Abstract
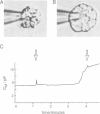
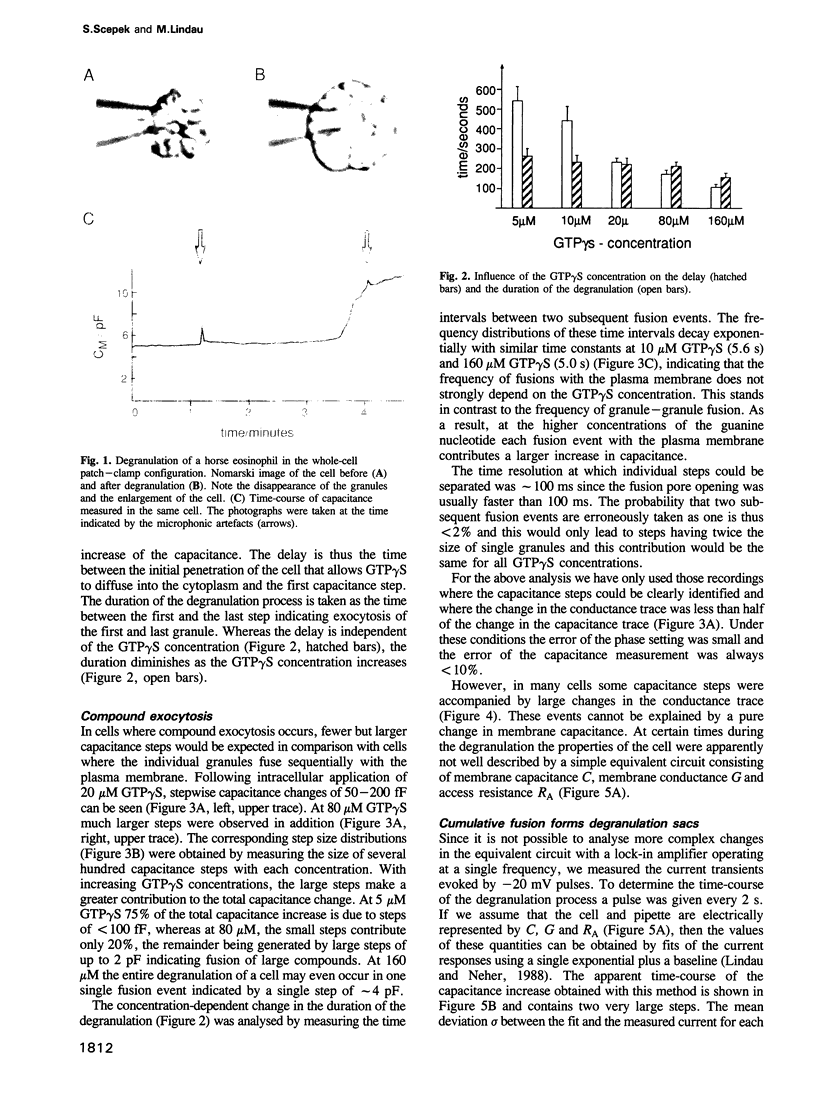
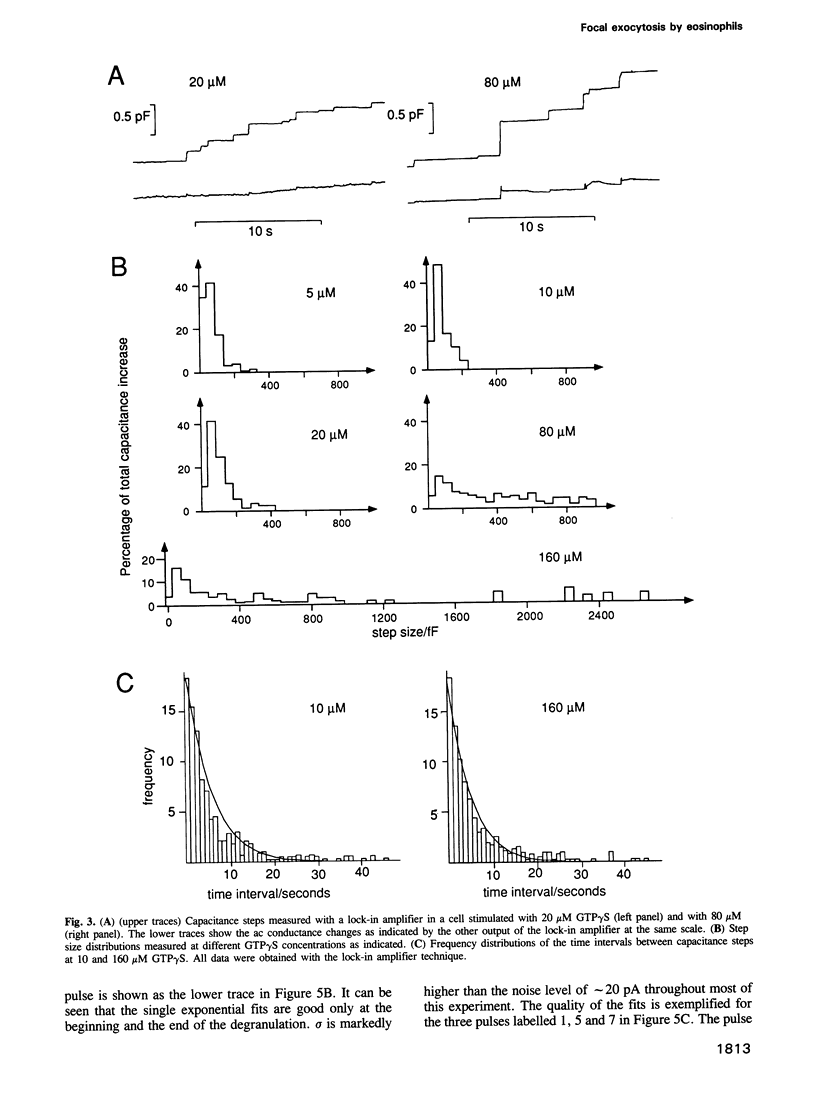
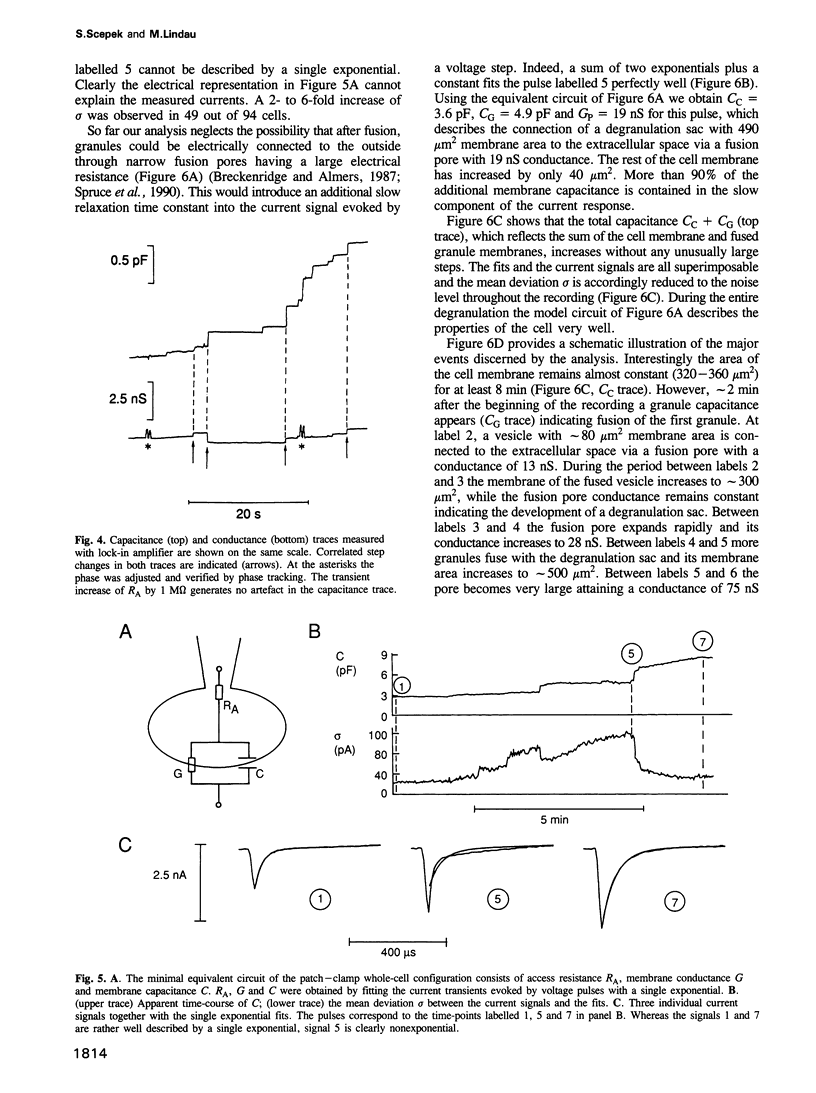
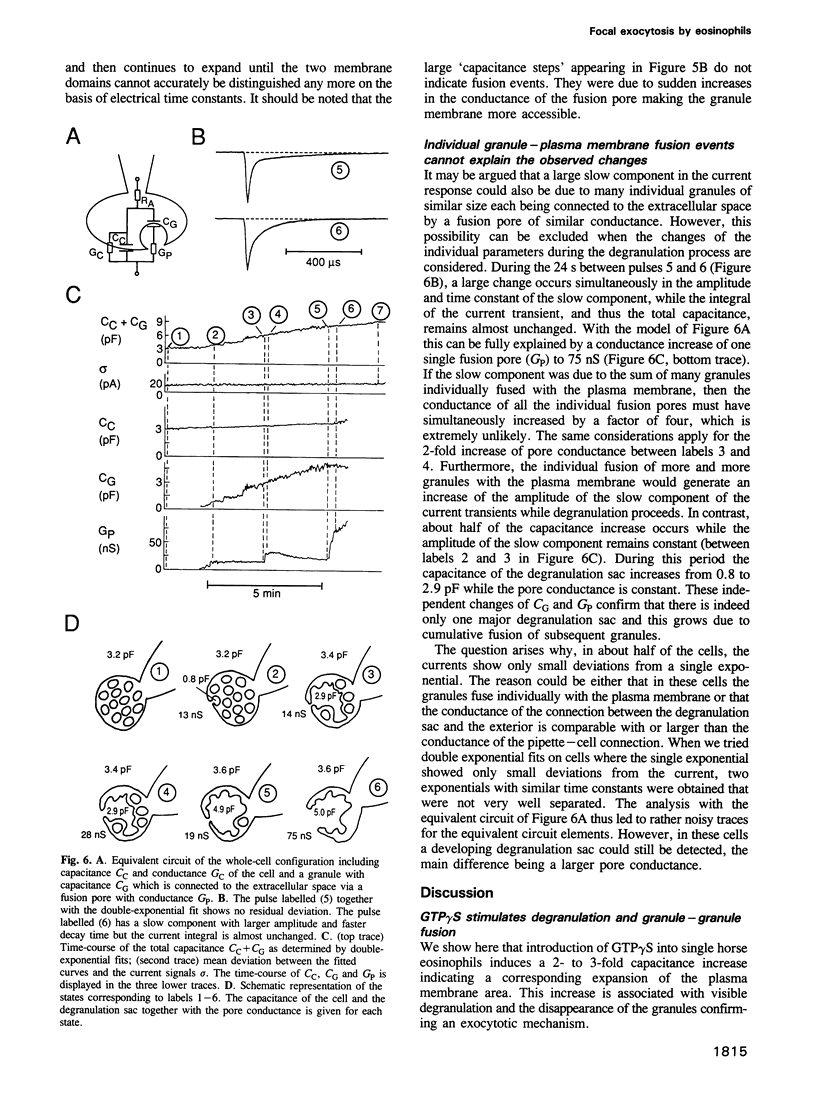
We have investigated the granule fusion events during exocytosis in horse eosinophils by time-resolved patch-clamp capacitance measurements. Stimulation with intracellular GTP gamma S leads to a stepwise capacitance increase by 4.0 +/- 0.9 pF. At GTP gamma S concentrations < 20 microM the step size distribution is in agreement with the granule size distribution in resting cells. Above 80 microM the number of steps is reduced and very large steps occur. The total capacitance increase, however, is unaffected. These results show that at high GTP gamma S concentrations granule--granule fusion occurs inside the cell forming large compound granules, which then fuse with the plasma membrane (compound exocytosis). The electrical equivalent circuit of the cell during degranulation indicates the formation of a degranulation sac by cumulative fusion events. Fusion of the first granule with the plasma membrane induces fusion of further granules with this granule directing the release of all the granular