Abstract
Excess water-soluble vitamins are thought to be eliminated in the urine. We have reported a strong relationship between water-soluble vitamin intake and urinary excretion in females. The relationship, however, is not well understood in males. In the present experiment, 10 Japanese male subjects were given a standard Japanese diet for the first week. The subjects remained on the same diet, and a synthesized water-soluble vitamin mixture containing one time the Dietary Reference Intakes (DRIs) for Japanese was given for the second week, three times the DRIs for the third week, and six times the DRIs for the fourth week. Twenty-four-hour urine samples were collected each week. Urinary excretion levels for seven of the nine water-soluble vitamin levels, excluding vitamin B12 and folate, increased linearly and sharply in a dose-dependent manner. These results suggest that measuring urinary water-soluble vitamins can be good nutritional markers for assessing vitamin intakes in humans.
Keywords: water-soluble vitamins, blood, urine, biomarker, human
Introduction
Evaluating the nutritional levels of individuals is important because metabolism differs among people. Calculating the nutritional intake from recorded dietary intakes has been the most frequently used method. Measuring biomarkers in blood and urine samples has been another common approach.
We have previously reported that urinary excretion of water-soluble vitamins closely reflects the excess water-soluble vitamins in rats1–15 and humans.16–27 Nutritional assessment using biomarkers is persuasive and leads to quick changes in dietary habits. We have not yet assessed whether there is a strong relationship between urinary excretion and vitamin intake in males. We sought to answer this question in the present experiment.
Materials and Methods
This study, conducted from November 13, 2006 to December 8, 2006, was reviewed and approved by the Ethical Committee of The University of Shiga Prefecture. All participants provided written informed consent.
Chemicals
Thiamin hydrochloride (C12H17ClN4OS-HCl; molecular weight = 337.27), riboflavin (C17H20N4O6; molecular weight = 376.37), pyridoxine hydrochloride (C8H11NO3-HCl; molecular weight = 205.63), pyridoxal phosphate (PLP) monohydrate (C8H10NO6P-H2O; molecular weight = 265.16) cyanocobalamin (C63H88CoN14O14P; molecular weight = 1355.40), nicotinamide (C6H6N2O; molecular weight = 122.13), calcium pantothenate (C18H32N2O10-Ca; molecular weight = 476.54), folic acid (C19H19N7O6; molecular weight = 441.40), D(+)-biotin (C10H16N2O3S; molecular weight = 244.31), and L(+)-ascorbic acid (C6H8O6; molecular weight = 176.13) were purchased from Wako Pure Chemical Industries. 4-Pyridoxic acid (4-PIC; C8H9NO4; molecular weight = 183.16) was made by ICN Pharmaceuticals (Costa Mesa, CA, USA) and obtained through (Wako Pure Chemical Industries, Osaka, Japan).
N1-methylnicotinamide (MNA) chloride (C7H9N2O-HCl; = molecular weight = 159.61) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). N1-methyl-2-pyridone-5-carboxamide (2-Py, C7H8N2O2; molecular weight = 152.15) and N1-methyl-4-pyridone-3-carboxamide (4-Py, C7H8N2O2; = 152.15) were synthesized employing the methods of Pullman and Colowick28 and Shibata et al,29 respectively. All other chemicals used were of the highest purity available from commercial sources.
Subjects
Male Japanese university students and faculty members were recruited from The University of Shiga Prefecture. The purpose and protocol of this study were explained to all participants, and written informed consent was obtained. Participants diagnosed with cold or influenza, and those who had taken multi-vitamin supplements at least once during the previous month, were excluded. All subjects passed the regular medical examination in the university. Of the 12 apparently healthy male Japanese who participated in this study, 10 subjects, aged 19–55 years (means ± SD = 26.8 ± 11.0 years) completed the study (Table 1).
Table 1.
Characteristics of Japanese male subjects.
| SUBJECTS | AGE, Y | HEIGHT, m | BODY WEIGHT, g | BMI, kg/m2 |
|---|---|---|---|---|
| 1 | 25 | 167.7 | 63.6 | 22.6 |
| 2 | 23 | 170.4 | 58.5 | 20.1 |
| 3 | 23 | 182.6 | 68.3 | 20.5 |
| 4 | 22 | 171.9 | 60.2 | 20.4 |
| 5 | 20 | 173.0 | 55.4 | 18.5 |
| 6 | 20 | 175.2 | 68.7 | 22.4 |
| 7 | 25 | 178.0 | 63.9 | 20.2 |
| 8 | 19 | 173.3 | 69.9 | 23.3 |
| 9 | 36 | 174.1 | 87.7 | 28.9 |
| 10 | 55 | 177.2 | 68.5 | 21.8 |
| Mean | 26.8 | 174.3 | 66.5 | 21.9 |
| SD | 11.0 | 4.2 | 8.3 | 2.3 |
All subjects (n = 10) were housed in the same facility and given the same diet. The height, body weight, and body mass index (BMI) of the 10 subjects were 174.3 ± 4.2 cm, 66.5 ± 8.3 kg, and 21.9 ± 2.3 kg/m2, respectively (Table 1). The experimental period was 4 weeks. Diet (Fig. 1) consisted of bread (126 g), butter (7 g), ham (38 g), yoghurt (90 g), tomato (40 g), lettuce (40 g), and milk (200 mL) as breakfast; rice (300 g), toasted and seasoned laver (1.4 g), luncheon meat (95 g), boiled egg (55 g), raw cabbage (50 g), mayonnaise (10 g), miso-soup (miso, 10 g), and Japanese tea (200 mL) as lunch; rice (300 g), Pacific saury of the soy sauce taste (70 g), tofu (soybean curd; 150 g), katsuo-bushi (1.5 g), spinach (leaves, boiled; 50 g), soy source (6 g), sesame seeds (1 g), kiwifruit (50 g), and Japanese tea (200 mL) as dinner; and cheese (25 g) and jelly fruit mix (200 g) as midnight snacks. The nutrient elements are shown in Table 2. The subjects were allowed to drink freely natural mineral water (Asahi Oisii Mizu Rokko [Japanese]) obtained from Asahi Soft Drinks Co. (Tokyo, Japan). Nutrients were calculated by using Standard Tables of Food Composition in Japan-2010-.30 In addition, only water-soluble vitamins in food were measured by us.
Figure 1.
Photographs of the food that were administered to the Japanese male subjects during the experiment.
Table 2.
Composition of diet fed to Japanese male subjects during the study.
| BREAKFAST | LUNCH | DINNER | SNACK | TOTAL | |
|---|---|---|---|---|---|
| Energy, kcal, MJ | 694 | 977 | 830 | 225 | 2,726 |
| 2.90 | 4.08 | 3.47 | 1.07 | 11.39 | |
| Protein, g | 28.6 | 30.7 | 31.7 | 10.3 | 101.2 |
| Fat, g | 26.9 | 38.2 | 19.5 | 6.5 | 91.1 |
| Carbohydrate, g | 84.7 | 123.3 | 127.8 | 30.9 | 366.7 |
| Vitamins | |||||
| Vitamin A, μgRE | 152 | 166 | 271 | 65 | 653 |
| Vitamin D, μg | 0.9 | 1.1 | 9.2 | 0.0 | 11.1 |
| Vitamin E, mg | 1.66 | 2.26 | 4.50 | 0.28 | 8.69 |
| Vitamin K, μg | 21 | 81 | 158 | 1 | 260 |
| Vitamin B1, mg | 0.47 | 0.35 | 0.26 | 0.05 | 1.12 |
| (0.37) | (0.34) | (0.50) | (0.05) | (1.26) | |
| Vitamin B2, mg | 0.56 | 0.73 | 0.33 | 0.10 | 1.72 |
| (0.45) | (0.50) | (0.29) | (0.05) | (1.29) | |
| Vitamin B6, mg | 0.27 | 0.33 | 0.50 | 0.02 | 1.12 |
| (0.23) | (0.23) | (0.36) | (0.02) | (0.84) | |
| Vitamin B12, μg | 1.0 | 2.7 | 8.8 | 0.8 | 13.3 |
| (1.01) | (3.11) | (5.60) | (0) | (9.72) | |
| Vitamins | |||||
| Niacin, mgNE1 | 9.8 | 9.2 | 10.5 | 2.0 | 31.5 |
| (10.3) | (10.0) | (14.7) | (1.3) | (36.3) | |
| Pantothenic acid, mg | 2.4 | 2.6 | 1.5 | 0.1 | 6.6 |
| (2.8) | (2.4) | (1.4) | (0.2) | (6.8) | |
| Folate, μg | 95 | 126 | 132 | 14 | 367 |
| (98) | (81) | (97) | (10) | (286) | |
| Biotin, μg | 10.8 | 18.4 | 4.8 | 0.0 | 34.0 |
| (15.6) | (14.5) | (9.0) | (1.7) | (42.4) | |
| Vitamin C, mg | 36 | 37 | 45 | 7 | 125 |
| (22) | (34) | (45) | (43) | (144) | |
| Minerals | |||||
| Na, mg | 1,190 | 1,777 | 741 | 289 | 3,998 |
| K, mg | 872 | 556 | 856 | 123 | 2,406 |
| Ca, mg | 382 | 148 | 335 | 164 | 1,028 |
| Mg, mg | 72 | 85 | 148 | 11 | 315 |
| P, mg | 531 | 470 | 533 | 195 | 1,728 |
| Fe, mg | 1.2 | 3.9 | 3.7 | 0.3 | 9.2 |
| Zn, mg | 2.8 | 4.4 | 3.9 | 0.8 | 11.9 |
| Cu, mg | 0.23 | 0.54 | 0.77 | 0.02 | 1.56 |
| Mn, μg | 0.39 | 1.53 | 1.82 | 0.02 | 3.76 |
| I, μg | 51 | 94 | 1 | 0 | 146 |
| Se, μg | 39 | 24 | 10 | 0 | 74 |
| Cr, μg | 1 | 1 | 1 | 0 | 3 |
| Mo, μg | 36 | 104 | 95 | 0 | 235 |
Notes: Values were calculated from the data of “Standard Tables of Food Composition in Japan-2010-.” Numbers in parentheses state the water-soluble vitamin content as measured by us.
