Abstract
Multiple system atrophy (MSA) is a neurodegenerative disease with two motor phenotypes: parkinsonian (MSA-P) and cerebellar (MSA-C). To elucidate whether in addition to the motor abnormalities there are other significant differences between these phenotypes we performed a retrospective review of 100 patients (61 males, 39 females) with a diagnosis of possible (12%), or probable (88%). Four patients eventually had postmortem confirmation (i.e., definite MSA). Sixty percent were classified as having MSAP and 40% as MSA-C. MSA-C and MSA-P patients had similar male prevalence (60%), age of onset (56±9 years), and frequency of OH (69%). Brain MRI abnormalities were more frequent in MSA-C patients (p<0.001). Mean survival was 8±3 years for MSA-C and 9±4 years for MSA-P patients (p=0.22). Disease onset before 55 years predicted longer survival in both phenotypes. Initial autonomic involvement did not influence survival. We conclude that patients with both motor phenotypes have mostly similar survivals and demographic distributions. The differences here identified could help counseling of patients with MSA.
Keywords: MSA, MSA-P, MSA-C, parkinsonism, cerebellar, autonomic, MRI
INTRODUCTION
Multiple system atrophy (MSA) is a sporadic, adult-onset, fatally progressive degenerative synucleinopathy with two motor phenotypes: parkinsonian (MSA-P) and cerebellar (MSA-C) (Gilman et al. 2008). Autonomic failure with symptomatic orthostatic hypotension (OH) and urogenital abnormalities is reported in both phenotypes (Stemberger and Wenning 2011).
Although several studies have investigated the natural history of the disease, it is still unclear whether there are significant differences in demographics and non-motor domains between the C and P phenotypes. MSA-C is more prevalent in Japan (Watanabe et al. 2002) whereas MSA-P is more prevalent in Europe and the US (Kollensperger et al. 2010; Wenning et al. 2013). Although the course of MSA is generally rapidly progressive, some patients have slower progression and longer life span (Kim and Jeon 2012; Petrovic et al. 2012). Whether survival is different in patients with MSA-C or MSA-P is debated (O’Sullivan et al. 2008). What clinical features, if any, predict progression and survival in these patients is controversial. In this regard, some reports suggest that early autonomic involvement is an independent risk factor for rapid disease progression and shorter survival (Tada et al. 2007).
Here we describe the clinical features and survival of 100 MSA patients followed in a single center with emphasis on the differences and similarities between patients with MSA-C and MSA-P and on factors affecting survival.
METHODS
We retrospectively reviewed the medical records of patients included in the database of the Dysautonomia Center at the New York University from January 1994 to December 2011. We identified 100 patients with complete clinical and hemodynamic data who on the initial visit received a diagnosis of possible (12%), or probable (88%) MSA according to Consensus criteria (Gilman et al. 2008). Four patients eventually had post mortem neuropathological confirmation of the diagnosis and were reclassified as definite MSA.
Brain magnetic resonance imaging (MRI) scans were available from 56 patients. Of those, 39 were performed on a 1.5-T MRI machine, with a protocol that included sagittal T1-weighted images (repetition time/echo time, 600/14 seconds; slice thickness, 5 mm), axial intermediate and T2-weighted sequences (repetition time/echo time, 2500/30-90 seconds; slice thickness, 5 mm), and inversion recovery axial T1 images (repetition time/echo time/inversion time, 2500/20/800 seconds; slice thickness, 4 mm). In the remaining 17 cases, the brain MRI studies were performed elsewhere and provided by the patients. The interval between initial visit and brain MRI was no more than 18 months.
Complete Unified Multiple System Atrophy Rating Scale (UMSARS) scores (Wenning et al. 2004) were available in 30 patients. Scales for Outcomes in Parkinson Disease – Autonomic (SCOPA-AUT) scores (Visser et al. 2004) were available in 36 patients. L-dopa equivalent dose at time of first visit was calculated as previously described (Katzenschlager et al. 2005). L-dopa response was clinically assessed by the treating investigator.
We defined “disease onset” as the age of the patient when he/she was first aware of a characteristic motor or autonomic sign/symptom, excluding erectile dysfunction; “time to full expression” as the time from the onset of the first symptom to the involvement of the second domain (either motor or autonomic depending on the sequence of clinical features); “survival time” as either: 1) time from disease onset to death, 2) time from disease onset to present day for surviving patients or 3) time from disease onset to last office visit for patients lost to follow up.
