Abstract
Free radical formation and oxidative damage have been extensively investigated and validated as important contributors to the pathophysiology of acute central nervous system injury. The generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is an early event following injury occurring within minutes of mechanical impact. A key component in this event is peroxynitrite-induced lipid peroxidation. As discussed in this review, peroxynitrite formation and lipid peroxidation irreversibly damages neuronal membrane lipids and protein function, which results in subsequent disruptions in ion homeostasis, glutamate-mediated excitotoxicity, mitochondrial respiratory failure and microvascular damage. Antioxidant approaches include the inhibition and/or scavenging of superoxide, peroxynitrite, or carbonyl compounds, the inhibition of lipid peroxidation and the targeting of the endogenous antioxidant defense system. This review covers the preclinical and clinical literature supporting the role of ROS and RNS and their derived oxygen free radicals in the secondary injury response following acute traumatic brain injury (TBI) and spinal cord injury (SCI) and reviews the past and current trends in the development of antioxidant therapeutic strategies. Combinatorial treatment with the suggested mechanistically complementary antioxidants will also be discussed as a promising neuroprotective approach in TBI and SCI therapeutic research. This article is part of a Special Issue entitled: Antioxidants and antioxidant treatment in disease.
Keywords: Antioxidant, Reactive oxygen species, Oxidative damage, Traumatic brain injury, Spinal cord injury, Lipid peroxidation
1. Introduction and background
Hallmarks of the secondary injury response in TBI and SCI include the loss of ionic homeostasis, glutamate excitotoxicity, mitochondrial dysfunction and microvascular disruption, which all occur immediately following the primary mechanical injury. These complex and integrated secondary injury cascades feed into pathways that yield free radical formation, which induces oxidative damage, another pathophysiological hallmark of central nervous system (CNS) injury. Uncontrolled reactive oxygen chain reactions triggered by secondary injury cascades can feed back into the secondary injury response creating an endless pool of ROS and the ultimate consequence is massive neuronal death. This review discusses both the role of ROS in the propagation of CNS injury and past and current therapeutic strategies used for the treatment of TBI and SCI.
1.1. Reactive oxygen species
ROS are oxygen-derived radicals and include the highly reactive superoxide (O2•−), hydroxyl (•OH) and peroxyl (RO2•) as well as non-radicals such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) (Table 1). The cascade of oxygen radical reactions begins with the production of O2•−, which occurs in response to rapid elevations in intracellular Ca2+ immediately following the primary mechanical injury in TBI and SCI. A single electron reduction of oxygen results in the formation of , which then acts as either an oxidant or a reductant. Superoxide dismutase (SOD) rapidly catalyzes the dismutation of O2•− into H2O2 and oxygen and at low pH, O2•− can dismutate spontaneously. The formation of highly reactive oxygen radicals, which have unpaired electron(s) in their outer molecular orbitals and the propagation of chain reactions are fueled by non-radical ROS, which do not have unpaired electron(s), but are chemically reactive. For example, •OH radicals are generated in the iron-catalyzed Fenton reaction where ferrous iron (Fe2+) is oxidized to form •OH in the presence of H2O2.
Fe2+ + H2O2→Fe3+ + ·OH + OH−
Table 1.
Reactive oxygen species and their sources.
| ROS | Symbols | Source |
|---|---|---|
| Superoxide anion | O2•− | Arachidonic acid metabolism, xanthine oxidase activity, mitochondrial leak |
| Hydrogen peroxide | H2O2 | Dismutation of superoxide catalyzed by SOD |
| Hydroxyl radical | HO• | Fenton reaction |
| Nitric oxide | NO− | Nitric oxide synthase (NOS) activity |
| Peroxynitrite | OONO− | The reaction of •NO with O2•− |
| Lipid peroxyl | LOO• | The reaction of a radical (L•) with oxygen, lipid hydroperoxide (LOOH) decomposition by Fe3+ |
| Lipid alkoxyl | LO• | LOOH decomposition by Fe2+ |
ROS and their sources implicated in the secondary injury response following trauma-induced injury.
Superoxide acting as a reducing agent can donate electrons to ferric iron (Fe3+), cycling it back to the ferrous state in the Haber–Weiss reaction, thus driving subsequent Fenton reactions and increased production of •OH.
O2•− + Fe3+→Fe2+ + O2
Under physiological conditions, iron is tightly regulated by its transport protein, transferrin and storage protein, ferritin, both of which bind the ferric (Fe3+) form. This reversible bond of transferrin and ferritin with iron decreases with declining pH (below pH7), as is the case after CNS injury resulting in the release of iron and initiation of oxygen radical production. A second source of iron comes from hemoglobin upon its release after mechanical induced hemorrhage.
Although O2•− itself is less reactive than •OH radical, its reaction with nitric oxide (•NO) radical forms the highly reactive oxidizing agent, peroxynitrite (PN: ONOO−) and hydroxyl radical as a byproduct (Table 1).
O2•− + ·NO→ONOO−
Subsequent PN decomposition results in the formation of additional highly reactive cytotoxic free radicals including nitrogen dioxide (•NO2) and carbonate radical (•CO3). •NO2 and •CO3 are formed by either the protonation of PN to peroxynitrous acid (ONOOH) or by PN reaction with carbon dioxide to form nitrosoperoxocarbonate (ONOOCO2). ONOOH and ONOOCO2 are separately decomposed to form highly reactive NO2 and •OH and •NO2 and •CO3, respectively.
ONOOH→ · NO2 + ·OH
ONOOCO2→ · NO2 + ·CO3
The PN-derived radicals induce oxidative damage to proteins, lipids (cellular and mitochondrial membranes) and nucleic acids [1, 2]. For example, PN-derived NO2 induces protein nitration resulting in posttranslational modification of protein-bound tyrosine to 3-nitrotyrosine (3-NT). Thus, 3-NT serves as a biological marker of ONOO- action [3, 4]. Additionally, PN products can instigate lipid peroxidation (LP) further propagating oxidative and irreversible cellular damage.
1.2. Lipid peroxidation
Lipid peroxidation defined as the oxidative degradation of lipids occurs when oxygen radicals react with polyunsaturated fatty acids such as arachidonic acid, linoleic acid, eicosapentaenoic (EPA) acid and docosahexaenoic acid (DHA) resulting in disruptions in cellular and membrane integrity. The process is a radical chain reaction characterized by three distinct steps: initiation, propagation and termination [5]. Briefly, initiation begins with ROS-induced hydrogen atom abstraction from polyunsaturated fatty acids, which yields a lipid radical (L•). In the second propagation step, the unstable L• reacts with oxygen to form a lipid peroxyl radical (LOO•). The LOO• in turn abstracts a hydrogen atom from adjacent polyunsaturated fatty acids yielding a lipid hydroperoxide (LOOH) and a second L•, which sets off a series of chain reactions. These propagation reactions are terminated in the third step when the substrate becomes depleted and a lipid radical reacts with another radical or radical scavenger to yield a stable non-radical end product. Two highly toxic products of LP are 4-hyroxynonenal (4-HNE) and acrolein, both of which have been well characterized in TBI and SCI experimental models. These aldehydic peroxidation end products covalently bind proteins and amino acids altering their structure and functional properties. Amino acids (lysine, histidine and arginine) are also targeted by oxygen radicals resulting in the formation of protein carbonyl moieties.
Overproduction of the ROS described above overwhelms the antioxidant response resulting in ROS interactions with proteins, lipids, carbohydrates and nucleic acids. Oxidative modification of these bio-molecules alters their functions ultimately leading to irreversible cellular damage. This cascade of damage is collectively referred to as “oxidative stress” or “oxidative damage”.