NE (niacin equivalent): calculated by assuming that 1 mg of nicotinamide can be synthesized from 60 mg of tryptophan and that 100 g of protein contains 1.2 g of tryptophan.
Diet and study design
The subjects took the diet from day 1 to day 5 in each week (experimental period, 4 weeks). But, they lived freely to soften the restraint at day 6 and day 7 in each week. Approximately, 1, 3-, and 6-folds of the synthesized water-soluble vitamin mixture as vitamin mixture α, β, and γ shown in DRIs for Japanese-2010-31 were made (Table 3). They were given the diet only for the first week, the diet with vitamin mixture α for the second week, the diet with vitamin mixture β for the third week, and the diet with vitamin mixture γ for the fourth week. One-third of the dose was put into a small gelatinous capsule, and the capsule was administered three times daily after breakfast, lunch, and dinner. The folate level in vitamin mixture γ exceeds the tolerable upper limit intake of folate (1 mg/d),31 however, does not the no-observed-adverse-effect-level (5 mg/d);31 that figure has been set on toxicological consideration. Thus, the administration of vitamin mixture γ for a week was approved by the Ethical Committee of The University of Shiga Prefecture.
Table 3.
Composition of vitamin mixtures administered during weeks 2, 3, and 4.
| WEEK 2 VITAMIN MIXTURE α |
WEEK 3 VITAMIN MIXTURE β |
WEEK 4 VITAMIN MIXTURE γ |
|
|---|---|---|---|
| Thiamin,1 mg | 1.4 | 4.2 | 8.4 |
| mg as dietary vitamin B1* | 2.4 | 7.0 | 13.9 |
| Riboflavin, mg | 1.6 | 4.8 | 9.6 |
| mg as dietary vitamin B2* | 2.4 | 7.2 | 14.4 |
| Pyridoxine,2 mg | 1.4 | 4.2 | 8.4 |
| mg as dietary vitamin B6* | 2.0 | 5.9 | 11.8 |
| Cyanocobalamin, μg | 2.4 | 7.2 | 14.4 |
| μg as dietary vitamin B12* | 4.8 | 14.4 | 28.8 |
| Nicotinamide, mg | 15 | 45 | 90 |
| mg as dietary niacin* | 25.5 | 76.5 | 153 |
| Pantothenic acid,3 mg | 6 | 18 | 36 |
| mg as dietary pantothenic acid* | 8.4 | 25.2 | 50.4 |
| Pteroylmonoglutamic acid, mg | 0.24 | 0.72 | 1.44 |
| mg as dietary folate* | 0.48 | 1.44 | 2.88 |
| Biotin, μg | 50 | 150 | 300 |
| μg as dietary biotin* | 65 | 195 | 390 |
| Ascorbic acid, mg | 100 | 300 | 600 |
| mg as dietary vitamin C* | 100 | 300 | 600 |
Notes:
Thiamin hydrochloride was used; value was expressed as thiamin itself.
Pyridoxine hydrochloride was used; value was expressed as pyridoxine itself.
Ca pantothenate was used; value was expressed as pantothenic acid itself.
Analytical methods
General biomarkers in blood
Whole blood samples were sent to the Institute of Shiga Health Center (Shiga, Japan) for measurements of general biomarkers in blood. The numbers and percentages of blood cells are measured by XE-2100 automated hematology analyzer. Enzyme activities and the concentrations of nutrients were measured by automatic biochemical analysis systems LABOSPECT 008.
Measurement of the levels of water-soluble vitamins in whole blood and plasma
The whole blood samples (3 mL × 2) were taken from the brachial vein and placed directly into a tube Venoject II (code No. VP-DK052K [Terumo Corporation, Tokyo, Japan]). A portion of the whole blood (0.6 mL) was retained to measure nicotinamide and riboflavin levels, and stored at −80°C until needed. Additional collected blood samples were centrifuged for 30 minutes at 1,500 × g to separate the plasma and particles at room temperature. The plasma samples were divided into six new tubes (0.3 mL each) to measure PLP, vitamin B12, pantothenic acid, folates, biotin, and vitamin C stored at −80°C until needed.
Vitamin B1
Frozen plasma samples (0.15 mL) were thawed, 5% trichloroacetic acid (0.3 mL) was added, and mixed well and kept for 10 minutes at 4°C. The suspension was centrifuged at 10,000 × g for 10 minutes at 4°C. The measurement method32 was modified as follows. The vitamin B1 in the supernatant (0.2 mL) was reacted with 1% cyanogen bromide (40 μL) in a strong alkali medium (5% NaOH 40 μL) at room temperature (25°C). After being kept for 10 minutes, 1.5 mol/L HCl (80 μL) and water (0.38 mL) were added. Then, the mixture was centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatant was passed through a 0.45-μm microfilter. The filtrate (50 μL) was injected directly into a high-performance liquid chromatography (HPLC) system to measure the thiocrome level. This method employs adsorption on a Tosoh ODS-100S (15 × 3.2 mm, inner diameter (I.D.), average particle size: 5 μm, used as pre-column) and a Tosoh ODS-100S (250 × 4.6 mm, I.D., average particle size: 5 μm) column, elution with 0.1 mol/L KH2PO4–K2HPO4 buffer (pH 8.6) containing 3% acetonitrile at a flow rate of 1.5 mL/min, and estimation at excitation wavelength of 375 nm as well as emission wavelength of 430 nm. The column temperature was kept at 40°C. The thiocrome of thiamin was eluted ca. 5.5 minutes under the conditions.
Vitamin B2
Frozen ethylenediaminetetraacetic acid (EDTA)-treated whole blood samples (0.1 mL) stored at −80°C were thawed. The measurement method33 was modified as follows. To 0.1 mL of each of thawed whole blood, 0.44 mL of water and 0.26 mL of 0.5 mol/L H2SO4 were added and kept at 80°C for 15 minutes. After cooling on ice, 0.2 mL of 10% trichloroacetic acid was added and the mixture was centrifuged at 10,000 × g for 3 minutes at 4°C. From the supernatant obtained, 0.2 mL was withdrawn and added to 0.2 mL of 1 mol/L NaOH. The alkalized mixture was irradiated at a height of 20 cm from the liquid with two fluorescent lamps (20 W) for 30 minutes at room temperature. Then, 20 μL of glacial acetic acid was added to the mixture. The neutralized mixture was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system to measure the lumiflavin level. This method employs adsorption on a Tosoh ODS-80Ts (250 × 4.6 mm, I.D., average particle size: 5 μm) column, elution with a mixture of 10 mmol/L NaH2PO4–NaOH buffer (pH 5.5):acetonitrile (85:15, v/v) at a flow rate of 0.8 mL/min, and estimation at excitation wavelength of 445 nm as well as emission wavelength of 530 nm. The column temperature was kept at 40°C. The lumiflavin was eluted ca. 19.0 minutes under the conditions.
PLP (a coenzyme of vitamin B6)
Frozen plasma samples (0.1 mL) were thawed and then added to 0.1 mL of 5% metaphosphoric acid and mixed well for 5 minutes at room temperature. The mixture was centrifuged for 15 minutes at 10,000 × g at 4°C. The resulting supernatant was retained. The acidified supernatant (0.1 mL) was added to 0.1 mL of methylene chloride and mixed well for 2 minutes. The mixture was centrifuged for 15 minutes at 10,000 × g at 4°C. The supernatant was retained and filtered through a 0.45-μm microfilter. The filtrate (20 μL) was directly injected into the HPLC system. The analytical method was based on the report by Rybak and Pfeiffer34 and slightly modified as follows. Separation of PLP in plasma was carried out using a Tosoh ODS 80Ts (250 × 4.6 mm, I.D., average particle size: 5 μm) column with pump-1 and column oven-1. The mobile phase consisted of 50 mmol/L NaH2PO4–H3PO4 buffer (pH 3.1):acetonitrile (95:5, v/v); a flow rate of 0.7 mL/min was used and column temperature was maintained at 35°C. After the separation was completed, the column effluent was subjected to post-column through a T-connector attached to the polytetrafluoroethylene reactor tube (550 × 0.5 mm i.D.) in column oven-2 which was maintained at 75°C and reacted with 22 mmol/L NaClO2, which was delivered via pump-2 at a flow rate of 0.5 mL/min. The reacted PLP was measured at an excitation wavelength of 325 nm and an emission wavelength of 425 nm. The total analysis time was 20 minutes. PLP is eluted at ≈10 minutes under these conditions.
