Abstract
Febuxostat is a nonpurine xanthine oxidase (XO) inhibitor, which recently received marketing approval. However, information regarding the experience with this agent among advanced chronic kidney disease (CKD) patients is limited. In the current study, we investigated the effects of oral febuxostat in patients with advanced CKD with asymptomatic hyperuricemia. We demonstrated, for the first time, that not only the serum levels of uric acid (UA) but also those of 8-hydroxydeoxyguanosine, an oxidative stress marker, were significantly reduced after six months of febuxostat treatment, with no adverse events. These results encouraged us to pursue further investigations regarding the clinical impact of lowering the serum UA levels with febuxostat in advanced CKD patients in terms of concomitantly reducing oxidative stress via the blockade of XO. More detailed studies with a larger number of subjects and assessments of the effects of multiple factors affecting hyperuricemia, such as age, sex, and dietary habits, would shed light on the therapeutic challenges of treating asymptomatic hyperuricemia in patients with various stages of CKD.
Keywords: febuxostat, chronic kidney disease, hemodialysis, uric acid, oxidative stress
Introduction
Hyperuricemia, defined as a serum urate level exceeding the limit of solubility, mirrors supersaturation of the extracellular fluid with urate, and predisposes affected subjects to gout, which is characterized by the tissue deposition of monosodium urate crystals, although it is a necessary but not a substantial factor for the development of the disease.1 The current urate-lowering strategies include reducing the urate production with xanthine oxidase (XO) inhibitors and accelerating the urinary excretion of uric acid (UA) with uricosuric agents.2,3 Uricosuric agents, such as probenecid and benzbromarone, may have limited effectiveness in patients with reduced renal function.3,4 The purine analog XO inhibitor, allopurinol, has remained widely prescribed for the treatment of hyperuricemia, but requires dose adjustment in subjects with renal impairment, which may lead to a reduced benefit.2,3,5,6
Febuxostat, a nonpurine XO inhibitor that recently received marketing approval, has been focused on as an alternative option for the treatment of hyperuricemia in patients with chronic kidney disease (CKD) because it undergoes hepatic metabolism and may require less dose adjustment in association with the renal function.6,7 Moreover, several lines of evidence have focused on the blockade of XO activity as a potential therapeutic strategy for various other kinds of oxidative stress-mediated tissue and vascular injuries.8,9 However, information regarding the experience with this therapeutic agent among patients with advanced CKD is limited.7 In this regard, the current study investigated the effects of febuxostat in patients with advanced CKD with hyperuricemia in terms of the reduction of the serum UA levels and the longitudinal changes in several serum indicators for oxidative stress.
Materials and Methods
Seventeen patients on chronic hemodialysis (HD) treatment who had serum UA levels above 8.0 mg/dL and who were not receiving anti-hyperuricemic agents participated in the study. All subjects had oliguria or anuria. The subjects had to be in stable condition, and they had no history of active liver diseases or any other significant medical status, no change in diuretics or steroid therapy within one month of study enrollment and were not chronic users of any nonsteroidal anti-inflammatory drugs. The usual medications, such as anti-hypertensive agents, erythropoietin, and phosphate binders, were continued during the study period. Sex was not considered. The exclusion criteria were as follows: age <20 years or >90 years, type I diabetes mellitus or type II diabetes mellitus with poor glucose control (glycosylated hemoglobin >9% at the start of the observation period), treatment with mercaptopurine hydrate or azathiopurine, pregnancy, and any medical or surgical conditions that made patients unsuitable for this study as judged by the attending physician. All patients were assigned to oral febuxostat and entered the six-month treatment period from July through August 2012, during which they initially received febuxostat 10 mg orally once daily in the morning. The target serum UA level was <6.0 mg/dL, and the dose of febuxostat was titrated or increased up to a maximum of 40 mg/day.