The impact of ROS production is heightened when oxygen radicals feed back to secondary injury pathways creating a continuous cycle of ion imbalance, Ca2+ buffering impairment, mitochondrial dysfunction, glutamate-induced excitotoxicity and microvascular disruption. One example of ROS-induced ionic disruption arises from LP induced damage to the plasma membrane Ca2+ pump and Na+/K+-ATPase, which contributes to increases in intracellular Ca2+ concentrations, mitochondrial dysfunction and additional ROS production. Both Ca2+ pump and Na+/K+-ATPase disruptions result in further increases in intracellular Ca2+ and Na+ accumulation respectively, the latter causing reversal of the Na+/Ca++ exchanger [6, 7]. PN formed from mitochondrial Ca2+ overload contribute to mitochondrial dysfunction. Nitric oxide, formed from mitochondrial NOS, in turn reacts with O2•− to produce the highly toxic PN, which impairs respiratory and Ca2+ buffering capacity via its derived free radicals [8]. Indeed increased PN-derived 3NT and 4HNE has been detected during the time of mitochondrial dysfunction and correlates with respiratory and Ca2+ buffering impairment [9]. Increased synaptosomal 4-HNE content is associated with impaired synaptosomal glutamate and amino acid uptake [10, 11]. Glutamate and NMDA induced damage in neuronal cultures is attenuated with LP inhibition confirming LP and oxidative damage as mediators of glutamate excitotoxicity [12]. The occurrence of LP-induced spinal microvascular damage was demonstrated with treatment with LP inhibitors vitamin E and ascorbic acid, which inhibited the reduction in white matter spinal cord blood flow [13].
2. Oxidative damage in the pathophysiology of CNS injury
The current body of evidence of ROS-induced cellular damage in CNS injury is vast. Furthermore, the time course of ROS production and damage has been well characterized in established experimental models of TBI and SCI. Highlights of some of the most significant findings is presented here.
2.1. Evidence for oxidative damage in TBI
One of the first lines of evidence of oxygen radical formation in secondary injury came from cat fluid percussion TBI models demonstrating an immediate post-injury increase in O2•− that continued at least 1 h post-injury [14, 15]. This was followed by studies in experimental rodent TBI models demonstrating a rapid transient increase in brain •OH levels as early as 5 min post injury [16, 17]. The immediate post-traumatic burst of •OH was followed by increases in the LP product, phosphatidylcholine hydroperoxide (PCOOH) within 1 h post-injury [18].
Subsequent studies demonstrated PN formation in various established rodent TBI models via indirect detection methods. Endothelial, neuronal and inducible NOS, which can produce both precursors of PN (•NO and O2•−) are up-regulated within 24 h after TBI in rat controlled cortical impact (CCI) and weight drop models [19–21]. Mesenge and colleagues [22] demonstrated PN action by quantifying increases in tyrosine nitration at 4 h and 24 h post-injury in a mouse closed head injury model. In addition, PN induced oxidative damage is evidenced by the increase in the PN biomarker 3-NT, which has been detected within the first hour post-injury with elevations lasting for several days [23, 24] (Fig. 1). Similar increases in the LP marker, 4HNE have also been demonstrated early post-injury and coincide with elevations in 3-NT [23]. Furthermore, a substantial amount of evidence supporting oxidative damage in the pathophysiology of TBI comes from studies demonstrating neuroprotection of post-traumatic damaged tissue by treatment with antioxidant compounds [25–28].
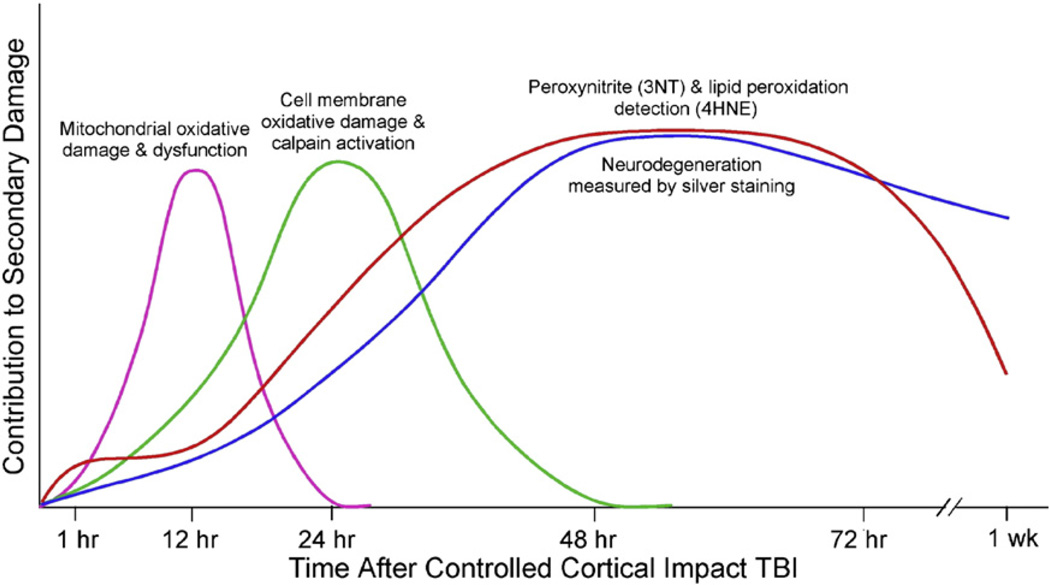
Fig. 1.
Time course of measured secondary injury events in the mouse CCI model of TBI. Mitochondrial oxidative damage and dysfunction precedes the onset (by 3 h) of neurodegeneration as measured by the respiratory control ratio and mitochondrial calcium-buffering capacity following CCI-TBI-induced injury [134]. Oxidative damage-induced increases in cytoplasmic Ca2+ and subsequent cell membrane damage as measured by calpain-mediated spectrin degradation begins by 6 h and continues through 48 h (peak at 24 h) post-TBI [135]. PN-induced oxidative damage as measured by immunohistochemical elevations in the oxidative markers, 3-NT and 4-HNE, occurs by 1 h and remains elevated for several days [23]. Post-traumatic time course of neurodegeneration as demonstrated by de olmos silver staining begins at 12 h and reaches its peak by 72 h following injury [135].
2.2. Evidence for oxidative damage in SCI
Increases in O2•− and •OH have been well documented in contused spinal cord injuries. Specifically, Liu et al. [29] reported an immediate increase in O2•− production following rat spinal cord impact injury, which remained elevated over 10 h [29]. Early increases in •OH levels occur by 5 min extending through 3 h following impact injury to the rat spinal cord [30, 31].
Initial studies demonstrating free radical production and oxidative damage in CNS injury comes from experimentally injured cat spinal cord. The LP product, malondialdehyde (MDA) measured by electron resonance spectrometry was one of the first lines of evidence for free radical production in SCI and was increased within 5 h following impact SCI [32]. Hall et al. [33] detected a similar increase in MDA and increase in free radical-induced cyclic guanosine minophosphate (cGMP) levels by 1 h. Early MDA production in rat models of SCI has also been well established to increase as early as 2 h following injury [34].