Vitamin B12
Frozen plasma samples (50 μL) were thawed and then added to 0.57 mmol/L acetate buffer, pH 4.5 (0.25 mL), 0.05% KCN (10 μL), and water (0.5 mL). The vitamin B12 was converted to cyanocobalamin by immersing it to boiling water bath for 30 minutes. After cooling on ice, 10% methaphosphoric acid (15 μL) was added and then the solution was centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatants were used for measuring levels of vitamin B12 by the microbioassay method using Lactobacillus leichmanii ATCC 7830.35
Nicotinamide
Frozen whole blood samples (75 μL) in a screw-capped vial were thawed and added to 1.425 mL of 2 μg/L isonicotinamide (used as an internal standard), which was autoclaved at 120°C for 10 minutes to convert pyridine nucleotide coenzymes to nicotinamide. After cooling on ice, the sample was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant (1.2 mL) was removed and 70 μL of 70% perchloric acid was added, which was kept for 5 minutes in room temperature. The sample was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant (1.0 mL) was removed, retained, and used for measuring nicotinamide. The supernatant sample (1.0 mL) was twice extracted with diethylether (5 mL) in the presence of K2CO3 (1.2 g).29 The dried materials were dissolved in 0.5 mL of water, which was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system.29 This method employs adsorption on a Tosoh ODS-80Ts (250 × 4.6 mm, I.D., average particle size: 5 μm) column, elution with a mixture of 10 mmol/L KH2PO4 (pH was adjusted to 3.0 by addition of H3PO4):methanol (22:3, v/v) at a flow rate of 0.8 mL/min. The wavelength was set at 260 nm and the column temperature was maintained at 30°C. The isoniotinamide (used as an internal standard) and nicotinamide were eluted ca. 6.5 minutes and 7.0 minutes, respectively, under the conditions.
Pantothenic acid
Frozen plasma sample (0.1 mL) was thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was used for measuring pantothenic acid by the microbioassay method using Lactobacillus plantarum ATCC 8014.36
Folates
Frozen plasma sample was thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was used for measuring folates by the microbioassay method using Lactobacillus rhamnosus ATCC 27773.37
Biotin
Frozen plasma sample was thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was used for measuring biotin by the microbioassay method using L. plantarum ATCC 8014.38
Vitamin C
Freshly prepared plasma (0.1 mL) was added to 0.9 mL of ice-cold 20% metaphosphoric acid containing 1% stannous chloride, mixed well, and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was used for measuring total vitamin C (ascorbic acid, dehydroascorbic acid, and diketogulonic acid). The employed method39 was modified as follows. The supernatant (0.1 mL) was consequently added to 0.1 mL of 0.2% 2,6-dichloroindophenol, 50 μL of 5% metaphosphoric acid containing 1% stannous chloride, and 0.12 mL of 2% dinitrophenylhydrazine in 4.5 mol/L H2SO4, and mixed well. The mixture was stirred at 37°C for 3 hours. The reacted product, dehydroascorbic acid bis(dinitrophenyl)hydrazone), was added to 1 mL of water and then to 1 mL of ethyl acetate, and vigorously shacked for 5 minutes. After centrifuged at 600 × g for 1 minute at room temperature, the organic layer (0.6 mL) was removed and retained. The retained layer of ethyl acetate (0.6 mL) was dried up using Centrifuge Evaporator HITACHI CE1 equipped with Cold Trap TP6D and Diaphragm Vacuum Pump HITACHI VD3 at 35°C for 30 minutes. The dried materials were dissolved in 0.2 mL of acetonitrile, and the solution was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system.39 This method employs adsorption on a μBobdasphere 5 μm C18-100A (150 × 3.9 mm, average particle size: 5 μm) column, elution with a mixture of 0.1% triethylamine:acetonitrile (50:50, v/v) at a flow rate of 1.0 mL/min, and estimation at 505 nm. The column temperature was kept at 40°C. The reacted product, dehydroascorbic acid bis(dinitrophenyl)hydrazone), was eluted ca. 8.1 minutes under the conditions.
Measurement of the levels of water-soluble vitamins in urine
Twenty-four-hour urine samples were collected in amber bottles with 10 mL of 1 mol/L HCl and these urine samples were stored at −80°C until needed.
Vitamin B1
The acidified urine samples were thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained and used for measurement of the levels of vitamin B1. The vitamin B1 in the supernatant (0.5 mL) was reacted with 1% cyanogen bromide (0.1 mL) in a strong alkali medium (5% NaOH 0.5 mL) at room temperature. After being kept for 10 minutes, 1.5 mol/L HCl (80 μL) was added. Then, the mixture was centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatant was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system to measure the thiocrome level.32 This method employs adsorption on a Tosoh ODS-100S (15 × 3.2 mm, I.D., average particle size: 5 μm, used as pre-column) and Tosoh ODS-100S (250 × 4.6 mm, I.D., average particle size: 5 μm) column, elution with 0.05 mol/L KH2PO4–K2HPO4 buffer (pH 8.6) containing 1% acetonitrile at a flow rate of 1.0 mL/min, and estimation at excitation wavelength of 375 nm as well as emission wavelength of 430 nm. The column temperature was kept at 40°C. The thiocrome of thiamin was eluted ca. 17.5 minutes under the conditions.
Vitamin B2
The acidified urine samples were thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained and used for measurement of the levels of vitamin B2. The resulting supernatant was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system.44 This method employs adsorption on a Tosoh ODS-80Ts (250 × 4.6 mm, I.D., average particle size: 5 μm) column, elution with a mixture of 10 mmol/L NaH2PO4–NaOH buffer (pH 5.5):acetonitrile (85:15, v/v) at a flow rate of 0.8 mL/min, and estimation at excitation wavelength of 445 nm as well as emission wavelength of 530 nm. The column temperature was kept at 40°C. The riboflavin was eluted ca. 10.5 minutes under the conditions.
Vitamin B6
The acidified urine samples were thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained and used for measurement of the levels of 4-PIC, a catabolite of vitamin B6.41 The resulting supernatant was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system. This method employs adsorption on a Tosoh ODS-120A (250 × 4.6 mm, I.D., average particle size: 5 μm) column, elution with a mixture of 40 mmol/L H3PO4–KOH (pH 2.2):acetonitrile (95:5, v/v) at a flow rate of 1.0 mL/min, and estimation at excitation wavelength of 355 nm as well as emission wavelength of 436 nm. The column temperature was kept at 40°C. The 4-PIC was eluted ca. 5.0 minutes under the conditions.
Vitamin B12
Frozen acidified urine samples (0.9 mL) were added to 0.1 mmol/L acetate buffer, pH 4.8 (0.18 mL), 0.025% KCN (0.02 mL), and water (0.68 mL). The vitamin B12 was converted to cyanocobalamin by autoclaving for 5 minutes at 121°C. After cooling, 10% methaphosphoric acid (20 μL) was added and then the solution was centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatants were used for measuring levels of vitamin B12 by the microbioassay method using L. leichmanii ATCC 7830.35
Nicotinamide
Frozen urine samples (1.5 mL) were thawed and then centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant (1.0 mL) was withdrawn and added to 10 μL of 1.0 mg/mL isonicotinamide and added with diethylether (5.0 mL) in the presence of K2CO3 (1.2 g) to extract nicotinamide, 2-Py, and 4-Py in the water layer into organic solvent layer.29 This extraction procedure was repeated twice. The combined diethylether layer was dried up at 40°C and the dried materials were dissolved in 0.5 mL of water. The water solution was passed through a 0.45-μm microfilter. The filtrate (20 μL) was injected directly into a HPLC system to measure the nicotinamide, 2-Py, and 4-Py levels as reported previously29 and modified as follows. This method employs adsorption on a Chemcosorb 7-ODS-L (250 × 4.6 mm, I.D., average particle size: 7 μm) column, elution with a mixture of 10 mmol/L potassium dihydrogen phosphate (pH was adjusted to 3.0 by addition of phosphoric acid):methanol (22:3, v/v) at a flow rate of 0.8 mL/min. The wavelength was set at 260 nm and the column temperature was maintained at 30°C. The isonicotinamide (used as internal standard), nicotinamide, 2-Py, and 4-Py were eluted ca. 6.2, 7.0, 7.9, and 8.9 minutes, respectively, under the conditions.
An another catabolite MNA in urine sample (0.8 mL) reacted with 0.1 mol/L acetophenone in ethanol (0.5 mL) in a strong alkali medium (1.0 mL of 6 mol/L NaOH) at 0°C in the presence of a large amount of isonicotinamide (0.20 mL of 1 mol/L isonicotinamide).42 After being kept for 10 minutes, formic acid was added and the mixture was kept for another 15 minutes at 0°C. Then, the mixture was heated at above 93°C for 5 minutes. The reaction product, 1-methyl-7-phenyl-1,5-dihydro-5-oxo-1,6-naphthyridine, was analyzed by HPLC described previously and modified as follows.17 The method employs adsorption on a Tosoh ODS-80Ts (250 × 4.6 mm, I.D., 5 μm) column, elution with a mixture of 30 mmol/L KH2PO4 (pH was adjusted to 3.0 by H3PO4) containing 1 mmol/L EDTA and 1 g/L 1-heptanesulfonic acid sodium salt:acetonitrile (1000:290, v/v), at a flow rate of 1.0 mL/min, and estimation at excitation wavelength of 382 nm as well as emission wavelength of 440 nm. The column temperature was maintained at 30°C. The derivatized product of MNA was eluted ca. 7.5 minutes under the conditions.
Pantothenic acid
Frozen acidified urine samples were thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatants were used for measuring pantothenic acid by the microbioassay method using L. plantarum ATCC 8014.36
Folates
Frozen acidified urine samples were thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatants were used for measuring folates by the microbioassay method using Lactobacillus rhamnosus ATCC 27773.37
Biotin
Frozen acidified urine samples were thawed and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatants were used for measuring biotin by the microbioassay method using L. plantarum ATCC 8014.38
Vitamin C
Frozen acidified urine sample (0.1 mL) was thawed and consequently added to 0.1 mL of 0.2% 2,6-dichloroindophenol, 50 μL of 1% stannous chloride, and 0.12 mL of 2% dinitrophenylhydrazine in 4.5 mol/L H2SO4, and mixed well.39 The resulting mixture was stirred at 37°C for 3 hours. The reacted product, dehydroascorbic acid bis(dinitrophenyl)hydrazone), was added to 1 mL of water and then to 1 mL of ethyl acetate, and vigorously shacked for 5 minutes. After centrifuged at 600 × g for 1 minute at room temperature, the organic layer (0.6 mL) was removed and retained. The remaining procedures were exactly the same as followed for plasma vitamin C.