The blood pressure (BP) was measured before all HD sessions, and the data regarding the systolic BP and diastolic BP were the average of each value on the last HD day of the week. The blood samples were obtained from vascular access, including arteriovenous fistulas and arteriovenous grafts, before HD sessions. The hemoglobin (Hb), hematocrit (Hct), platelet count (Plt), serum levels of UA, blood urea nitrogen (BUN), creatinine (Cr), sodium (Na), chloride (Cl), potassium (K), calcium (Ca), inorganic phosphate (Pi), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were measured at baseline (week 0) and every four weeks during the observation period. The serum levels of 8-hydroxydeoxyguanosine (8-OHdG), 3-nitrotyrosine-modified proteins (3-NT), and protein carbonyls were determined at baseline and at weeks 4, 12, and 24 during the treatment period. The serum levels of 8-OHdG were measured by an enzyme-linked immunosorbent assay (ELISA) as described previously.10 The ELISA method was also used to measure the serum levels of 3-NT (Japan Institute for the Control of Aging, Nikken SEIL Co, Shizuoka, Japan) and protein carbonyls (BioCell Co, Auckland, New Zealand). This study was performed in accordance with the Declaration of Helsinki and was approved by the medical ethics committee of Jichi Medical University, and all patients included in the present study provided their informed consent.
The data were expressed either as the number of participants or as the percentage (%) of the study population. The remaining data were expressed as the means ± standard deviation (SD), or as medians and interquartile ranges (IR) for variables with a skewed distribution. A repeated measures analysis of variance combined with Fisher’s protected least significant difference test for normal distributions and the Kruskal–Wallis test with Dunn’s method for skewed distributions were used to compare the time course data, when appropriate. Values of P < 0.05 were considered to be statistically significant. The statistical analyses were performed using the SigmaPlot 12 software program for Windows (Systat Software, Inc., San Jose, CA) unless otherwise stated.
Results and Discussion
The demographic profiles of the 17 patients included in the present study are summarized in Table 1. No subjects had a history of gouty attacks. The patients had been treated with chronic HD for a median of four years. The causes of advanced CKD included diabetic nephropathy, chronic glomerulonephritis, hypertensive nephrosclerosis, and polycystic kidney disease. All subjects were on the optimum tolerated medical management. Febuxostat lowered the serum UA levels (8.9 ± 1.0 at baseline) significantly from one month after the initiation of the treatment (Fig. 1), and the target serum UA level (<6.0 mg/dL) was achieved in 12 patients (70.5%) after one month of treatment, compared to 13 (76.4%) and 14 (82.3%) patients after three and six months of treatment, respectively.
Table 1.
Demographic profiles of the patients at the start of the study.
| DEMOGRAPHIC CHARACTERISTICS | |
|---|---|
| Age (years) | 64 ± 10 |
| Sex (male/female) | 15/2 |
| HD duration (years) | 6.4 ± 5.7 |
| UNDERLYING CAUSES OF CKD, N (%) | |
| Diabetic nephropathy | 9 (53) |
| Chronic glomerulonephritis | 3 (18) |
| Hypertensive nephrosclerosis | 3 (18) |
| Polycystic kidney disease | 2 (12) |
| MEDICATIONS, N (%) | |
| Calcium channel antagonist(s) | 7 (41) |
| Angiotensin-converting-enzyme inhibitor | 1 (6) |
| Angiotensin receptor blocker(s) | 7 (41) |
| Renin inhibitor | 1 (6) |
| Other anti-hypertensive agent(s) | 4 (24) |
| Loop diuretic(s) | 3 (18) |
Figure 1.
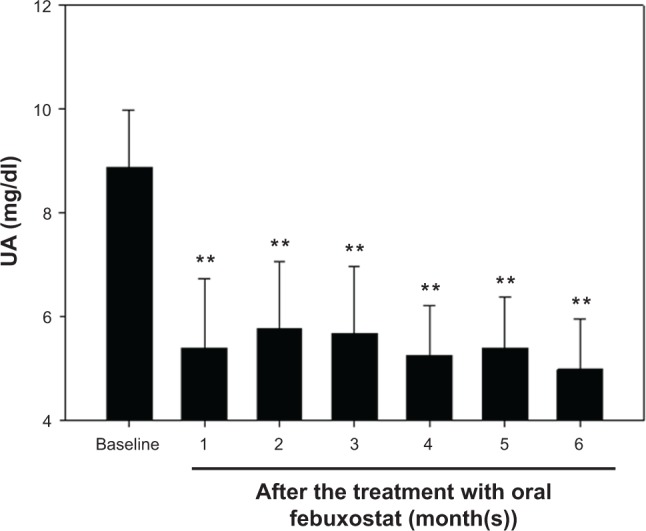
The serum UA levels before and after the initiation of the treatment with oral febuxostat. After six months of febuxostat treatment, the serum UA levels were significantly decreased from those at baseline. All patients were entered into the six-month treatment period from July through August 2012. Note that the decrease in the serum UA levels was already significant after one month treatment with febuxostat.