Alternative approaches used to measure and support LP include immunohistochemical analysis of LP-derived aldehydic breakdown products 4-HNE and acrolein and free-iron correlations with increased LP products. Time courses in contused rat spinal cord revealed increases in 3-NT and 4-HNE at 3 h post injury with peak levels for both at 24 h [35]. While 3-NT levels returned to control conditions by 2 weeks, 4-HNE levels remained elevated up to 2 weeks post injury [35]. Others also report similar immunohistochemical and immunoblot increases in the levels of 4-HNE [10, 36, 37] and acrolein [38, 39].
Evidence for PN-induced oxidative damage via 3-NT immunological detection occurs as early as 1 h post rat contused spinal cord [35]. This increase in 3-NT overlaps with the temporal and special time course of 4-HNE, suggesting PN as the culprit of both forms of post-injury oxidative damage [35]. Further support for PN-induced damage in SCI comes from studies that infused the PN-generating compound SIN-1 into non-injured spinal cord to mimic production of 3-NT and subsequent neuronal damage in contused spinal cord [40–43].
It should be noted that the reported time course of ROS generation and oxidative stress varies across the different head injury models, but this is not surprising considering each model represents a different magnitude of injury: focal, multifocal and diffuse. Regardless of the timing differences, it is evident from the cumulative literature that oxidative stress plays an early role in the secondary sequelae underlying human CNS injury and the observed variations translate to the complexity of clinical CNS injury. Collectively these data demonstrate the significant early contribution of oxidative damage in the secondary injury response in TBI and SCI supporting the need for improved antioxidant therapies in CNS injury.
3. Antioxidant therapy strategies
Based on the literature characterizing the oxidative secondary injury response in CNS injury, potential antioxidant therapeutic interventions include: (1) compounds that either inhibit the formation of or scavenge ROS and RNS prior to LP initiation or (2) compounds that inhibit LP propagation reactions or scavenge lipid radicals (LOO•) and alkoxyl radical (LO•) after LP is initiated. The major limitation of the first approach is the narrow therapeutic window to block the initial burst of post-traumatic radical species production that is responsible for the free radical chain reactions of oxidative damage. Thus, it may be argued that pharmacological inhibition of this almost immediate free radical formation is impractical. The second approach of inhibition of LP propagation reactions requires compounds that target neural cell membranes to block LOO• and LO• interaction with adjacent polyunsaturated fatty acids. This antioxidant strategy has a more feasible therapeutic window and has proven successful with administration of high doses of the glucocorticoid steroid methyloprednisolone (MP). A more detailed discussion of glucocorticoid antioxidant mechanisms is described below.
4. Pharmacological antioxidants therapies for SCI
4.1. High-dose methylprednisolone therapy for SCI
The use of glucocorticoids in the treatment of CNS injury was not novel as dexamethasone and MP were already routinely employed in the clinical treatment of SCI. However, their use in this regard as far back as the 1960s was based on their ability to decrease cerebral edema [44].
The idea of glucocorticoid antioxidant therapy in CNS trauma grew attention in the early 1980s following studies characterizing glucocorticoid effects on spinal cord function, motor neuron excitability and synaptic transmission in cat spinal cord [45–47]. The idea that glucocorticoid steroids could inhibit post-traumatic LP was based upon their lipophilic nature allowing them to insert themselves within the cell membrane and thus potentially inhibit LP propagation reactions in the phopholipid bilayer. A series of studies soon emerged using high-dose MP treatment in the contused cat spinal cord experimental model. These studies characterized MP as an effective inhibitor of free-radical reactions and LP if administered in high doses intraveneously [47, 48]. Subsequent studies described additional neuroprotective effects of MP including the ability to inhibit posttraumatic spinal cord ischemia, support aerobic energy metabolism, decrease intracellular calcium overload and reduce calpain-mediated neurofilament loss [49–52]. However, the dose–response of MP in relation to LP and these other multimodal effects was found to follow a U-shape pattern [53]. The antioxidant efficacy of certain glucocorticoids was also found to be independent of steroid receptor activity and did not correlate with their anti-inflammatory potency. Moreover the LP-inhibiting actions of MP required much higher doses that exceed what is required for classic glucocorticoid receptor-mediated actions [54]. It should be noted that lower doses of MP are likely to produce anti-inflammatory effects, which is a well-established function of glucocorticoids [55].
4.2. NASCIS trials I–III
During the rapid pre-clinical advances in the understanding of glucocorticoid antioxidant mechanisms, clinical studies of MP had already been initiated. In 1985 the first National Acute Spinal Cord Injury Study (NASCIS I) using MP for the treatment of spinal cord injury was initiated, but the 10-day, low-dose therapy of MP failed to demonstrate any significant efficacy [56, 57]. The significant evidence obtained from preclinical experiments using high-dose MP treatment in cat spinal cord injury described above prompted the second NASCIS published in 1990. In the NASCIS II trial, a 24 h intensive dosing with MP (30 mg/kg i.v. bolus plus 23 h infusion as 5.4 mg/kg) was compared to placebo in acute SCI [58]. Indeed when the 24 h dosing with MP was initiated within the first 8 h, it resulted in significant improvements in both neurologically complete (i.e., plegic) and incomplete (i.e., paretic) patients with sustained recovery lasting through 1 yr follow-ups [59, 60]. The outcomes of NASCIS were replicated and confirmed in subsequent trials in SCI patients [61, 62]. Unfortunately, when the 24 h dosing with MP was delayed until after 8 h, it resulted in decreased neurological recovery, thus limiting the therapeutic window to within 8 h [60]. Additional predictable side effects related to steroid therapy were also described including gastrointestinal bleeding, wound infections and delayed healing all of which were not significantly more frequent than those in placebo-treated patients [58].
The occurrence of the steroid therapy side effects prompted the development of a steroid compound that retained the anti-LP effects of MP without producing the typical glucocorticoid effects of the steroid. Thus emerged the 21-aminosteroids or “lazaroids,” a novel group of LP inhibitors designed to be devoid of glucocorticoid receptor interactions, which limited the clinical use of high-dose MP. Of these, tirilazad (U-74006F) was selected for further studies based on positive results in animal SCI models [63–65].
With the development of tirilazad came the third NASCIS trial, which compared three groups of patients: those that received (1) a 24 h intensive MP dosing (30 mg/kg i.v. bolus plus 23 h infusion at 5.4 mg/kg) described in NASCIS II, (2) a 48 h intensive MP dosing (30 mg/kg i.v. bolus plus 47 h infusion at 5.4 mg/kg) and (3) a single MP dose (30 mg/kg i.v. bolus) followed by 47 h administration of tirilazad [66, 67]. The groups were also assessed for recovery within a 3 h window compared to a cohort that received delayed treatment 3–8 h post SCI. All three groups recovered comparably when treatment was begun within the 3 h window. However differences in the effectiveness emerged when the treatment was delayed 3–8 h post SCI. In the 3–8 h cohort, the 48 h MP dosing group recovered significantly better than the 24 h MP dosing group. Recovery in the tirilazad 3–8 h post SCI cohort was better then the 24 h MP group but not significant, and poorer than the 48 h MP group. Collectively, these results demonstrated that initiation of high dose MP treatment is optimal within 3 h, tirilazad is as effective as 24 h MP therapy and if treatment is delayed after 3 h post SCI, MP therapy should be extended from 24 h to 48 h. However, prolonged dosing with MP resulted in significantly more glucocorticoid related immunosuppression related side effects including gastrointestinal bleeding, wound infections, and delayed healing [67]. As expected the non-glucocorticoid, tirilazad did not show evidence of steroid related side effects and would thus be a safer compound for extended dosing studies.