Measurement of the contents of water-soluble vitamins in food
Preparation and measurement of the extracts of B-group vitamins from breakfast, lunch, dinner, and snacks was described below. Whole foods served at breakfast, lunch, dinner, and snacks were mixed, and minced well by a kitchen knife. Each of the whole minced materials was added to 5 volumes of 1% TAKA-DIASTASE® (obtained from Sankyo Co., Ltd., Tokyo, Japan. Now the company name was changed to Daiichi-Sankyo Co., Ltd.) solution dissolved in water and incubated for 3 hours at 37°C in order to digest biopolymers such as protein, fat, and carbohydrate. These homogenates were designated as the treated homogenates of foods.
Vitamin B1
The treated homogenates of foods were added to 10 volumes of cold 5% trichloroacetic acid and mixed well for 5 minutes at room temperature. The acidified homogenates were centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained and used immediately for measurement of the vitamin B1 level. The measurement method32 was modified as follows. The vitamin B1 in the supernatant (0.2 mL) reacted with 1% cyanogen bromide (40 μL) in a strong alkali medium (5% NaOH 40 μL) at room temperature (25°C). After 10 minutes, 1.5 mol/L HCl (80 μL) and water (0.38 mL) were added. The mixture was then centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatant was passed through a 0.45-μm microfilter. The filtrate (50 μL) was injected directly into a HPLC system to measure the thiocrome level.32 The remaining procedures were exactly the same as followed for plasma vitamin B1.
Vitamin B2
The treated homogenates of foods were added to 5 volumes of 50 mmol/L KH2PO4–K2HPO4 buffer (pH 7.0). The measurement method33 was modified as follows. To 0.1 mL of each homogenates, 0.44 mL of water and 0.26 mL of 0.5 mol/L H2SO4 were added, and the mixture were kept at 80°C for 15 minutes. After cooling, 0.2 mL of 10% trichloroacetic acid was added, and the mixture was centrifuged at 10,000 × g for 3 minutes at 4°C. The supernatants were used for measuring vitamin B2. The remaining procedures were exactly the same as followed for plasma vitamin B2.
Vitamin B6
The treated homogenates of foods were added to 2 volumes of 0.11 mol/L HCl and the mixture was mixed well for 10 minutes at room temperature. The acidified homogenate was autoclaved at 121°C for 3 hours to convert vitamin B6 coenzyme to the free form of vitamin B6. After cooling, the mixture was adjusted to pH 5.0 with 1 mol/L NaOH and then increased to 100 mL with water. The solution was filtered with qualitative filter number 2 (Advantec, Tokyo, Japan). The filtrate was used to measure the level of vitamin B6 using Saccharomyces carlsbergenisis strain 4228 ATCC 9080.43
Vitamin B12
The treated homogenates of foods (0.5 mL) were added to 2.5 mL of 0.57 mol/L acetic acid–sodium acetate buffer (pH 4.5) plus 5 mL of water and 0.1 mL of 0.05% KCN. The homogenate was then put into a boiling water bath for 5 minutes. After cooling, 0.15 mL of 10% metaphosphoric acid was added and increased to 10 mL with water. The solution was centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatants were used to measure the level of vitamin B12 by the microbioassay method using L. leichmanii ATCC 7830.35
Nicotinamide
The treated homogenates of foods (1 mL) were withdrawn, added to 4 mL of water, and then autoclaved at 121°C for 10 minutes to convert pyridine nucleotide coenzymes to nicotinamide. After cooling, the mixture was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained, the precipitated materials re-extracted with 5 mL of water, and the supernatant retained again. Both retained supernatants were combined and were used for measuring nicotinamide. The remaining procedures were exactly the same as followed for blood nicotinamide.
Pantothenic acid
The treated homogenates of foods were added to 5 volumes of 50 mmol/L KH2PO4–K2HPO4 buffer (pH 7.0) and were incubated overnight at 37°C to convert free pantothenic acid from the bound type of pantothenate compounds. The reaction was stopped by placing the mixture into a boiling water bath for 5 minutes. After cooling, the mixture was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained, the precipitated materials were re-extracted with 2 mL of water, and the supernatant again retained. Both retained supernatants were combined, and the extract was used to measure the level of pantothenic acid by the microbioassay method using L. plantarum ATCC 8014.36
Folate
The treated homogenates of foods were added to 5 volumes of 0.1 mol/L KH2PO4–K2HPO4 buffer (pH 6.1) and were autoclaved at 121°C for 5 minutes. After cooling, 2.5 mL of proteinase MS (200 U/mL of water; Kaken Pharmaceutical Co., Ltd., Tokyo, Japan) was added. The mixture was incubated at 37°C for 3 hours to digest proteins and release polyglutamated folate from the protein-bound types. The reaction was stopped by placing the mixture into a boiling water bath for 10 minutes. After cooling, 0.5 mL of conjugase (extract from porcine kidney acetone powder [type II]; Sigma-Aldrich, St Louis, MO, USA) was added to the mixture, which was then incubated overnight at 37°C to convert polyglutamated folates to monoglutamated folates. The reaction was stopped by placing into a boiling water bath for 10 minutes. After cooling, the mixture was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was retained, the precipitated materials re-extracted with 3 mL of water, and the supernatant again retained. Both retained supernatants were combined, and the extract was used to measure the folate levels by the microbioassay method using Lactobacillus rhamnosus ATCC 27773.37
The conjugase solution was made up as follows: 60 mL of 50 mmol/L KH2PO4–K2HPO4 buffer (pH 7.0) was added to 20 g of porcine kidney acetone powder and stirred for 30 minutes at 4°C. The suspension was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was dialyzed against a large amount of 50 mmol/L KH2PO4–K2HPO4 buffer (pH 7.0) to remove endogenous folate from the kidney acetone powder. The dialyzed conjugase solution was used.
Biotin
The treated homogenates of foods were added to 2 volumes of 2.25 mol/L H2SO4 and were autoclaved at 121°C for 1 hour to convert bound biotin to the free form of biotin. After cooling, the suspension was centrifuged at 10,000 × g for 10 minutes at 4°C. The resulting supernatants were retained and used to measure the biotin level by the microbioassay method using L. plantarum ATCC 8014.38
Vitamin C
The treated homogenates of foods (1 mL) were added to 9 mL of ice-cold 20% metaphosphoric acid containing 1% stannous chloride, mixed well, and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was used for measuring total vitamin C (ascorbic acid, dehydroascorbic acid, and diketogulonic acid). The remaining procedures were exactly the same as followed for plasma vitamin C.
Urinary Excretion Percentages of Water-soluble Vitamins
The urinary excretion of percentages of water-soluble vitamins over intakes of water-soluble vitamins during the study was calculated as follows: (24-hour urinary excretion of vitamin mol/d)/(vitamin intake, mol/d) × 100.
Statistical Methods
Intra- and inter-individual variations were calculated with analysis of variance. Nonparametric Friedman test for repeated measures following Dunn’s post-test was used to analyze statistical differences. Pearson coefficients were calculated to determine correlation between dietary vitamin intake and the concentration of blood or urine vitamin. A p value of <0.05 was considered statistically significant. Prism version 5.0 (Graph Pad Software, San Diego, CA, USA) was used for all statistical analyses.
Results
Basic characteristics
The body weight and height of the 10 Japanese male subjects did not change during the course of the study. Table 1 shows their age, height, body weight, and BMI. Table 2 shows the composition of the diet fed throughout the study. Table 3 shows the vitamin mixtures α, β, and γ administered in weeks 2, 3, and 4, respectively. Table 4 shows the general biomarkers in the blood at the end of weeks 1, 2, 3, and 4; all values were within the reference range.
Table 4.
General biomarkers in the blood of Japanese male subjects.