Notes: N = 17, **P < 0.01 versus baseline.
After six months, 16 subjects were still under the treatment with oral febuxostat (10 mg/day), and there was one subject who was being treated with a reduced dose of 5 mg/day. Also, only two patients were treated with the agent at a dose of above 10 mg/day at that point, one with 20 mg/day and one with 40 mg/day. All doses of febuxostat were well tolerated by the patients with no withdrawals because of side effects or allergic reactions. Although there was a significant increase in the systolic BP values obtained at five and six months compared to those observed at baseline, no significant changes in diastolic BP, Hb, Hct, Plt, BUN, or the serum levels of Cr, Na, Cl, K, Ca, Pi, AST, ALT, or LDH were noted during the observation period (Table 2). There were similar trends in the serum levels of protein carbonyls and 3-NT, while the serum 8-OHdG levels measured after six months were significantly lower than those at the baseline (Table 3). No patients experienced symptoms of gouty arthritis, including joint pain, swelling, or redness,2 during the observation period.
Table 2.
Changes in clinical parameters during the observation period.
| AFTER THE INITIATION OF ORAL FEBUXOSTAT TREATMENT | P VALUE | |||||||
|---|---|---|---|---|---|---|---|---|
| BASELINE | 1 MONTH | 2 MONTHS | 3 MONTHS | 4 MONTHS | 5 MONTHS | 6 MONTHS | ||
| Systolic BP (mmHg) | 141 ± 20 | 137 ± 16 | 141 ± 29 | 141 ± 16 | 142 ± 21 | 148 ± 21* | 149 ± 19* | <0.001 |
| Diastolic BP (mmHg) | 74 ± 11 | 73 ± 9 | 73 ± 10 | 76 ± 10 | 76 ± 12 | 74 ± 11 | 73 ± 11 | 0.186 |
| Hb (g/dL) | 10.7 ± 1.0 | 10.8 ± 1.0 | 10.9 ± 1.1 | 10.8 ± 1.6 | 10.9 ± 1.3 | 10.9 ± 1.3 | 10.6 ± 1.0 | 0.989 |
| Hct (%) | 33.9 ± 3.1 | 33.9 ± 3.2 | 33.5 ± 3.4 | 34.1 ± 3.4 | 34.0 ± 4.3 | 34.0 ± 3.6 | 33.1 ± 2.9 | 0.976 |
| Plt (×104/μl) | 16.8 (IR: 14.2–22.7) | 15.6 (IR: 13.6–25.1) | 17.8 (IR: 14.2–24.6) | 18.4 (IR: 14.9–25.2) | 17.7 (IR: 12.8–23.1) | 16.7 (IR: 12.8–21.6) | 18.3 (IR: 11.7–21.8) | 0.887 |
| BUN (mg/dL) | 62.1 ± 11.7 | 64.8 ± 18.6 | 65.1 ± 15.8 | 63.2 ± 14.1 | 64.7 ± 19.1 | 67.0 ± 21.4 | 69.9 ± 16.5 | 0.886 |
| Cr (mg/dL) | 11.4 ± 1.9 | 11.8 ± 2.0 | 11.8 ± 1.9 | 11.8 ± 2.3 | 11.9 ± 2.2 | 11.5 ± 2.2 | 11.5 ± 2.2 | 0.991 |
| Na (mmol/l) | 138 ± 4 | 139 ± 4 | 140 ± 4 | 139 ± 4 | 139 ± 4 | 139 ± 3 | 138 ± 2 | 0.805 |
| K (mmol/l) | 4.9 ± 0.9 | 4.9 ± 0.8 | 5.0 ± 1.0 | 5.0 ± 0.9 | 4.9 ± 0.7 | 5.0 ± 0.8 | 5.0 ± 0.9 | 0.999 |
| CI (mmol/l) | 102 ± 5 | 103 ± 4 | 103 ± 4 | 103 ± 4 | 103 ± 5 | 102 ± 4 | 103 ± 3 | 0.93 |
| Ca (mg/dL) | 8.8 ± 0.8 | 9.0 ± 0.9 | 8.8 ± 0.6 | 8.8 ± 0.6 | 8.9 ± 0.7 | 9.1 ± 0.8 | 9.0 ± 0.9 | 0.852 |
| Pi (mg/dL) | 4.5 ± 1.3 | 5.1 ± 1.2 | 5.3 ± 1.4 | 5.0 ± 0.9 | 5.1 ± 1.5 | 5.6 ± 1.5 | 4.9 ± 1.0 | 0.296 |
| AST (U/l) | 16.5 ± 15.8 | 18.0 ± 15.4 | 15.0 ± 6.7 | 15.0 ± 6.4 | 17.2 ± 7.5 | 18.4 ± 8.4 | 18.3 ± 13.6 | 0.938 |
| ALT (U/l) | 17.9 ± 17.6 | 18.8 ± 16.7 | 15.2 ± 9.4 | 14.4 ± 8.3 | 14.0 ± 8.0 | 17.5 ± 10.4 | 19.4 ± 18.2 | 0.857 |