Two important parameters of the NASCIS trials were the defined antioxidant therapeutic window and treatment duration, both of which prompted controversies and confusion surrounding the data quality, statistical analysis and interpretation [68–72]. In addition, the observed associated complications with steroid therapy (sepsis, hyperglycemia, delayed wound healing) prompted questions regarding the benefit-risk ratio of MP in the treatment of human SCI even though high-dose MP therapy was not found to significantly increase these side effects compared to placebo-treated patients. If initiated soon after injury (within 8 h) and with continued treatment for 24 to 48 h, high-dose MP is beneficial in improving motor neurologic function. If treatment is delayed until 3 to 8 h after injury, then a 48 h dosing regimen is required for improved neurologic outcome. Recognizing these two important parameters (therapeutic window and duration) as well as the narrow U-shaped dose response of MP is crucial in commencing the appropriate therapy for SCI. Despite the criticisms, MP steroid therapy is the only pharmacological therapy to have shown efficacy in a phase III randomized trial and to date remains the only available acute neuroprotective treatment for SCI. However, normal neurological function is not achieved on average with MP treatment demonstrating the need for more research and randomized clinical trials on steroid therapy including the possibility of combined neuroprotective treatment.
Despite the inability of the above-mentioned SCI therapies to achieve robust neuroprotection and motor recovery the SCI clinical trials to date have provided positive valuable information and paved the way for the development of more effective therapeutics and clinical evaluation. Indeed a number of recent publications have established guidelines and recommendations for future SCI clinical trials [73–76].
5. Pharmacological antioxidants therapies for TBI
The antioxidant TBI trials thus so far, which include polyethylene glycol-conjugated-SOD (PEG-SOD), tirilazad and dexanabinol, have unfortunately failed in their efforts to provide neuroprotection in moderately to severely injured TBI patients. A brief discussion of the PEG-SOD and tirilazad trials and their inadequacies follows.
5.1. PEG-SOD
SOD was previously shown in experimental head injury models to inhibit the post-traumatic microvascular dysfunction induced by rapid up-regulation of O2•− following injury. Soon after these studies a small clinical trial was initiated using a more stable PEG-conjugated SOD (PEG-SOD) in TBI patients, in which treatment was initiated within 8 h post injury. Initial results indicated a trend towards improved neurological outcomes [77], however a later multi-center phase III study did not amount to significant effects on increased survival or neurologic recovery [78]. PEG-SOD was limited in its therapeutic value based on it large size and rather narrow therapeutic window to scavenge the short-lived primordial O2•− radical. A target further downstream in the post-traumatic free radical pathways would seemingly be more feasible.
5.2. Tirilazad
The non-glucocorticoid LP inhibitor tirilazad, which inhibits LP propagation reactions by membrane stabilization and scavenging LOO• was tested as a TBI therapeutic in the early 1990s around the time of its use in the NASCIS III study. After successfully passing a small phase safety trial, tirilazad entered two (North America and Europe) phase III multi-center trials in moderately and severely injured TBI patients. Tirilazad or placebo was initiated within 4 h following injury at a dose of 2.5 mg/kg every 6 h for a duration of 5 days. Both trials failed for different reasons. The data from the North American study was stopped because of improper randomization and an initial concern of mortality amongst the tirilazad-treated group. The European data was published in 1998 and the study failed to show any significant benefit for tirilazad in the treatment of TBI in either the moderate or severely injured patient groups [79]. A subsequent post hoc analysis revealed significantly less mortality in moderately and severely injured male patients that also had subarachnoid hemorrhage. Additional clinical trials of tirilazad in TBI have not since been pursued. Factors contributing to the failure of the tirilazad study were the apparent inability of the agent to cross the blood–brain barrier in high enough concentrations in severely injured patients as well as gender differences in treatment outcomes and drug metabolism [80, 81].
The disappointing results of the TBI clinical trials have prompted an intense debate on the need for major improvements in the design of future TBI clinical trials. Included is the need for in depth preclinical testing of potential agents in multiple species and head injury models, alternative strategies in randomization of patient populations, adequate pharmacokinetic data and distinguishing subgroups of TBI patients prior to trial initiation [81, 82]. Another major challenge in TBI clinical trials is the heterogeneous nature of head trauma. Keeping this mind it is plausible that some of the aforementioned agents that have failed may indeed be effective within subgroups of TBI populations. This has been shown in various post hoc analyses of specific patient subgroups that were found to benefit from the proposed treatment compared to placebo. Thus, the need for improved clinical trial design and analyses is imperative for the future of novel TBI therapeutics.
6. Recent advances in antioxidant therapeutic strategies
A detailed summary of antioxidant approaches in SCI and TBI was recently published [83, 84]. Here, an update on the current promising neuroprotective mechanisms for CNS injury will be reviewed.
6.1. U-83836E
U-83836E is a second-generation lazaroid with a non-steroidal structure characterized by a ring portion of alpha-tocopherol bonded to various amine groups. Its structure enables the dual functionality of LP inhibition and scavenging LOO•, thereby making it muchmore effective than the endogenous scavenger vitamin E. U-83836E has been shown to decrease post traumatic LP and protein nitration as well as preserve mitochondrial respiratory function and calcium buffering capacity in the mouse CCI TBI model (Fig. 1) [85]. More recently U-83836E has also been shown to inhibit calpain-mediated cytoskeletal degradation in the same model (Fig. 1) signifying the intricate relationship between post-traumatic LP, disruptions in neuronal Ca2+ homeostasis and calpain-mediated cytoskeletal damage [86]. In this regard, U-83836E is able to inhibit an early event in a series of linked secondary injury pathways, thereby providing neuroprotection at multiple biochemical levels (See Fig. 2 for U-83836E molecular targets).
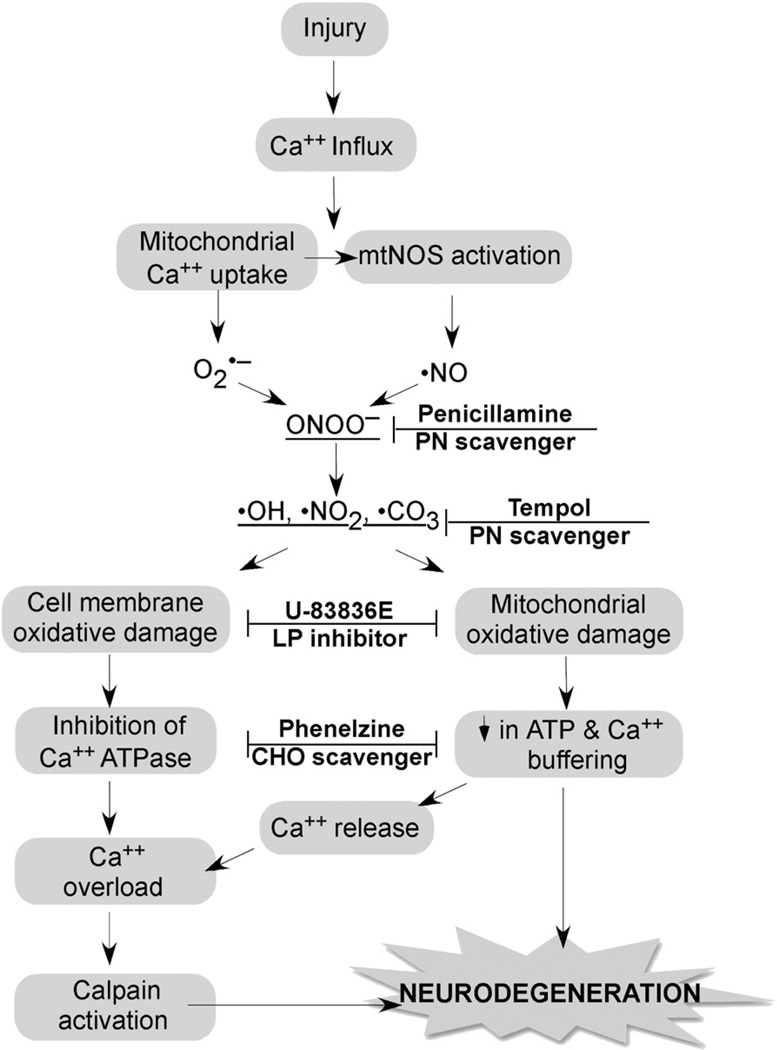
Fig. 2.