| REFERENCE VALUE | WEEK 1 (DAY 5) |
WEEK 2 (DAY 12) |
WEEK 3 (DAY 19) |
WEEK 4 (DAY 26) |
|
|---|---|---|---|---|---|
| Erythrocyte count, ×104/μL | 430–570 | 542 ± 17 | 529 ± 18 | 531 ± 20 | 525 ± 18 |
| Leukocyte count, μL | 3300–9000 | 5040 ± 1146 | 5150 ± 1339 | 5120 ± 1155 | 5210 ± 1333 |
| Hemoglobin, g/dL | 13.5–17.5 | 16.3 ± 0.5 | 15.8 ± 0.4 | 15.7 ± 0.5 | 15.7 ± 0.3 |
| Hematocrit, % | 39.7–52.4 | 48.7 ± 2.0 | 47.6 ± 1.9 | 47.1 ± 1.9 | 47.4 ± 1.8 |
| MCV, fL | 85–102 | 90.0 ± 3.7 | 90.0 ± 3.4 | 88.8 ± 3.4 | 90.5 ± 3.7 |
| MCH, pg | 28.0–34.0 | 30.1 ± 1.0 | 30.0 ± 0.8 | 29.5 ± 0.9 | 29.9 ± 0.8 |
| MCHC, % | 30.2–35.1 | 33.5 ± 0.7 | 33.3 ± 0.7 | 33.3 ± 0.5 | 33.1 ± 0.7 |
| AST, U/L | 10–40 | 16.4 ± 4.6 | 20.0 ± 6.1 | 22.6 ± 5.3 | 21.1 ± 6.7 |
| ALT, U/L | 5–45 | 17.6 ± 6.0 | 21.9 ± 7.4 | 19.3 ± 8.4 | 21.9 ± 5.5 |
| γ-GTP, U/L | >80 | 21.6 ± 12.9 | 21.1 ± 13.0 | 21.8 ± 14.1 | 22.2 ± 14.4 |
| HDL-Cho, mg/dL | 40–70 | 50 ± 6 | 49 ± 8 | 48 ± 6 | 50 ± 8 |
| Total-Cho, mg/dL | 120–219 | 175 ± 31 | 169 ± 22 | 174 ± 22 | 180 ± 27 |
| Triglyceride, mg/dL | 30–149 | 96 ± 60 | 93 ± 58 | 55.4 ± 24.8 | 54.9 ± 17.3 |
| Total lipid, mg/dL | 400–800 | 513 ± 74 | 493 ± 44 | 543 ± 106 | 539 ± 91 |
| Blood glucose, mg/dL | 70–109 | 86 ± 5 | 85 ± 5 | 86 ± 5 | 86 ± 5 |
| Total protein, mg/dL | 6.7–8.3 | 7.9 ± 0.4 | 7.5 ± 0.3 | 7.5 ± 0.4 | 7.6 ± 0.4 |
| Albumin, mg/dL | 3.8–5.3 | 5.2 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 |
| Albumin/Globurin | 1.1–2.0 | 1.9 ± 0.3 | 2.1 ± 0.3 | 2.1 ± 0.2 | 2.0 ± 0.3 |
| Creatinine, mg/dL | 0.61–1.04 | 0.91 ± 0.06 | 0.85 ± 0.06 | 0.84 ± 0.08 | 0.85 ± 0.09 |
| Uric acid, mg/dL | >7.0 | 6.1 ± 1.1 | 6.0 ± 1.0 | 6.0 ± 1.1 | 5.9 ± 1.0 |
| Homocysteine, nmol/mL | 3.7–13.5 | 7.1 ± 0.8 | 7.1 ± 0.9 | 6.6 ± 0.8 | 6.5 ± 0.8 |
| Total bilirubin, mg/dL | 0.–1.1 | 1.1 ± 0.4 | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.1 ± 0.3 |
| PIVKA-II, μg/mL | >1 | >1 | >1 | >1 | >1 |
Notes:
Values are means ± SD for the 10 subjects. Nonparametric Friedman test for repeated measures following Dunn’s post-test was used to analyze statistical differences; no significant differences were observed at all.
Abbreviations: MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
Blood Water-soluble Vitamins
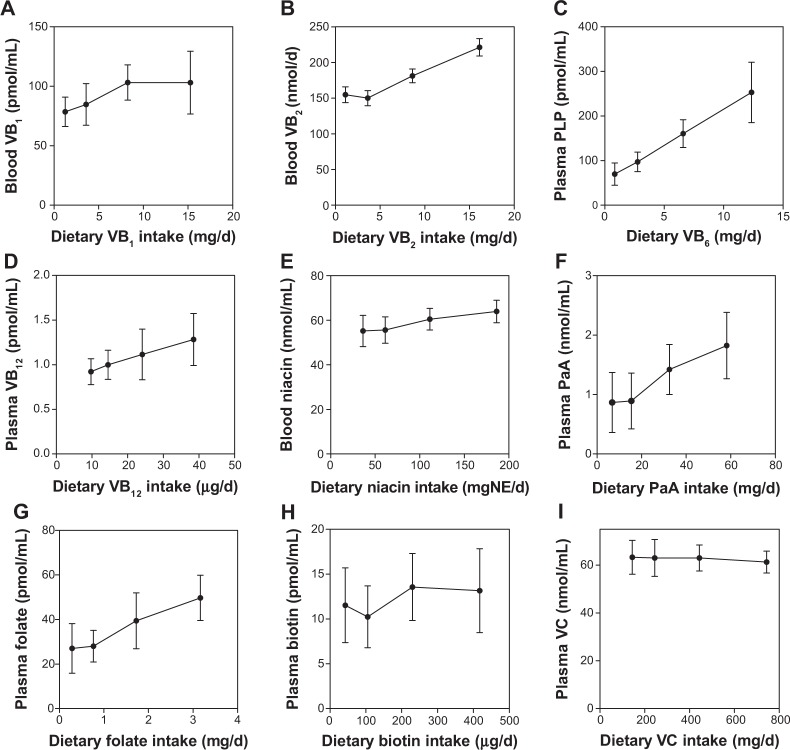
Figure 2 shows the correlation between water-soluble vitamins ingested and circulating in the blood. The correlation was significant for vitamin B2, PLP (coenzyme form of vitamin B6), vitamin B12, nicotinamide, pantothenic acid, and folate, but the slopes were not steep against the amounts ingested. No significant correlation existed for vitamin B1, biotin, and vitamin C.
Figure 2.
Correlation of vitamin intake and concentration of water-soluble vitamins in the blood of the Japanese male subjects.
Notes: Each point represents the mean ± SD of the 10 subjects. Pearson coefficients were calculated to determine the correlation between vitamin intake and the vitamin concentration in the blood or urine. (A) VB1 = vitamin B1 (r = 0.888, p = 0.0581; not significant); (B) VB2 = vitamin B2 (r = 0.976, p = 0.024; significant); (C) PLP, a coenzyme form of vitamin B6 (r = 1.000, p = 0.0002; significant); (D) VB12 = vitamin B12 (r = 0.999, p = 0.002; significant); (E) nicotinamide (r = 0.984, p = 0.016; significant); (F) PaA = pantothenic acid (r = 0.984, p = 0.016; significant); (G) folate (r = 0.989, p = 0.011; significant); (H) biotin (r = 0.713, p = 0.289; not significant); (I) VC = vitamin C (r = −0.927, p = 0.074; not significant).
Urine Volume
Figure 3 shows the daily changes of the urine volumes of each subject. Twenty-four-hour urine samples were collected eight times and the results are presented in Table 5. The intra-individual coefficient of variation was lower than the inter-individual coefficient of variation. The lowest urine volume was 700 mL/d and the highest 3,030 mL/d. The median was 1,595 mL/d, and the mean ± SD of all 80 samples was 1,678 ± 630 mL/d.
Figure 3.
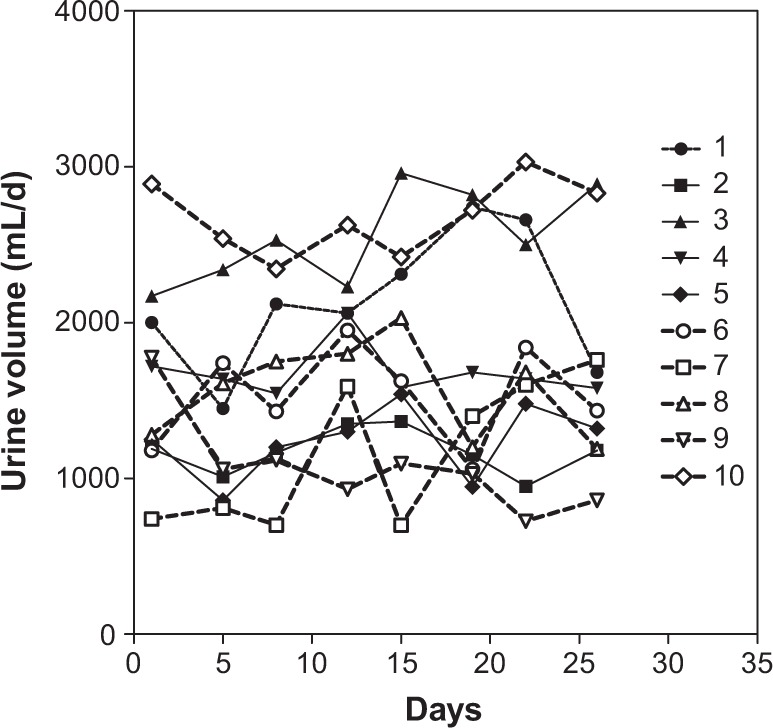
Changes in urine volumes for each Japanese male subject during the study.
Notes: Each symbol represents the value for each subject. Numbers indicate the subject number.
Table 5.
Averages of the eight daily urine volumes collected from each Japanese male subject.
| SUBJECT NO. | AVERAGE URINE VOLUME, mL/d |
|---|---|
| 1 | 2127 ± 443 |
| 2 | 1170 ± 144 |
| 3 | 2555 ± 305 |
| 4 | 1681 ± 163 |
| 5 | 1237 ± 236 |
| 6 | 1533 ± 311 |
| 7 | 1163 ± 466 |
| 8 | 1568 ± 311 |
| 9 | 1074 ± 313 |
| 10 | 2675 ± 237 |
Notes: Values are means ± SD for the eight urinary volumes collected. Intra-individual coefficient of variation is 7.0% and inter-individual coefficient of variation is 34.1%.
Urinary Creatinine
Figure 4 shows the daily changes in the urinary creatinine in each subject. Twenty-four-hour urine samples were collected eight times. The average urinary creatinine for each individual is shown in Table 6. The intra-individual coefficient of variation was lower than the inter-individual coefficient of variation. The lowest level of urinary creatinine was 11.42 mmol/d and the highest level was 20.35 mmol/d. The median was 15.58 mmol/d, and the mean ± SD of all 80 samples was 15.60 ± 1.55 mmol/d.
Figure 4.