| LDH (U/l) | 179 ± 40 | 189 ± 39 | 179 ± 28 | 178 ± 26 | 180 ± 30 | 191 ± 45 | 188 ± 48 | 0.923 |
Note:
P < 0.05 versus baseline.
Table 3.
The serum levels of several oxidative stress markers before and after the initiation of the treatment with oral febuxostat.
| AFTER THE INITIATION OF ORAL FEBUXOSTAT TREATMENT | P VALUE | ||||
|---|---|---|---|---|---|
| BASELINE | 1 MONTH | 3 MONTHS | 6 MONTHS | ||
| Protein carbonyls (nmol/mg protein) | 0.07 (IR: 0.03–0.12) | 0.10 (IR: 0.06–0.14) | 0.07 (IR: 0.06–0.15) | 0.05 (IR: 0.03–0.11) | 0.329 |
| 3-NT (nM) | 30.4 (IR: 25.2–40.4) | 33.2 (IR: 25.5–42.2) | 37.3 (IR: 25.3–45.3) | 28.7 (IR: 24.7–43.9) | 0.928 |
| 8-OHdG (ng/ml) | 1.64 (IR: 1.24–2.95) | 2.51 (IR: 1.24–4.74) | 1.05 (IR: 0.84–1.87) | 0.54 (IR: 0.204–1.16)* | 0.002 |
Note:
P < 0.05 versus baseline.
The treatment of patients with asymptomatic hyperuricemia (a serum UA level higher than 8 mg/dL) with urate-lowering agents has been recommended and applied in Japan,7,11 while the appropriate dose of febuxostat among subjects with advanced CKD has not yet been established. The current observations suggest that even relatively low doses of febuxostat, which is approved at a dose of 40 to 60 mg/day as the standard dose for the treatment of hyperuricemia with or without gouty arthritis in Japan, may also work effectively among chronic HD patients for reducing the serum UA to a level that has been arbitrarily proposed as a therapeutic target for hyperuricemia.7,11,12 The validity of the indications for urate-lowering agents among overall subjects with asymptomatic hyperuricemia remains to be delineated, and clinicians should bear in mind that the prevalence of refractory gout and/or gouty tophi is much lower in Japan in comparison to that in the United States and Europe, where negative opinions regarding pharmaceutical interventions predominate.11–14 One may argue that the clinical benefit of using urate-lowering agents requires careful evaluation, especially in subjects on chronic HD treatment, since an incipient gouty attack is quite rare in hyperuricemic long-term HD patients, and the frequency of gouty arthritis has been shown to decrease after the initiation of a periodic HD program in advanced CKD subjects.15,16 Moreover, a significant association between higher serum UA levels and lower mortality, which may be dependent on the favorable nutritional status, has been demonstrated in the HD population.17 Otherwise, it may be necessary to focus on the fact that some of the reactive oxygen species are produced as a by-product of urate formation through a XO-dependent pathway and the pharmacological nature of febuxostat, which is characterized by a higher bioavailability and a more potent blockade of XO activity than the traditional XO inhibitor allopurinol.1,2,8,18 Indeed, the superior potency of febuxostat to allopurinol for the inhibition of reactive oxygen synthesis has been demonstrated in several reports,19,20 and the serum UA levels could be used as a surrogate indicator of the XO activity.