Strategy for combination antioxidant therapy for TBI and SCI. Injury triggers an increase in cytoplasmic Ca2+ via voltage dependent and glutamate receptor-operated channels, which initiates the depicted cascade of events. Mitochondria Ca2+ uptake causes O2•− leakage from the electron transport chain and activation of mitochondrial nitric oxide synthase (NOS). O2•− and •NO combine to form the highly reactive nitrogen species PN (ONOO−) giving rise to nitrogen dioxide •NO2, hydroxyl •OH and •CO3. These PN-derived radicals induce cell membrane and mitochondrial oxidative damage resulting in the inhibition of Ca2+ ATPase and a decrease in the mitochondrial ATP production and membrane potential (ΔΨ), respectively. Mitochondrial dysfunction causes the dumping of mitochondrial Ca2+ into the cytoplasm where it exacerbates cytoplasmic calcium overload and calpain activation. Calpain initiates the proteolysis of cytoskeletal proteins and other substrates ultimately contributing to neurodegeneration. The combination of the antioxidant penicillamine or tempol, which catalytically reacts with PN-derived radicals with a chain-breaking LP inhibitor such as U-83836E or a carbonyl (CHO) scavenging compound (Phenelzine) should produce a better neuroprotective effect than any of these compounds alone.
6.2. Melatonin
Melatonin (n-acetyl-5-mehoxytryptophan), a pleiotropic compound that primarily functions in the regulation of circadian rhythms and sleep also has known antioxidant and neuroprotective properties. The antioxidant actions of melatonin include the direct scavenging of free radicals as well as indirect regulation of endogenous antioxidant enzyme expression [87]. The ability of melatonin to scavenge LOO• and react with PN has been previously described [88–90]. There are extensive studies in models of experimental SCI demonstrating melatonin-induced decrease in lipid peroxidation, preservation of neuronal structure and increased functional recovery following injury [91–94]. A more recent combination therapy of melatonin and dexamethasone demonstrated significant anti-inflammatory effects and tissue and motor recovery in an experimental model of mouse SCI [95]. Melatonin administered in combination with exercise in the contused rat SCI model is associated with an increased number of motor neurons, a decrease in iNOS mRNA levels and increased hind limb movement compared to no treatment and exercise only controls [96]. In experimental TBI models, melatonin has been shown to increase brain antioxidant levels, decrease NF-kappaB activation and improve cognitive function [97–99]. A recent combinational treatment of melatonin and minocycline in a rat CCI model failed to demonstrate significant histopathological or cognitive functional recovery alone or in combination [100]. However, it was suggested that dosing, treatment initiation and duration played a role in the negative outcome. Advantages of melatonin as a CNS injury therapeutic include its lipophilicity, brain penetrability and potential for low side effects [94].
6.3. Carbonyl scavenging: penicillamine and phenelzine
As discussed earlier, the LP-derived breakdown products 4-HNE and acrolein can bind protein amino acid residues (histidine, lysine, arginine and cysteine) resulting in the functional disruption of enzymatic proteins [101]. Spinal cord and brain mitochondrial function are highly sensitive to 4-HNE and acrolein with spinal cord mitochondria demonstrating increased sensitivity to these LP-derived aldehydes [102]. Compounds that covalently bind LP-derived aldehydes act as carbonyl scavengers and have demonstrated neuroprotective potential in experimental trauma models. For example, D-penicillamine irreversibly binds primary aldehydes and has been shown to scavenge PN [103]. In isolated rat brain mitochondria, application of D-penicillamine protected against PN-induced mitochondrial respiratory dysfunction and also concomitantly decreased 4-HNE levels [104]. Moreover, acute penicillamine was previously shown to improve neurological recovery in the mouse concussive head injury model [105].
In addition to thiol-containing compounds, Hamann and colleagues have demonstrated that chemical scavenging of 4-HNE and acrolein by treatment with hydrazine (−NH-NH2)-containing compounds is neuroprotective in SCI models [39, 106, 107]. Protection from 4-HNE-induced cellular cytotoxicity by hydrazines can occur even after 4-HNE has bound cellular proteins suggesting an advantageous therapeutic window for treatment [108]. The hydrazine containing compound, phenelzine, a well-characterized monoamine oxidase inhibitor antidepressant also has the ability to react with carbonyl moieties of 4-HNE or acrolein preventing their interactions with targeted amino acids and proteins [108]. Although phenelzine has yet to be investigated in TBI and SCI models, recent studies from the author's laboratory demonstrate neuroprotective effects of phenelzine on the preservation of mitochondrial bioenergetics in an in vitro model of 4-HNE-induced mitochondrial respiratory dysfunction (unpublished data).
6.4. Scavengers of PN and PN-derived free radicals: tempol
Nitroxide-containing antioxidants referred to as “spin trapping” agents gained some attention for modulating oxidative injury. Of these agents, tempol, a stable membrane permeable nitroxide, is the most potent in its antioxidant effects and is involved in the metabolism of many ROS and RNS including O2•−, H2O2 and PN-derived •NO2 and •CO3 [109, 110] The neuroprotective effects of tempol have been described in both models of TBI and SCI injury [111]. In the mouse CCI-TBI model, tempol reduced post-traumatic LP and protein nitration-induced oxidative damage, which resulted in preserved mitochondrial bioenergetics, reduced calpain-mediated cytoskeletal damage and reduced neurodegeneration (Fig. 1) [112]. Additionally, tempol decreased post-traumatic brain edema and improved neurological recovery in the rat contusion head injury model [113]. Similarly, in SCI models tempol protected against post-traumatic PN-induced mitochondrial impairment, cytoskeletal degradation [114], white matter loss and loss of locomotor function [115]. Although, therapeutic window analyses experiments revealed a short 1 h treatment window for tempol to achieve mitochondrial protective effects [114], others reported a therapeutic treatment-window of several days for tempol-induced locomotor recovery [115]. Thus, the observed recovery of function effects of tempol in SCI remains promising and requires further investigation. In addition, tempol may be an ideal candidate for combination therapy with other neuroprotective approaches (Fig. 2).
6.5. Resveratrol
The popularity of resveratrol, a polyphenolic compound enriched in grapes and red wine, as an anti-oxidant neuroprotective agent emerged in the early 2000s. The beneficial effects of resveratrol have been investigated in cancer as well as neurodegenerative models of neurotrauma, stroke, Parkinson's, Huntington's and Alzheimer's disease [116]. In SCI models, resveratrol has been reported to decrease oxidative stress [117], improve post-injury edema, Na+, K+-ATPase activity [118] and improve neurologic recovery [119]. A more recent study by Liu et al. [120] demonstrated that the observed resveratrol-induced neurologic and histopathologic improvements were mechanistically associated with increased SOD activity and decreased expression of MDA, inflammatory cytokines and pro-apoptotic proteins. These effects translate to TBI models as well with reported reductions in MDA, xanthine oxidase and •NO and associated decreased tissue lesion volume [121] and hippocampal functional recovery [122].