Kaplan-Meier curves were used to compare survival according to dominant motor phenotype (parkinsonian vs. cerebellar), presenting feature (autonomic vs. motor), hastiness of motor/autonomic progression (progression of motor and autonomic domains ≥3 years vs. < 3 years), age of onset (<55 vs. ≥55 years), and gender distribution. Log-rank (Mantel-Cox) test was used to examine whether Kaplan-Meier survival curves differed statistically among subgroups. Censored data included patients alive and lost to follow-up. Uncensored data included only patients with a known date of death. Data was tested for normality and parametric and non-parametric tests were used as appropriate. Unpaired student’s t tests were used to compare continuous variables. Contingency tables were analyzed by Fisher’s exact test or the Chi-square test, as appropriate. Correlation analysis (Pearson’s coefficient) was used to assess the relationship between UMSARS and SCOPA-AUT. All tests were two-sided and significance was set at p≤0.05. Analyses were preformed with Prism 6 (GraphPad Software, Inc). Ethical approval was obtained for this study and all subjects gave informed consent. No patient refused participation.
RESULTS
Demographics
Table 1 summarizes demographics and clinical features of the patients on their initial visit. Of those with probable MSA there were 56 males and 32 females and of those with possible MSA there were 6 males and 6 females. Four cases eventually had pathological confirmation, and were re-classified as definite MSA (3 MSA-P, 1 MSA-C). Sixty percent were classified as having MSA-P and 40% as MSA-C. Age of onset was similar in both phenotypes at 56 years old (56±8 years in MSA-C and 56±10 in MSA-P). Patients were seen on average 5 years after disease onset (5±2 in MSA-C, 5±3 in MSAP). There was a trend for male predominance in both motor phenotypes.
Table 1.
Demographics and clinical features on initial visit. P value denotes statistical difference between MSA-C and MSA-P. Statistically significant P values are denoted in bold. Values are presented as means ± standard deviation where appropriate.
| MSA | MSA-C | MSA-P | P value | |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Patients, n | 100 | 40 | 60 | |
| Male: female | 61:39 | 65:35 | 58:42 | 0.21 |
| Age at first symptom (y) | 56±9 | 56±8 | 56±10 | 0.95 |
| Duration of disease before initial visit (y) | 5±3 | 5±2 | 5±3 | 0.69 |
| PRESENTING FEATURES | ||||
| Autonomic (%) | 48 | 54 | 43 | 0.32 |
| Motor (%) | 34 | 29 | 38 | 0.37 |
| Combined (%) | 18 | 17 | 19 | 0.84 |
| CLINICAL CHARACTERISTICS | ||||
| Neurological | ||||
| Bradykinesia (%) | 72 | 45 | 90 | <0.001 |
| Rigidity (%) | 67 | 28 | 93 | <0.001 |
| Postural instability (%) | 51 | 40 | 58 | <0.01 |
| Tremor (%) | 41 | 18 | 57 | <0.001 |
| Gait Ataxia (%) | 48 | 73 | 32 | <0.001 |
| Limb Ataxia (%) | 45 | 75 | 25 | <0.001 |
| Dysarthria (%) | 47 | 75 | 28 | <0.001 |
| Nystagmus (%) | 19 | 43 | 3 | <0.001 |
| Babinski sign (%) | 39 | 43 | 37 | 0.39 |
| Hyperreflexia (%) | 49 | 48 | 50 | 0.78 |
| UMSARS (units) | (n=30) 38.3±14 |
(n=15) 36.8±13 |
(n=15) 38.7±15 |
0.57 |
| Part I | 18.5±6 | 17.4±6 | 19.5±7 | 0.39 |
| Part II | 20.0±7 | 19.4±8 | 20.7±8 | 0.23 |