In the current study, 8-OHdG, 3-NT, and protein carbonyls, which have been included in the list of the most common oxidative stress biomarkers in various settings,20,21 were used as the indices of the condition of the present patients. Numerous processes other than the XO-mediated pathway have been implicated in the oxidative stress among the subjects with CKD.21,22 In addition, it has been shown that chronic HD treatment also leads to excessive radical production and impairment of the anti-oxidant capacity.23 Despite the lack of qualitative information regarding the significance of individual oxidative stress markers,24 we feel that it is reasonable to consider that 8-OHdG, but not 3-NT and protein carbonyls, may help to detect the inhibitory effects of febuxostat on XO-mediated oxidative stress among the chronic HD patients. Although the precise role of febuxostat in reducing the serum levels of 8-OHdG remains to be delineated, our findings suggest that the generation of 8-OHdG may be more dependent on XO than that of 3-NT or protein carbonyls. On the other hand, it has been proposed that the XO inhibitors may be utilized as adjunctive anti-hypertensive agents in some subsets of hyperuricemic and hypertensive subjects associated with or without advanced CKD,25,26 while we found that the systolic BP levels after five and six months of treatment with febuxostat were higher than those at baseline, despite the titration of anti-hypertensive agents corresponding to the context. We have no explanation for this discrepancy; however, a seasonal bias may have been involved. Indeed, it has been reported that the systolic BP shows seasonal changes in chronic HD patients, with peak BP noted in the winter.27
Finally, the number of patients included in the present series was quite small, thus implying that this study may be statistically underpowered or that the clinical parameters may have been overestimated. As such, our findings should be interpreted with caution. Nevertheless, our results encourage us to pursue further investigations regarding the clinical impact of lowering the serum UA level with febuxostat in chronic HD patients in terms of determining the appropriate dose of the agent as well as concomitantly reducing oxidative stress by blocking XO. Indeed, despite the comparable serum levels of low-density lipoprotein (LDL) between baseline and week 24, we found that the serum levels of the oxidative form of LDL, another oxidative stress marker,20 at week 24 were significantly decreased compared to those observed at baseline among the 12 subjects who led us to determine the serum levels of these parameters (data not shown). Obviously, more detailed studies with a larger number of subjects and assessments of the effects of multiple factors affecting hyperuricemia, such as age, sex, and dietary habits,2,14 would shed light on the therapeutic challenges of treating asymptomatic hyperuricemia in patients with various stages of CKD.28
Footnotes
Author Contributions
TA drafted the manuscript. YM, CI, OI, ST, and YW made contributions to the acquisition of the clinical data. EK and DN provided a detailed review of the contents and structure of the manuscript, resulting in significant changes to the original document. All authors have read and approved the final manuscript.