6.6. Small molecule Nrf2/ARE signaling activators
The body's endogenous antioxidant defense is regulated by nuclear factor E2-related factor 2/antioxidant response element (Nrf2/ARE) signaling at the transcriptional level [123, 124]. Nrf2 activation and the up-regulation of antioxidant and anti-inflammatory genes represents a valid neurotherapeutic intervention in CNS injury and has been previously described in various experimental models of stroke and neurodegenerative diseases [125].More recently, the role of Nrf2/ARE activation in SCI has been explored as a targeted neuroprotective strategy. Indeed, studies in Nrf2 (−/−)mice demonstrated increased spinal cord edema and expression of inflammatory cytokines compared to wild-type Nrf2 mice following SCI [126, 127]. In mild rat thoracic SCI, Wang et al. [128] reported an increase in Nrf2 levels as early as 30 min post-injury lasting through 3 days. Application of sulforaphrane, a Nrf2/ARE signaling activator, significantly reduced contusion volume and increased coordination. These positive outcomes were a result of sulforaphrane-induced increases in Nrf2, glutamine and decreases in inflammatory cytokines, IL-1β and TBFα [128].
The mRNA levels of Nrf2-regulated antioxidant enzymes, heme oxygenase (HO-1) and NAD(P)H:quinonereductase-1 (NQO1) are up-regulated 24 h post TBI [129]. In addition, Nrf2-knockout mice are susceptible to increased oxidative stress and neurologic deficits following TBI compared to their wild-type counterparts [130]. Administration of sulforaphane is also neuroprotective in various animal models of TBI specifically reducing cerebral edema, and oxidative stress and improving BBB function and cognitive deficits [131]. Studies by Chen et al. [132] demonstrated increased expression of Nrf2 and HO-1 in the cortex of the rat subarachnoid hemorrhage model. Treatment with sulforaphane further increased the expression of Nrf2, HO-1, NQ01 and glutathione S-transferase-α1 (GST-α1) resulting in the reduction of brain edema, cortical neuronal death and motor deficits [132]. Tert-butylhydroquinone, another activator of Nrf2 protects against TBI-induced inflammation and damage via reduction in NF-KB activation and TNFα and IL-1β production following injury in the mouse closed head injury model [133]. Collectively these studies demonstrate a significant neuroprotective role of Nrf2 signaling through the activation of antioxidant enzymes and reduction of the inflammatory secondary injury response following CNS injury. Thus, Nrf2 activation may be a prime candidate for the attenuation of oxidative stress and subsequent neurotoxicity in SCI and TBI via the development of small-molecule activators of the Nrf2/ARE pathway.
7. Conclusions
Over the past several decades experimental research in TBI and SCI models has validated the contribution of free radical-induced oxidative damage in the multidimensional secondary injury response, which occurs following acute CNS injury. From these studies, two therapeutic agents that specifically targeted LP inhibition, MP and tirilazad were further explored in multi-center phase III SCI clinical trials where they were shown to improve neurological recovery. Unfortunately, the steroid related side effects with prolonged treatment of MP have resulted in the continued search for safer antioxidant agents for the treatment of acute SCI. To date TBI clinical trials have failed to reproduce the neuroprotective effects of several pharmacological agents shown to be protective in preclinical experimental head injury models suggesting a quandary in approaches in translational research design. Nevertheless, the development of potent antioxidant compounds remains an attractive and promising strategy for the treatment of CNS injury. Focusing on events downstream of LP initiation including the inhibition of LP propagation and scavenging of LP-derived aldehydes is ideal because these signaling events extend several days following injury and thus widen the treatment therapeutic window range. The vast majority of preclinical studies have focused on targeting a specific free radical producing pathway in the oxidative component of the secondary injury response. It is well established that the secondary injury response is a highly integrated and complex cascade of biochemical events. It is likely that targeting multiple points of the free radical signaling pathways would produce a greater neuroprotective effect. Such combinational approaches would require a significant amount of preclinical research to answer critical questions including appropriate dosing based on detailed pharmacokinetic studies as well as treatment initiation and duration times that would aid in the translation to clinical testing. This multi-mechanistic antioxidant therapy strategy is depicted in Fig. 2. Such improvements will open up a new avenue and spectrum of possibilities for the treatment of CNS injury.
Acknowledgements
Some of the work reviewed in this article was supported by funding from the Kentucky Spinal Cord & Head Injury Trust.
Abbreviations
- TBI
Traumatic brain injury
- SCI
Spinal cord injury
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- PN
Peroxynitrite
- LP
Lipid peroxidation
- MP
Methylprednisolone
- CCI
Controlled cortical impact
- 4-HNE
4-hydroxynonenal
- 3-NT
3-Nitrotyrosine
Footnotes
This article is part of a Special Issue entitled: Antioxidants and antioxidant treatment in disease.
References
- 1.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 2.Radi R. Peroxynitrite reactions and diffusion in biology. Chem. Res. Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- 3.Hall ED, Oostveen JA, Andrus PK, Anderson DK, Thomas CE. Immunocyto-chemical method for investigating in vivo neuronal oxygen radical-induced lipid peroxidation. J. Neurosci. Methods. 1997;76:115–122. doi: 10.1016/s0165-0270(97)00089-7. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 6.Rohn TT, Hinds TR, Vincenzi FF. Ion transport ATPases as targets for free radical damage. Protection by an aminosteroid of the Ca2+ pump ATPase and Na+/K+ pump ATPase of human red blood cell membranes. Biochem. Pharmacol. 1993;46:525–534. doi: 10.1016/0006-2952(93)90530-a. [DOI] [PubMed] [Google Scholar]
- 7.Rohn TT, Hinds TR, Vincenzi FF. Inhibition of Ca2+−pump ATPase and the Na +/K+−pump ATPase by iron-generated free radicals. Protection by 6,7-dimethyl-2,4-DI-1- pyrrolidinyl-7H-pyrrolo[2,3-d] pyrimidine sulfate (U-89843D), a potent, novel, antioxidant/free radical scavenger. Biochem. Pharmacol. 1996;51:471–476. doi: 10.1016/0006-2952(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 8.Bringold U, Ghafourifar P, Richter C. Peroxynitrite formed by mitochondrial NO synthase promotes mitochondrial Ca2+ release. Free Radic. Biol. Med. 2000;29:343–348. doi: 10.1016/s0891-5849(00)00318-x. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J. Neurotrauma. 2007;24:991–999. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- 10.Carrico KM, Vaishnav R, Hall ED. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J. Neurotrauma. 2009;26:1369–1378. doi: 10.1089/neu.2008-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang JR, Scherch HM, Hall ED. Direct measurement of lipid hydroperoxides in iron-dependent spinal neuronal injury. J. Neurochem. 1996;66:355–361. doi: 10.1046/j.1471-4159.1996.66010355.x. [DOI] [PubMed] [Google Scholar]