| L-dopa equivalent dose (mg) | (n=45) 426.6±290.2 |
(n=14) 528.5±284 |
(n=31) 380.6±285.6 |
0.12 |
| L-dopa response (%) | 31 | 8 | 39 | 0.07 |
| Autonomic | ||||
| Total SCOPA (units) | (n=36) 17.9±7 |
(n=18) 16.8±8 |
(n=18) 18.1±7 |
0.31 |
| Gastrointestinal | 4.2±3 | 3.9±3 | 4.5±3 | 0.46 |
| Urinary | 8.8±4 | 8.3±5 | 9.2±4 | 0.51 |
| Cardiovascular | 1.9±2 | 1.7±2 | 2.2±2 | 0.36 |
| Thermoregulatory | 1.6±2 | 1.4±2 | 1.7±2 | 0.58 |
| Pupillomotor | 0.4±1 | 0.4±1 | 0.4±1 | 0.94 |
| Sexual dysfunction | 1.6±1 | 1.5±1 | 1.8±1 | 0.31 |
| Fall in SBP after 3 min tilt (mmHg) | 38±29 | 34±20 | 40±34 | 0.34 |
| Frequency of OH (30/15 mmHg, %) | 69 | 69 | 69 | 1 |
| MRI FINDINGS (56 patients) | ||||
| Normal (%) | 30 | 7 | 48 | <0.001 |
| Cerebellar/pons atrophy (%) | 44 | 71 | 21 | <0.001 |
| Putaminal abnormalities (%) | 14 | 15 | 14 | 1 |
| Other (%) | 12 | 7 | 17 | 0.42 |
First symptoms and disease progression
Data on first symptom were available in 88 patients (35 MSA-C and 53 MSA-P). The remaining 12 patients were uncertain regarding their initial symptoms. Autonomic symptoms were the first manifestation of the disease in 43% of MSA-P and 54% MSA-C patients. Time from the first symptom to involvement of another (motor or autonomic) domain (i.e., time to full expression) was similar in both phenotypes. In those patients who reported autonomic symptoms first, motor impairment took additional 3.0±1.5 years to be noticed. In patients who reported motor impairment first, it took 2.4±1.5 years to notice autonomic involvement.
Clinical phenotype at initial visit
Patients with MSA-P most commonly presented with rigidity, followed by bradykinesia, tremor and postural instability. MSA-C patients most frequently presented with dysarthria, limb and gait ataxia, and less commonly with nystagmus. At the time of evaluation, UMSARS scores were similar in the 2 phenotypes.
Urogenital dysfunction, including high voiding frequency, retention and/or incontinence, was the most common autonomic complaint at the time of first evaluation. Erectile dysfunction was reported in 95% of men. OH with a fall of at least 30/15 mmHg was observed in 69% of patients with MSA-C and MSA-P; the magnitude of the fall was similar in both phenotypes. History of at least one syncopal episode was documented in 44% of the patients (54% MSA-C and 38% MSA-P). There were no differences in terms of SCOPA-AUT domain scores. SCOPA-AUT scores correlated with UMSARS-I (functional disability); (p=0.023), but not with UMSARS-II scores (motor impairment); (p=0.3).
Neuroimaging and L-dopa response
Overall, 70% of patients had poor or no motor response to L-dopa treatment, while 30% had an initial, but short-lived response. Of those with an initial good response, 93% had MSA-P. Of note, 13 patients who were on long-term L-dopa therapy and reported no benefit did report worsening of their symptoms when L-dopa was discontinued.
MSA-C patients more frequently had brain MRI abnormalities (p<0.001). Predominant abnormality was atrophy in the cerebellum and pons, more frequently in MSA-C (p<0.001). Putaminal abnormalities, presenting as either hyperintense rim or other changes in signal intensity, was equally present in patients with the P and C phenotypes.