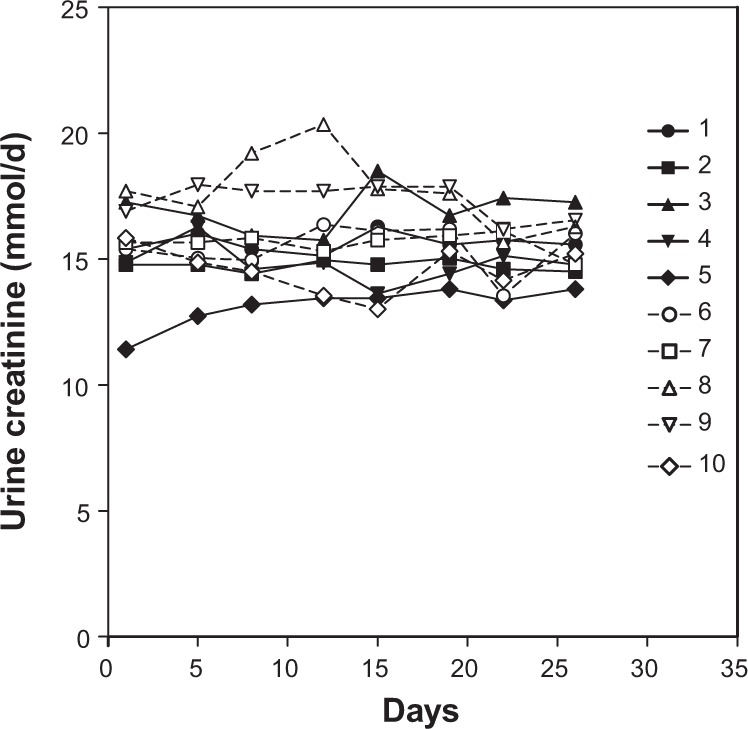
Changes in urinary creatinine for each Japanese male subject during the study.
Notes: Each symbol represents the value for each subject. Numbers indicate the subject number.
Table 6.
Averages of the eight daily urinary creatinine collected from each Japanese male subject.
| SUBJECT NO. | AVERAGE URINE CREATININE, mmol/d |
|---|---|
| 1 | 15.64 ± 0.37 |
| 2 | 14.73 ± 0.21 |
| 3 | 16.95 ± 0.88 |
| 4 | 14.82 ± 0.74 |
| 5 | 13.15 ± 0.78 |
| 6 | 15.45 ± 0.94 |
| 7 | 15.63 ± 0.41 |
| 8 | 17.71 ± 1.51 |
| 9 | 17.35 ± 0.68 |
| 10 | 14.56 ± 0.95 |
Notes: Values are means ± SD for the eight urinary creatinines collected. Intra-individual coefficient of variation is 7.4% and inter-individual coefficient of variation is 34.8%.
Urinary Water-soluble Vitamins
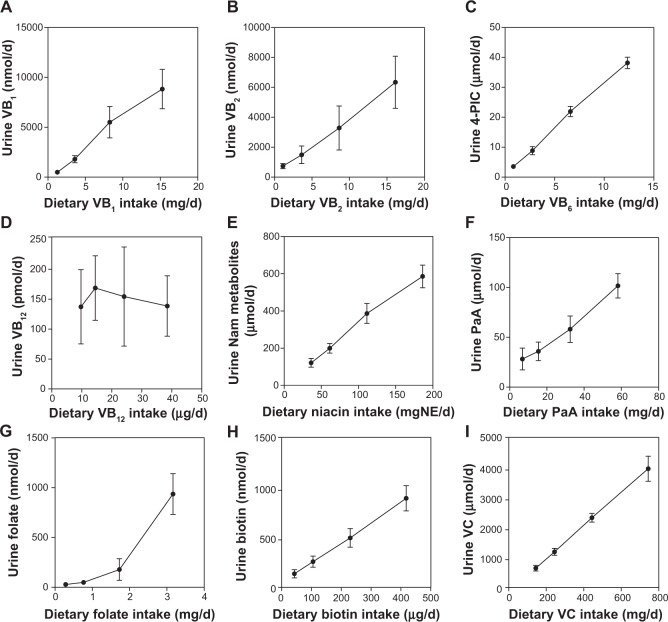
Figure 5 shows the correlation between the water-soluble vitamins excreted in the urine and ingested. A significant correlation was observed for seven of the nine water-soluble vitamins.
Figure 5.
Correlation of vitamin intake and urinary concentrations of water-soluble vitamins in Japanese male subjects.
Notes: Each point represents the mean ± SD of the 10 subjects. Pearson coefficients were calculated to determine the correlation between vitamin intake and the vitamin concentration in the blood or urine. (A) VB1 = vitamin B1 (r = 0.993, p = 0.007; significant); (B) VB2 = vitamin B2 (r = 0.999, p = 0.0013; significant); (C) 4-PIC, a catabolite of vitamin B6 (r = 0.999, p = 0.0009; significant); (D) VB12 = vitamin B12 (r = −0.288, p = 0.714; not significant); (E) nicotinamide (r = 0.997, p = 0.0031; significant); (F) PaA = pantothenic acid (r = 0.995, p = 0.0052; significant); (G) folate (r = 0.943, p = 0.0574; not significant); (H) biotin (r = 1.000, p = 0.0003; significant); (I) VC = vitamin C (r = 1.000, p < 0.0001; significant).
Urinary excretion of vitamin B12 did not increase with vitamin B12 intake (Fig. 5D).
Urinary excretion of folate increased with folate intake, but the line was not straight; which had a broken point (Fig. 5G). Figure 6 shows the breaking point between dietary folate intake and urinary folate. The breaking point was observed at approximately 1.6 mg of dietary folate/d, which was obtained by intersecting the graph lines of weeks 1 and 2 and weeks 3 and 4.
Figure 6.

Breaking point in the relationship between dietary folate intake and urinary folate in Japanese male subjects.
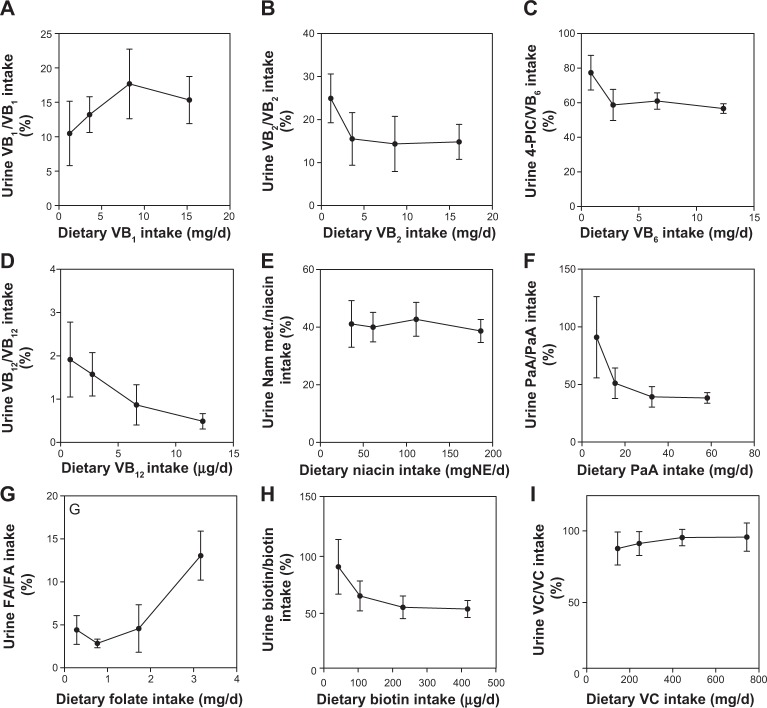
Figure 7 shows the percentage of water-soluble vitamins excreted in the urine. The percentages of vitamin B1, vitamin B2, vitamin B6, niacin, pantothenic acid, biotin, and vitamin C were relatively constant regardless of their intake. The percentage of vitamin B12 excreted decreased with intake, suggesting that vitamin B12 absorption is limited by a special mechanism.44
Figure 7.
Correlation of vitamin intake and the percentage of urinary vitamin/vitamin intake in Japanese male subjects.
Notes: Each point represents the mean ± SD of the 10 subjects. Pearson coefficients were calculated to determine the correlation between vitamin intake and the vitamin concentration in the blood or urine. (A) VB1 = vitamin B1 (r = 0.678, p = 0.322; not significant); (B) VB2 = vitamin B2 (r = −0.664, p = 0.336; not significant); (C) 4-PIC, a catabolite of vitamin B6 (r = −0.703, p = 0.297; not significant); (D) VB12 = vitamin B12 (r = −970, p = 0.030; significant); (E) nicotinamide (r = −0.445, p = 0.555; not significant); (F) PaA = pantothenic acid (r = −0.767, p = 0.234; not significant); (G) folate (r = 0.891, p = 0.109; not significant); (H) biotin (r = −0.802, p = 0.198; not significant); (I) VC = vitamin C (r = 0.883, p = 0.117; not significant).
Figure 8 shows the significant correlation between urinary vitamin B12 and urine volume.
Figure 8.
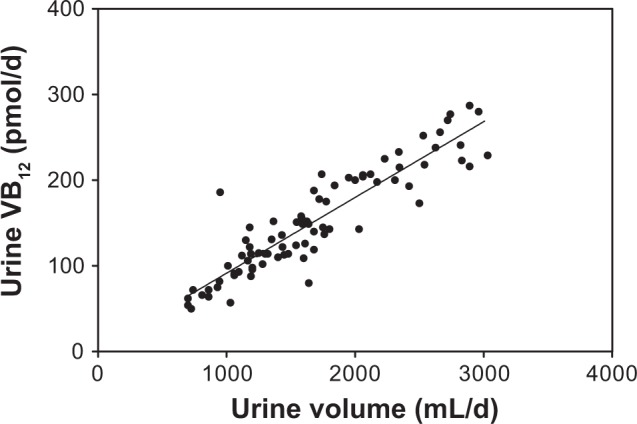
Correlation between urinary vitamin B12 and urine volume of Japanese male subjects.