- 12.Monyer H, Hartley DM, Choi DW. 21-Aminosteroids attenuate excitotoxic neuronal injury in cortical cell cultures. Neuron. 1990;5:121–126. doi: 10.1016/0896-6273(90)90302-v. [DOI] [PubMed] [Google Scholar]
- 13.Hall ED, Wolf DL. A pharmacological analysis of the pathophysiological mechanisms of posttraumatic spinal cord ischemia. J. Neurosurg. 1986;64:951–961. doi: 10.3171/jns.1986.64.6.0951. [DOI] [PubMed] [Google Scholar]
- 14.Kontos HA, Povlishock JT. Oxygen radicals in brain injury. Cent. Nerv. Syst. Trauma. 1986;3:257–263. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- 15.Kontos HA, Wei EP. Superoxide production in experimental brain injury. J. Neurosurg. 1986;64:803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- 16.Hall ED, Andrus PK, Yonkers PA. Brain hydroxyl radical generation in acute experimental head injury. J. Neurochem. 1993;60:588–594. doi: 10.1111/j.1471-4159.1993.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 17.Hall ED, Andrus PK, Yonkers PA, Smith SL, Zhang JR, Taylor BM, Sun FF. Generation and detection of hydroxyl radical following experimental head injury. Ann. N. Y. Acad. Sci. 1994;738:15–24. doi: 10.1111/j.1749-6632.1994.tb21785.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith SL, Andrus PK, Zhang JR, Hall ED. Direct measurement of hydroxyl radicals, lipid peroxidation, and blood–brain barrier disruption following unilateral cortical impact head injury in the rat. J. Neurotrauma. 1994;11:393–404. doi: 10.1089/neu.1994.11.393. [DOI] [PubMed] [Google Scholar]
- 19.Cobbs CS, Fenoy A, Bredt DS, Noble LJ. Expression of nitric oxide synthase in the cerebral microvasculature after traumatic brain injury in the rat. Brain Res. 1997;751:336–338. doi: 10.1016/s0006-8993(96)01429-1. [DOI] [PubMed] [Google Scholar]
- 20.Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic injury to rat brain upregulates neuronal nitric oxide synthase expression and L-[3H]nitroarginine binding. J. Neurotrauma. 1999;16:865–877. doi: 10.1089/neu.1999.16.865. [DOI] [PubMed] [Google Scholar]
- 21.Gahm C, Holmin S, Mathiesen T. Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery. 2000;46:169–177. [PubMed] [Google Scholar]
- 22.Mesenge C, Charriaut-Marlangue C, Verrecchia C, Allix M, Boulu RR, Plotkine M. Reduction of tyrosine nitration afterN(omega)-nitro-L-arginine-methylester treatment of mice with traumatic brain injury. Eur. J. Pharmacol. 1998;353:53–57. doi: 10.1016/s0014-2999(98)00432-4. [DOI] [PubMed] [Google Scholar]
- 23.Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- 24.Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 2005;25:673–684. doi: 10.1038/sj.jcbfm.9600068. [DOI] [PubMed] [Google Scholar]
- 25.Hall ED, McCall JM, Means ED. Therapeutic potential of the lazaroids (21-aminosteroids) in acute central nervous system trauma, ischemia and subarachnoid hemorrhage. Adv. Pharmacol. 1994;28:221–268. doi: 10.1016/s1054-3589(08)60497-4. [DOI] [PubMed] [Google Scholar]
- 26.Awasthi D, Church DF, Torbati D, Carey ME, Pryor WA. Oxidative stress following traumatic brain injury in rats. Surg. Neurol. 1997;47:575–581. doi: 10.1016/s0090-3019(96)00461-2. discussion 581–572. [DOI] [PubMed] [Google Scholar]
- 27.Mori T, Kawamata T, Katayama Y, Maeda T, Aoyama N, Kikuchi T, Uwahodo Y. Antioxidant, OPC-14117, attenuates edema formation, and subsequent tissue damage following cortical contusion in rats. Acta Neurochir. 1998;(Suppl. 71):120–122. doi: 10.1007/978-3-7091-6475-4_36. [DOI] [PubMed] [Google Scholar]
- 28.Marklund N, Clausen F, Lewen A, Hovda DA, Olsson Y, Hillered L. alpha-Phenyl-tert-N-butyl nitrone (PBN) improves functional and morphological outcome after cortical contusion injury in the rat. Acta Neurochir. (Wien) 2001;143:73–81. doi: 10.1007/s007010170141. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Sybert TE, Qian H, Liu J. Superoxide production after spinal injury detected by microperfusion of cytochrome c. Free Radic. Biol. Med. 1998;25:298–304. doi: 10.1016/s0891-5849(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu JB, Tang TS, Xiao DS. Changes of free iron contents and its correlation with lipid peroxidation after experimental spinal cord injury. Chin. J. Traumatol. 2004;7:229–232. [PubMed] [Google Scholar]
- 31.Bao F, Liu D. Hydroxyl radicals generated in the rat spinal cord at the level produced by impact injury induce cell death by necrosis and apoptosis: protection by a metalloporphyrin. Neuroscience. 2004;126:285–295. doi: 10.1016/j.neuroscience.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 32.Seligman ML, Flamm ES, Goldstein BD, Poser RG, Demopoulos HB, Ransohoff J. Spectrofluorescent detection of malonaldehyde as a measure of lipid free radical damage in response to ethanol potentiation of spinal cord trauma. Lipids. 1977;12:945–950. doi: 10.1007/BF02533316. [DOI] [PubMed] [Google Scholar]
- 33.Hall ED, Braughler JM. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na+ + K+)-ATPase activity. Dose–response analysis during 1st hour after contusion injury in the cat. J. Neurosurg. 1982;57:247–253. doi: 10.3171/jns.1982.57.2.0247. [DOI] [PubMed] [Google Scholar]
- 34.Qian H, Liu D. The time course of malondialdehyde production following impact injury to rat spinal cord as measured by microdialysis and high pressure liquid chromatography. Neurochem. Res. 1997;22:1231–1236. doi: 10.1023/a:1021980929422. [DOI] [PubMed] [Google Scholar]
- 35.Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin SA, Broderick R, Osbourne D, Waeg G, Blades DA, Scheff SW. The presence of 4-hydroxynonenal/protein complex as an indicator of oxidative stress after experimental spinal cord contusion in a rat model. J. Neurosurg. 1998;88:874–883. doi: 10.3171/jns.1998.88.5.0874. [DOI] [PubMed] [Google Scholar]
- 37.Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Mattson MP. 4-hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J. Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- 38.Luo J, Uchida K, Shi R. Accumulation of acrolein–protein adducts after traumatic spinal cord injury. Neurochem. Res. 2005;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- 39.Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J. Neurochem. 2008;107:712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces apoptotic cell death and activates caspase-3. Neuroscience. 2003;116:59–70. doi: 10.1016/s0306-4522(02)00571-7. [DOI] [PubMed] [Google Scholar]
- 41.Bao F, DeWitt DS, Prough DS, Liu D. Peroxynitrite generated in the rat spinal cord induces oxidation and nitration of proteins: reduction by Mn (III) tetrakis (4-benzoic acid) porphyrin. J. Neurosci. Res. 2003;71:220–227. doi: 10.1002/jnr.10481. [DOI] [PubMed] [Google Scholar]
- 42.Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience. 2002;115:839–849. doi: 10.1016/s0306-4522(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Bao F, Prough DS, Dewitt DS. Peroxynitrite generated at the level produced by spinal cord injury induces peroxidation of membrane phospholipids in normal rat cord: reduction by a metalloporphyrin. J. Neurotrauma. 2005;22:1123–1133. doi: 10.1089/neu.2005.22.1123. [DOI] [PubMed] [Google Scholar]
- 44.Reulen HJ, Schurmann K, editors. Steroids and Brain Edema. Berlin: Springer-Verlag Inc.; 1972. [Google Scholar]