Survival
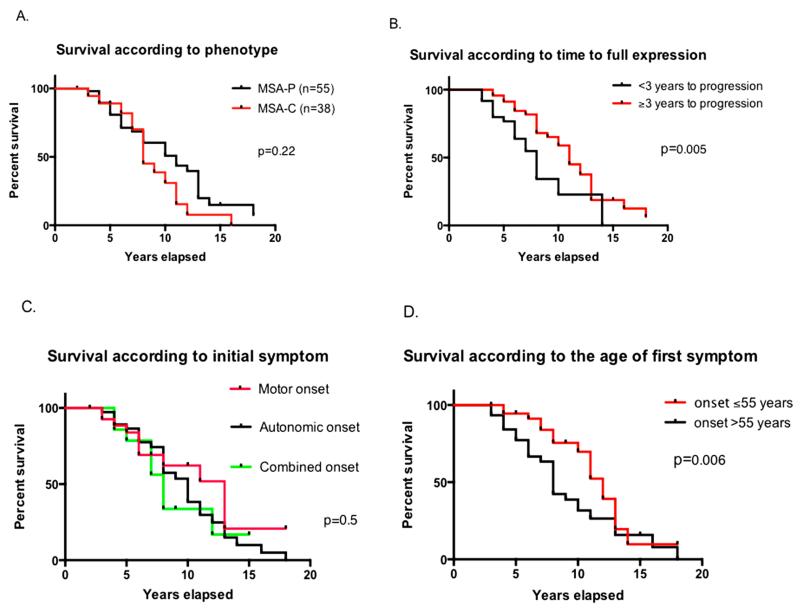
Date of death was obtained in 50 patients; 10 patients were lost to follow-up. Survival (estimated using data from all patients) was between 2 and 18 years with a mean survival of 8±3 years for MSA-C and 9±4 years for MSA-P patients. Survival curves were not significantly different for overall survival (Figure 1A); however, the curves crossed at year 7, after which point there was a trend towards longer survival in patients with MSA-P. We recorded survival of more than 15 years in 4 patients (3 MSA-P and 1 MSA-C). Patients who developed both motor and autonomic features in less than 3 years had a shorter survival than patients who took 3 years or longer to show full disease expression, i.e., motor and autonomic features (p=0.005, Figure 1B). To determine whether early autonomic involvement predicted shorter survival, we divided patients into three groups: those who first presented with autonomic, motor, or combined autonomic and motor symptoms. There were no significant differences in survival between these groups, i.e. early autonomic involvement did not predict shorter survival (p=0.5, log-rank test, Figure 1C). Gender did not influence survival either (data not shown). Patients who reported the first symptom, either motor or autonomic, before or at age 55 lived longer after disease onset than those who had their first symptom after age 55 (p=0.005, Gehan-Breslow-Wilcoxon test, Figure 1D).
Figure 1.
Kaplan-Meier curve depicting survival in MSA-C vs. MSA-P (A), Survival according to time to full expression (B), according to type of initial symptom (C) and according to age of first symptom (D).
DISCUSSION
This single-center retrospective study showed that patients with MSA-C and MSAP had similar survival (~8 years from the time of first symptom), gender distribution (mild male preponderance), same age of onset (mid fifties), and similar frequency of OH (~70%). These similarities between MSA-P and MSA-C underscore the “one disease” concept.
The present series also confirms that MSA-P is more common in US (Iodice et al. 2012) and European (Wenning et al. 2013) cohorts, in contrast to the higher frequency of MSA-C reported in Japanese populations (Watanabe et al. 2002); that autonomic symptoms are frequently the presenting feature, with 50% of patients showing a “premotor phase” (i.e., isolated autonomic phenotype) up to 3 years before the onset of motor features (Iodice et al. 2012); that the frequency of OH is around 70%, similar to other US (Iodice et al. 2012) and Japanese (Watanabe et al. 2002) cohorts but higher than European studies (Kollensperger et al. 2010); that MRI abnormalities are more frequent in MSA-C; and that patients with MSA-P are more likely to respond to L-dopa therapy (Wenning et al. 2013).
Interestingly, and in accordance with previous data (Watanabe et al. 2002; O’Sullivan et al. 2008), we found that patients with an earlier disease onset (< 55 years old) lived longer. MSA patients who developed both motor and autonomic involvement within 3 years, died earlier (Watanabe et al. 2002). It is not known whether the trend for a longer life span in patients with MSA-P would reach significance with a larger number of subjects, but in a recent study (Wenning et al. 2013) MSA-P was associated with shorter rather than longer survival. We found that SCOPA-AUT scores correlated with UMSARS-I (which measure functional disability) but not with UMSARS-II scores (which measure motor disability), similarly to a recent report (Damon-Perriere et al. 2012).