Notes: Pearson coefficient (r) is 0.913 and p < 0.0001. The correlation is significant.
Discussion
Dietary assessments are an easy way to obtain dietary and nutrient intakes, but misreporting is common and, therefore, cannot be an accurate way of determining nutritional statuses. Furthermore, using dietary assessments to determine micronutrient intakes is difficult because of high variations in habitual micronutrient intake. It is important to evaluate the nutritional level of each person because metabolic ability varies with every individual. Two methods (recorded dietary assessments, and measurement of nutritional biomarkers in the blood and urine) are being used to speculate the nutrients intakes. Nutritional biomarkers, for examples, measurements of nutrients and their metabolites in urine and blood, are used as a more accurate way for measuring the nutritional status of dietary intake or the metabolism of the dietary constituents. We previously determined that measuring the biomarkers of water-soluble vitamins in urine samples were better than in blood samples. We also investigated the relationship between urinary excretion and intakes of water-soluble vitamins in humans and animals.1–27 We confirmed that water-soluble vitamins were available as nutritional biomarkers for assessing their intake in females. This experiment sought to verify this fact in males.
The urine samples of the male subjects were clearly better than the blood samples. The slopes were much steeper in the urine than in the blood. Of the nine water-soluble vitamins, vitamin B12 excretion did not reflect vitamin B12 intake. Instead, urinary vitamin B12 is correlated with urine volume. We previously reported this phenomenon23 and again observed it in the present experiment. The urinary concentration of vitamin B12 (pmol/mL) was constant at approximately 50–60 pmol/L of urine. Vitamin B12 has a mechanism of absorption and excretion route that is different from other water-soluble vitamins: it is excreted through the bile.44 The change in urinary vitamin B12 is too small to evaluate vitamin B12 intake.23
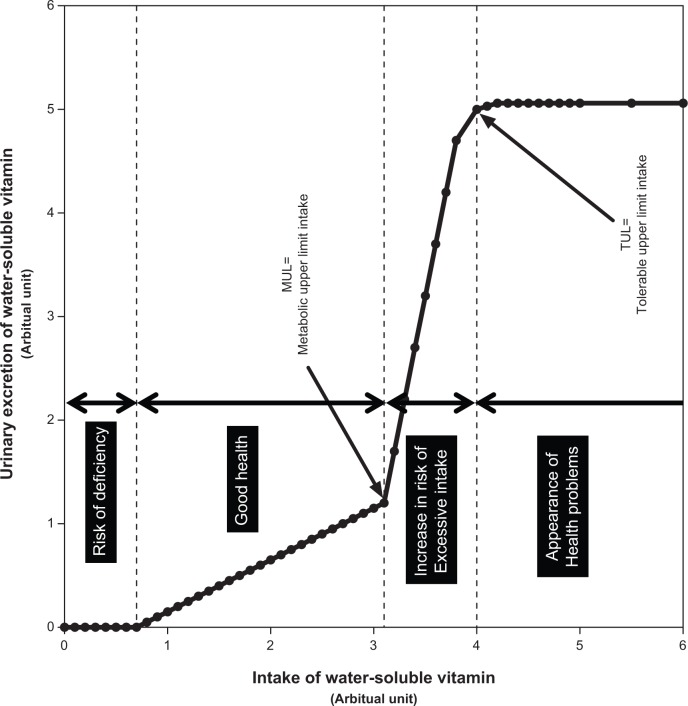
In Japanese females, folate had a lower linear regression coefficient value than the other water-soluble vitamins, except for vitamin B12.20 A similar observation was made in the present experiment. A breaking point was also observed at approximately 1.6 mg/d of dietary folate. Interestingly, administering 0.24 mg/d of synthetic folate (0.48 mg/d of dietary folate) did not change the urinary folate percentages, but administering more than 0.72 mg/d of synthetic folate (1.44 mg/d of dietary folate) changed the elimination process. We could not determine whether this was good or bad for health promotion. Figure 9 shows a proposed relationship between vitamin intake and urinary vitamin elimination. In general, urinary excretion of water-soluble vitamins cannot be detected when vitamin intake is below the required levels. When vitamin intake exceeds the requirements, they are absorbed passively into the body. The excreted vitamins and/or their metabolites are observed for the first time when they are eliminated linearly into the urine by passive diffusion. Therefore, urinary excretion of vitamins is constant. Such a phenomenon is thought to be good for health promotion. For folate intake, less than 1.6 mg/d is good for health promotion. When intake exceeds saturation in the body, the vitamins and/or their metabolites are actively excreted into urine to prevent excessive toxicity of the vitamins. Such a phenomenon is not good for health promotion, but toxicity does not appear. At this level, termed the tolerable upper limit, excretion does not increase according to vitamin intake because the body is no longer able to eliminate the vitamins. Habitual intakes above the tolerable upper limit increase the risks of health problems. For dietary folate, 1.6 mg/d is the tolerable upper limit. Apart from vitamin B12, no tolerable upper limits were seen in the other water-soluble vitamins.
Figure 9.
Proposed relationship between intake and elimination of water-soluble vitamins.
In conclusion, urinary water-soluble vitamin levels reflect their intakes and are suitable biomarkers for measuring their intakes. Vitamin B12 is an exception.
Acknowledgments
The authors express their sincere appreciation to their students for measuring the urinary excretions of vitamins.
Footnotes
Author Contributions
KS designed the study and drafted the manuscript. KS, JH, and TF performed the experiments, and JH and TF reviewed the manuscript and helped in the study design. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Joseph Zhou, Editor in Chief
FUNDING: This study was part of the project titled “Studies on the requirement of water-soluble vitamins in Japanese (principal investigator, Katsumi Shibata),” which was supported by The Ministry of Health, Labour and Welfare. This work is a publication of the Department of Nutrition, School of Human Cultures, at the University of Shiga Prefecture.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Shibata K, Fukuwatari T, Enomoto A, Sugimoto E. Increased conversion ratio of tryptophan to niacin by dietary di-n-butylphthalate. J Nutr Sci Vitaminol. 2001;47:263–266. doi: 10.3177/jnsv.47.263. [DOI] [PubMed] [Google Scholar]
- 2.Shibata K, Fukuwatari T, Sugimoto E. Effect of dietary pyrazinamide, an antituberculosis agent, on the metabolism of tryptophan to niacin and tryptophan to serotonin in rats. Biosci Biotechnol Biochem. 2001;65:1339–1346. doi: 10.1271/bbb.65.1339. [DOI] [PubMed] [Google Scholar]
- 3.Fukuwatari T, Morikawa Y, Hayakawa F, Sugimoto E, Shibata K. Influence of adenine-induced renal failure on tryptophan-niacin metabolism in rats. Biosci Biotechnol Biochem. 2001;65:2154–2161. doi: 10.1271/bbb.65.2154. [DOI] [PubMed] [Google Scholar]
- 4.Fukuwatari T, Suzuki Y, Sugimoto E, Shibata K. Elucidation of the toxic mechanism of plasticizers, phthalic acid esters, putative endocrine disrupters: effects of dietary di(2-ethylhexyl)phthalate on the metabolism of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 2002;66:705–710. doi: 10.1271/bbb.66.705. [DOI] [PubMed] [Google Scholar]
- 5.Fukuwatari T, Sugimoto E, Shibata K. Growth promoting activity of pyrazinoic acid, a putative active compound of antituberculosis drug pyrazinamide, in niacin-deficient rats through the inhibition of an ACMSD activity. Biosci Biotechnol Biochem. 2002;66:1435–1441. doi: 10.1271/bbb.66.1435. [DOI] [PubMed] [Google Scholar]
- 6.Fukuwatari T, Suzuki Y, Sugimoto E, Shibata K. Identification of toxic mechanism of plasticizers, phtahlic acid esters, which is a putative endocrine disrupters: time-dependent increase in quinolinic acid and its metabolites in rats fed di(2-ethylhexyl)phthalate diet. Biosci Biotechnol Biochem. 2002;66:2687–2691. doi: 10.1271/bbb.66.2687. [DOI] [PubMed] [Google Scholar]
- 7.Fukuwatari T, Ohta M, Sugimoto E, Sasaki R, Shibata K. Effects of dietary di(2-ethylhexyl)phthalate, a putative endocrine disrupter, on enzyme activities involved in the metabolism of tryptophan to niacin in rats. Biochim Biophys Acta. 2004;1672:67–75. doi: 10.1016/j.bbagen.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Fukuwatari T, Ohsaki S, Fukuoka S, Sasaki R, Shibata K. Phthalate esters enhance quinolinate production by inhibiting α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD), a key enzyme of the tryptophan pathway. Toxicol Sci. 2004;81:302–308. doi: 10.1093/toxsci/kfh204. [DOI] [PubMed] [Google Scholar]
- 9.Okuno A, Fukuwatari T, Shibata K. Urinary excretory ratio of anthranilic acid/kynurenic acid for an index of tolerable amount of tryptophan. Biosci Biotechnol Biochem. 2008;72:1667–1672. doi: 10.1271/bbb.70630. [DOI] [PubMed] [Google Scholar]
- 10.Shibata K, Takahashi C, Fukuwatari T, Sasaki R. Effects of excess pantothenic acid administration on the water-soluble vitamin metabolisms in rats. J Nutr Sci Vitaminol. 2005;51:385–391. doi: 10.3177/jnsv.51.385. [DOI] [PubMed] [Google Scholar]