material to the first fusion pore. The physiological function of eosinophils is the killing of parasites. Compound exocytosis and cumulative fusion enable the cells to focus the release of cytotoxic proteins to well defined target regions and prevent uncontrolled diffusion of this material, which would damage intact host cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez de Toledo G., Fernandez J. M. Compound versus multigranular exocytosis in peritoneal mast cells. J Gen Physiol. 1990 Mar;95(3):397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990 Dec;15(12):473–477. doi: 10.1016/0968-0004(90)90301-q. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Bennett J. P., Gomperts B. Freeze-fracture studies of chemotactic peptide-induced exocytosis in neutrophils: evidence for two patterns of secretory granule fusion. J Ultrastruct Res. 1983 Feb;82(2):221–232. doi: 10.1016/s0022-5320(83)90055-2. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Galli S. J., Morgan E., Galli A. S., Hammond M. E., Dvorak H. F. Anaphylactic degranulation of guinea pig basophilic leukocytes. I. Fusion of granule membranes and cytoplasmic vesicles formation and resolution of degranulation sacs. Lab Invest. 1981 Feb;44(2):174–191. [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Butterworth A. E., Sturrock R. F., Houba V. The mechansim of antibody-dependent, eosinophil-mediated damage to schistosomula of Schistosoma mansoni in vitro: a study by phase-contrast and electron microscopy. J Cell Sci. 1978 Dec;34:173–192. doi: 10.1242/jcs.34.1.173. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991 Mar 8;64(5):915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Jörg A., Klebanoff S. J. Horse eosinophil degranulation induced by the ionophore A23187. Ultrastructure and role of phospholipase A2. Am J Pathol. 1983 Jun;111(3):341–349. [PMC free article] [PubMed] [Google Scholar]
- Lawson D., Fewtrell C., Raff M. C. Localized mast cell degranulation induced by concanavalin A-sepharose beads. Implications for the Ca2+ hypothesis of stimulus-secretion coupling. J Cell Biol. 1978 Nov;79(2 Pt 1):394–400. doi: 10.1083/jcb.79.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Lindau M., Nüsse O., Bennett J., Cromwell O. The membrane fusion events in degranulating guinea pig eosinophils. J Cell Sci. 1993 Jan;104(Pt 1):203–210. doi: 10.1242/jcs.104.1.203. [DOI] [PubMed] [Google Scholar]
