.Macias J, Berenguer J, Japon MA, et al. Hepatic steatosis and steatohepatitis in human immunodeficiency virus/hepatitis C virus-coinfected patients. Hepatology. 2012;56:1261–70. doi: 10.1002/hep.25791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.