In contrast to some previous reports (Tada et al. 2007; O’Sullivan et al. 2008), early autonomic involvement did not predict shorter survival. Indeed, 3 out of 4 of our patients who survived longest (>15 years) had autonomic symptoms as presenting features. Furthermore, patients who start with autonomic symptoms do not progress to having motor symptoms faster than those who present initially with motor involvement (3.0±1.5 vs. 2.4±1.5 years of progression). The reasons of this discrepancy might be referral bias, because our center focuses on autonomic disorders MSA patients with autonomic features may be referred to us more frequently than to other centers.
We considered the possibility that the long surviving patients with MSA-P were misdiagnosed and actually had PD; close scrutiny of these cases, however, showed robust clinical support for the diagnosis of MSA (Kim and Jeon 2012; Petrovic et al. 2012). Also, the fact that not all patients underwent the same brain MRI protocol and that, in most of them, a T2* sequence (Kraft et al. 2002) was not routinely used might explain the low percentage of patients with putaminal abnormalities. Additional limitations of this study comprise the fact that the causes of death were not established; and the lack of regular assessments and quantitative outcome measures, which cannot be performed in retrospective chart reviews, as the present one.
In conclusion, MSA-C and MSA-P patients differ on their predominant motor deficits and the higher incidence of MRI abnormalities in those with MSA-C. Patients with both phenotypes, have similar survivals and demographic distributions. Fifty percent of all MSA patients had a “premotor phase” with an isolated autonomic phenotype lasting around 3 years. Onset before age 55 predicted longer survival in both phenotypes. Mean survival was 8 years. The differences here identified could help to improve the diagnosis and counseling of patients with MSA.
Acknowledgments
Funding source: National Institutes for Health (NIH) Rare Diseases Clinical Research Network grant (U54 S065736 to LJNK, JM and HK) and the Dysautonomia Foundation, Inc. (to DR, JAP, NG, and HK). The Rare Autonomic Disorders Consortium is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the NINDS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interests: The authors report no conflict of interests relevant to this article.
REFERENCES
- Damon-Perrière N, Foubert-Samier A, De Cock VC, Gerdelat-Mas A, Debs R, Pavy-Le Traon A, Senard JM, Rascol O, Tison F, Meissner WG. Assessment of the Scopa-Aut questionnaire in multiple system atrophy: relation to UMSARS scores and progression over time. Parkinsonism Relat Disord. 2012;18(5):612–615. doi: 10.1016/j.parkreldis.2011.12.009. doi:10.1016/j.parkreldis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. doi:10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iodice V, Lipp A, Ahlskog JE, et al. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry. 2012;83(4):453–459. doi: 10.1136/jnnp-2011-301068. doi:10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, Watt H, Bhatia K, Quinn N, Lees AJ. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord. 2005;20:151–157. doi: 10.1002/mds.20276. [DOI] [PubMed] [Google Scholar]
- Kraft E, Trenkwalder C, Auer DP. T2*-weighted MRI differentiates multiple system atrophy from Parkinson’s disease. Neurology. 2002;59(8):1265–1267. doi: 10.1212/01.wnl.0000032757.66992.3c. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jeon BS. Multiple system atrophy with prolonged survival. Mov Disord. 2012;27(14):1834. doi: 10.1002/mds.25289. doi:10.1002/mds.25289. [DOI] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Ndayisaba JP, et al. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord. 2010;25(15):2604–2612. doi: 10.1002/mds.23192. doi:10.1002/mds.23192. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(Pt 5):1362–1372. doi: 10.1093/brain/awn065. doi:10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- Petrovic IN, Ling H, Asi Y, et al. Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord. 2012;27(9):1186–1190. doi: 10.1002/mds.25115. doi:10.1002/mds.25115. [DOI] [PubMed] [Google Scholar]
- Stemberger S, Wenning GK. Modelling progressive autonomic failure in MSA: where are we now? J Neural Transm. 2011;118(5):841–847. doi: 10.1007/s00702-010-0576-3. doi:10.1007/s00702-010-0576-3. [DOI] [PubMed] [Google Scholar]
- Tada M, Onodera O, Tada M, et al. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol. 2007;64(2):256–260. doi: 10.1001/archneur.64.2.256. doi:10.1001/archneur.64.2.256. [DOI] [PubMed] [Google Scholar]
- Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19(11):1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125(Pt 5):1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264–274. doi: 10.1016/S1474-4422(12)70327-7. doi:10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19(12):1391–1402. doi: 10.1002/mds.20255. doi:10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]