ACADEMIC EDITOR: Anuj Chauhan, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Gaffo AL, Saag KG. Febuxostat: the evidence for its use in the treatment of hyperuricemia and gout. Core Evid. 2009;4:25–36. doi: 10.2147/ce.s5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker MA, Schumacher HR, Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 3.Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7–13. doi: 10.1097/RHU.0b013e318204aab4. [DOI] [PubMed] [Google Scholar]
- 4.Fujimori S, Ooyama K, Ooyama H, Moromizato H. Efficacy of benzbromarone in hyperuricemic patients associated with chronic kidney disease. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1035–1038. doi: 10.1080/15257770.2011.622732. [DOI] [PubMed] [Google Scholar]
- 5.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33(8):1646–1650. [PubMed] [Google Scholar]
- 6.Hosoya T, Ono I. A repeated oral administration study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with impaired renal function in Japan: pharmacokinetic and pharmacodynamic study. J Clin Rheumatol. 2011;17(4 suppl 2):S27–S34. doi: 10.1097/RHU.0b013e31821d36f2. [DOI] [PubMed] [Google Scholar]
- 7.Horikoshi R, Akimoto T, Inoue M, Morishita Y, Kusano E. Febuxostat for hyperuricemia: experience with patients on chronic hemodialysis treatment. Clin Exp Nephrol. 2013;17(1):149–150. doi: 10.1007/s10157-012-0763-7. [DOI] [PubMed] [Google Scholar]
- 8.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda H, Kawada N, Kaimori JY, et al. Febuxostat suppressed renal ischemia-reperfusion injury via reduced oxidative stress. Biochem Biophys Res Commun. 2012;427(2):266–272. doi: 10.1016/j.bbrc.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Morishita Y, Watanabe M, Hirahara I, Akimoto T, Muto S, Kusano E. Level of 8-OHdG in drained dialysate appears to be a marker of peritoneal damage in peritoneal dialysis. Int J Nephrol Renovasc Dis. 2012;5:9–14. doi: 10.2147/IJNRD.S27553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamanaka H. Japanese society of gout and nucleic acid metabolism. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1018–1029. doi: 10.1080/15257770.2011.596496. [DOI] [PubMed] [Google Scholar]
- 12.El-Zawawy H, Mandell BF. Managing gout: how is it different in patients with chronic kidney disease? Cleve Clin J Med. 2010;77(12):919–928. doi: 10.3949/ccjm.77a.09080. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1312–1324. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EU, Díaz-Torné C, Perez-Ruiz F, March LM. Epidemiology of gout: an update. Best Pract Res Clin Rheumatol. 2010;24(6):811–827. doi: 10.1016/j.berh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Ifudu O, Tan CC, Dulin AL, Delano BG, Friedman EA. Gouty arthritis in end-stage renal disease: clinical course and rarity of new cases. Am J Kidney Dis. 1994;23(3):347–351. doi: 10.1016/s0272-6386(12)80995-4. [DOI] [PubMed] [Google Scholar]
- 16.Ohno I, Ichida K, Okabe H, et al. Frequency of gouty arthritis in patients with end-stage renal disease in Japan. Intern Med. 2005;44(7):706–709. doi: 10.2169/internalmedicine.44.706. [DOI] [PubMed] [Google Scholar]
- 17.Latif W, Karaboyas A, Tong L, et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011;6(10):2470–2477. doi: 10.2215/CJN.00670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J, Struthers A. The role of urate and xanthine oxidase in vascular oxidative stress: future directions. Ther Clin Risk Manag. 2009;5:799–803. doi: 10.2147/tcrm.s5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik UZ, Hundley NJ, Romero G, et al. Febuxostat inhibition of endothelial-bound XO: implication for targeting vascular ROS production. Free Radic Biol Med. 2011;51(1):179–184. doi: 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial) Circ J. 2013;77(8):2043–2049. doi: 10.1253/circj.cj-13-0082. [DOI] [PubMed] [Google Scholar]
- 21.Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636–643. doi: 10.1111/j.1525-139X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths HR, Dias IH, Willetts RS, Devitt A. Redox regulation of protein damage in plasma. Redox Biol. 2014;2:430–435. doi: 10.1016/j.redox.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massy ZA, Stenvinkel P, Drueke TB. The role of oxidative stress in chronic kidney disease. Semin Dial. 2009;22(4):405–408. doi: 10.1111/j.1525-139X.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalalzadeh M, Nurcheshmeh Z, Mohammadi R, Mousavinasab N, Ghadiani MH. The effect of allopurinol on lowering blood pressure in hemodialysis patients with hyperuricemia. J Res Med Sci. 2012;17(11):1039–1046. [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2013;15(6):435–442. doi: 10.1111/j.1751-7176.2012.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argilés A, Mourad G, Mion C. Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N Engl J Med. 1998;339(19):1364–1370. doi: 10.1056/NEJM199811053391904. [DOI] [PubMed] [Google Scholar]
- 28.Murea M. Advanced kidney failure and hyperuricemia. Adv Chronic Kidney Dis. 2012;19(6):419–424. doi: 10.1053/j.ackd.2012.07.008. [DOI] [PubMed] [Google Scholar]