- 11.Sawamura H, Fukuwatari T, Shibata K. Effects of excess biotin administration on growth and urinary excretion of water-soluble vitamins in young rats. Biosci Biotechnol Biochem. 2007;71:2977–2984. doi: 10.1271/bbb.70381. [DOI] [PubMed] [Google Scholar]
- 12.Moriya A, Fukuwatari T, Sano M, Shibata K. Different variations of tissue B-group vitamin concentrations in short- and long-term starved rats. Br J Nutr. 2011;107:52–60. doi: 10.1017/S0007114511002339. [DOI] [PubMed] [Google Scholar]
- 13.Shibata K, Imai E, Sano M, Fukuwatari T. The urinary excretory ratio of nicotinamide catabolites was associated with the conversion ratio of tryptophan to nicotinamide in growing rats fed a niacin-free 20% casein diet. Biosci Biotechnol Biochem. 2012;76:186–188. doi: 10.1271/bbb.110564. [DOI] [PubMed] [Google Scholar]
- 14.Imai E, Sano M, Fukuwatari T, Shibata K. Urinary excretion of water-soluble vitamins increases in streptozotocin-induced diabetic rats without decreases in liver or blood vitamin content. J Nutr Sci Vitaminol. 2012;58:54–58. doi: 10.3177/jnsv.58.54. [DOI] [PubMed] [Google Scholar]
- 15.Shibata K, Sugita C, Sano M, Fukuwatari T. Urinary excretions of B-group vitamins reflect the nutritional status of B-group vitamins in rats. J Nutr Sci. 2013;2:e12. doi: 10.1017/jns.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto H, Ishikawa A, Yoshiike H, et al. Diurnal variations in human urinary excretion of nicotinamide catabolites. Effects of stress on the diurnal variations. Am J Clin Nutr. 2003;72:406–410. doi: 10.1093/ajcn/77.2.406. [DOI] [PubMed] [Google Scholar]
- 17.Shibata K, Fukuwatari T, Ohta M, et al. Values of water-soluble vitamins in blood and urine of Japanese young men and women consuming a semi-purified diet based on the Japanese Dietary Reference Intakes. J Nut Sci Vitaminol. 2005;51:319–328. doi: 10.3177/jnsv.51.319. [DOI] [PubMed] [Google Scholar]
- 18.Fukuwatari T, Ohta M, Kimura N, Sasaki R, Shibata K. Conversion ratio of tryptophan to niacin in Japanese women fed on a purified diet conforming to the Japanese Dietary Reference Intakes. J Nutr Sci Vitaminol. 2004;50:385–391. doi: 10.3177/jnsv.50.385. [DOI] [PubMed] [Google Scholar]
- 19.Fukuwatari T, Shibata K. Effect of nicotinamide administration on the tryptophan-nicotinamide pathway in humans. Int J Vitam Nutr Res. 2007;77:255–262. doi: 10.1024/0300-9831.77.4.255. [DOI] [PubMed] [Google Scholar]
- 20.Fukuwatari T, Shibata K. Urinary water-soluble vitamins and their metabolic contents as nutritional markers for evaluating vitamin intakes in young Japanese women. J Nutr Sci Vitaminol. 2008;54:223–229. doi: 10.3177/jnsv.54.223. [DOI] [PubMed] [Google Scholar]
- 21.Fukuwatari T, Shibata K. Consideration for diurnal variations in human blood NAD and NADP concentrations. J Nutr Sci Vitaminol. 2009;55:279–281. doi: 10.3177/jnsv.55.279. [DOI] [PubMed] [Google Scholar]
- 22.Shibata K, Fukuwatari T, Watanabe T, Nishimuta M. Intra- and inter-individual variations of blood and urinary water-soluble vitamins in Japanese young adults consuming a semi-purified diet for 7 days. J Nutr Sci Vitaminol. 2009;55:459–470. doi: 10.3177/jnsv.55.459. [DOI] [PubMed] [Google Scholar]
- 23.Fukuwatari T, Sugimoto E, Tsuji T, Hirose J, Fukui T, Shibata K. Urinary excretion of vitamin B12 depends on urine volume in female university students and elderly subjects in Japan. Nutr Res. 2009;29:839–845. doi: 10.1016/j.nutres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Imai E, Tsuji T, Sano M, Fukuwatari T, Shibata K. Association between 24 hour urinary α-tocopherol catabolite, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman (α-CEHC) and α-tocopherol intake in intervention and cross-sectional studies. Asian Pac J Clin Nutr. 2011;20:507–513. [PubMed] [Google Scholar]
- 25.Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Twenty-four-hour urinary watersoluble vitamins correlate to vitamin intakes in free-living Japanese university students. Eur J Clin Nutr. 2010;64:800–807. doi: 10.1038/ejcn.2010.72. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Urinary excretion of vitamin B1, B2, B6, niacin, pantothenic acid, folate, and vitamin C correlates with dietary intakes of free-living elderly, female Japanese. Nutr Res. 2010;30:171–178. doi: 10.1016/j.nutres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Twenty-four-hour urinary watersoluble vitamin levels correlate with their intakes in free-living Japanese school children. Public Health Nutr. 2011;14:327–333. doi: 10.1017/S1368980010001904. [DOI] [PubMed] [Google Scholar]
- 28.Pullman ME, Colowick SP. Preparation of 2- and 6-pyridones of N1-methylnicotinamide. J Biol Chem. 1954;206:121–127. [PubMed] [Google Scholar]
- 29.Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J Chromatogr. 1988;424:23–28. doi: 10.1016/s0378-4347(00)81072-5. [DOI] [PubMed] [Google Scholar]
- 30.Standard Tables of Food Composition in Japan Report of the Subdivision on Resources Japan: The Council for Science and Technology, Ministry of Education, Cultures, Sports, Science and Technology; 2010 [Google Scholar]
- 31.Dietary Reference Intakes for Japanese-2005. Tokyo: The Ministry of Health, Labour, and Welfare; 2004. (in Japanese) [Google Scholar]
- 32.Iwata H, Matsuda T, Tonomura H. Improved high-performance liquid chromatographic determination of thiamine and its phosphate esters in animal tissues. J Chromatogr. 1988;26:317–323. doi: 10.1016/s0021-9673(01)83586-x. [DOI] [PubMed] [Google Scholar]
- 33.Ohakawa H, Ohishi N, Yagi K. A simple method for micro-determination of flavin in human serum and whole blood by high-performance liquid chromatography. Biochem Int. 1982;4:187–194. [Google Scholar]
- 34.Rybak ME, Pfeiffer CM. Clinical analysis of vitamin B6: determination of pyridoxal 5′-phosphate and 4-pyridoxic acid in human serum by reversed-phase high-performance liquid chromatography with chlorite postcolumn derivatization. Anal Biochem. 2004;333:336–344. doi: 10.1016/j.ab.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe F, Takenaka S, Katsura H, et al. Pseudovitamin B12 is the predominant cobamide of an algal health food, spirulina tablets. J Agric Food Chem. 1999;47:4736–4741. doi: 10.1021/jf990541b. [DOI] [PubMed] [Google Scholar]
- 36.Skeggs HR, Wright LD. The use of Lactobacillus arabinosus in the microbiological determination of pantothenic acid. J Biol Chem. 1944;156:21–26. [Google Scholar]
- 37.Taisun H, Tamura T. Trienzyme extraction in combination with microbiologic assay in food folate analysis: an updated review. Exp Biol Med. 2005;230:444–454. doi: 10.1177/153537020523000702. [DOI] [PubMed] [Google Scholar]
- 38.Fukui T, Iinuma K, Oizumi J, Izumi J. Agar plate method using Lactobacillus plantarum for biotin determination in serum and urine. J Nutr Sci Vitaminol. 1994;40:491–498. doi: 10.3177/jnsv.40.491. [DOI] [PubMed] [Google Scholar]
- 39.Kishida K, Nishimoto Y, Kojo S. Specific determination of ascorbic acid with chemical derivatization and high-performance liquid chromatography. Anal Chem. 1992;64:1505–1507. [Google Scholar]
- 40.Ohkawa H, Ohishi N, Yagi K. New metabolites of riboflavin appear in human urine. J Biol Chem. 1983;258:5623–5628. [PubMed] [Google Scholar]
- 41.Gregpry JF, 3rd, Kirk JR. Determination of urinary 4-pyridovic acid using high performance liquid chromatography. Am J Clin Nutr. 1979;32:879–883. doi: 10.1093/ajcn/32.4.879. [DOI] [PubMed] [Google Scholar]
- 42.Shibata K. Ultramicro-determination of N1-methylnicotinamide in urine by high-performance liquid chromatography. Vitamins. (in Japanese) 1987;61:599–604. [Google Scholar]
- 43.Association of Official Analytical Chemists . Official Methods of Analysis. 17th ed. Arlington, VA, USA: AOAC Inc; 2000. [Google Scholar]
- 44.Shinton NK. Vitamin B12 and folate metabolism. Br Med J. 1972;1:556–559. doi: 10.1136/bmj.1.5799.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuwatari T, Shibata K. Relative availability of B-group vitamin in a test diet to free vitamin. J Home Eco Jpn (in Japanese) 2008;59:403–410. [Google Scholar]
- 46.Fukuwatari T, Shibata K. Relative availability of water-soluble vitamins in a white bread diet to free vitamin. J Home Eco Jpn (in Japanese) 2009;60:57–63. [Google Scholar]