- Lindau M. Time-resolved capacitance measurements: monitoring exocytosis in single cells. Q Rev Biophys. 1991 Feb;24(1):75–101. doi: 10.1017/s0033583500003279. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., Mackenzie C. D., Ramalho-Pinto F. J. Ultrastructural observations on the in vitro interaction between rat eosinophils and some parasitic helminths (Schistosoma mansoni, Trichinella spiralis and Nippostrongylus brasiliensis). Clin Exp Immunol. 1977 Oct;30(1):105–118. [PMC free article] [PubMed] [Google Scholar]
- Nüsse O., Lindau M., Cromwell O., Kay A. B., Gomperts B. D. Intracellular application of guanosine-5'-O-(3-thiotriphosphate) induces exocytotic granule fusion in guinea pig eosinophils. J Exp Med. 1990 Mar 1;171(3):775–786. doi: 10.1084/jem.171.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. The mechanisms which produce vacuolated and degranulated eosinophils. Br J Haematol. 1981 Oct;49(2):219–226. doi: 10.1111/j.1365-2141.1981.tb07218.x. [DOI] [PubMed] [Google Scholar]
- Vogel S. S., Zimmerberg J. Proteins on exocytic vesicles mediate calcium-triggered fusion. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4749–4753. doi: 10.1073/pnas.89.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]