- 45.Hall ED, Baker T, Riker WF., Jr Glucocorticoid effects on spinal cord function. J. Pharmacol. Exp. Ther. 1978;206:361–370. [PubMed] [Google Scholar]
- 46.Hall ED, Baker T. Further studies of glucocorticoid effects on spinal cord function: single and repetitive monosynaptic transmission and apparent Ia afferent transmitter turnover. J. Pharmacol. Exp. Ther. 1979;210:112–115. [PubMed] [Google Scholar]
- 47.Hall ED, Braughler JM. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg. Neurol. 1982;18:320–327. doi: 10.1016/0090-3019(82)90140-9. [DOI] [PubMed] [Google Scholar]
- 48.Hall ED, Braughler JM. Acute effects of intravenous glucocorticoid pretreatment on the in vitro peroxidation of cat spinal cord tissue. Exp. Neurol. 1981;73:321–324. doi: 10.1016/0014-4886(81)90067-4. [DOI] [PubMed] [Google Scholar]
- 49.Hall ED, Wolf DL, Braughler JM. Effects of a single large dose of methylprednisolone sodium succinate on experimental posttraumatic spinal cord ischemia. Dose–response and time–action analysis. J. Neurosurg. 1984;61:124–130. doi: 10.3171/jns.1984.61.1.0124. [DOI] [PubMed] [Google Scholar]
- 50.Young W, Flamm ES. Effect of high-dose corticosteroid therapy on blood flow, evoked potentials, and extracellular calcium in experimental spinal injury. J. Neurosurg. 1982;57:667–673. doi: 10.3171/jns.1982.57.5.0667. [DOI] [PubMed] [Google Scholar]
- 51.Anderson DK, Means ED, Waters TR, Green ES. Microvascular perfusion and metabolism in injured spinal cord after methylprednisolone treatment. J. Neurosurg. 1982;56:106–113. doi: 10.3171/jns.1982.56.1.0106. [DOI] [PubMed] [Google Scholar]
- 52.Braughler JM, Hall ED. Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+ + K+)-ATPase, lipid peroxidation, and alpha motor neuron function. J. Neurosurg. 1982;56:838–844. doi: 10.3171/jns.1982.56.6.0838. [DOI] [PubMed] [Google Scholar]
- 53.Hall ED. The neuroprotective pharmacology of methylprednisolone. J. Neurosurg. 1992;76:13–22. doi: 10.3171/jns.1992.76.1.0013. [DOI] [PubMed] [Google Scholar]
- 54.Hall ED, McCall JM, Chase RL, Yonkers PA, Braughler JM. A nonglucocorticoid steroid analog of methylprednisolone duplicates its high-dose pharmacology in models of central nervous system trauma and neuronal membrane damage. J. Pharmacol. Exp. Ther. 1987;242:137–142. [PubMed] [Google Scholar]
- 55.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J, Hunt WE, Perot PL, Jr, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 57.Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, Wagner FC, Flamm ES, Eisenberg HM, Goodman JH, et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J. Neurosurg. 1985;63:704–713. doi: 10.3171/jns.1985.63.5.0704. [DOI] [PubMed] [Google Scholar]
- 58.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 59.Bracken MB, Shepard MJ, Collins WF, Jr, Holford TR, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon JC, Marshall LF, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 60.Bracken MB, Holford TR. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2. J. Neurosurg. 1993;79:500–507. doi: 10.3171/jns.1993.79.4.0500. [DOI] [PubMed] [Google Scholar]
- 61.Otani K. Beneficial effect of methylprednisolone sodium succinate in the treatment of acute spinal cord injury. Sekitsui Sekizui. 1994;7:633–647. [Google Scholar]
- 62.Petitjean ME, Pointillart V, Dixmerias F, Wiart L, Sztark F, Lassie P, Thicoipe M, Dabadie P. Medical treatment of spinal cord injury in the acute stage. Ann. Fr. Anesth. Reanim. 1998;17:114–122. doi: 10.1016/s0750-7658(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 63.Hall ED, Braughler JM, McCall JM. New pharmacological treatment of acute spinal cord trauma. J. Neurotrauma. 1988;5:81–89. doi: 10.1089/neu.1988.5.81. [DOI] [PubMed] [Google Scholar]
- 64.Anderson DK, Braughler JM, Hall ED, Waters TR, McCall JM, Means ED. Effects of treatment with U-74006F on neurological outcome following experimental spinal cord injury. J. Neurosurg. 1988;69:562–567. doi: 10.3171/jns.1988.69.4.0562. [DOI] [PubMed] [Google Scholar]
- 65.Anderson DK, Hall ED, Braughler JM, McCall JM, Means ED. Effect of delayed administration of U74006F (tirilazad mesylate) on recovery of locomotor function after experimental spinal cord injury. J. Neurotrauma. 1991;8:187–192. doi: 10.1089/neu.1991.8.187. [DOI] [PubMed] [Google Scholar]
- 66.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 67.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J. Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 68.Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J. Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 69.Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury — a systematic review from a clinical perspective. Spinal Cord. 2000;38:273–286. doi: 10.1038/sj.sc.3100986. [DOI] [PubMed] [Google Scholar]
- 70.Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, Gropper MR, Goffin J, Madsen PW, III, Maiman DJ, Ondra SL, Rosner M, Sasso RC, Trost GR, Zeidman S. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J. Spinal Disord. 2000;13:185–199. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Fehlings MG. Editorial: recommendations regarding the use of methylprednisolone in acute spinal cord injury: making sense out of the controversy. Spine (Phila Pa 1976) 2001;26:S56–S57. doi: 10.1097/00007632-200112151-00012. [DOI] [PubMed] [Google Scholar]
- 72.Fehlings MG. Summary statement: the use of methylprednisolone in acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S55. doi: 10.1097/00007632-200112151-00011. [DOI] [PubMed] [Google Scholar]
- 73.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 74.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 75.Tuszynski MH, Steeves JD, Fawcett JW, Lammertse D, Kalichman M, Rask C, Curt A, Ditunno JF, Fehlings MG, Guest JD, Ellaway PH, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Privat A. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord. 2007;45:222–231. doi: 10.1038/sj.sc.3102009. [DOI] [PubMed] [Google Scholar]
- 76.Lammertse D, Tuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C, Ditunno JF, Fehlings MG, Guest JD, Ellaway PH, Kleitman N, Blight AR, Dobkin BH, Grossman R, Katoh H, Privat A, Kalichman M. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord. 2007;45:232–242. doi: 10.1038/sj.sc.3102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muizelaar JP, Marmarou A, Young HF, Choi SC, Wolf A, Schneider RL, Kontos HA. Improving the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated superoxide dismutase: a phase II trial. J. Neurosurg. 1993;78:375–382. doi: 10.3171/jns.1993.78.3.0375. [DOI] [PubMed] [Google Scholar]
- 78.Muizelaar JP, Kupiec JW, Rapp LA. PEG-SOD after head injury. J. Neurosurg. 1995;83:942. doi: 10.3171/jns.1995.83.5.0942. [DOI] [PubMed] [Google Scholar]
- 79.Marshall LF, Maas AI, Marshall SB, Bricolo A, Fearnside M, Iannotti F, Klauber MR, Lagarrigue J, Lobato R, Persson L, Pickard JD, Piek J, Servadei F, Wellis GN, Morris GF, Means ED, Musch B. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J. Neurosurg. 1998;89:519–525. doi: 10.3171/jns.1998.89.4.0519. [DOI] [PubMed] [Google Scholar]
- 80.Farin A, Deutsch R, Biegon A, Marshall LF. Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J. Neurosurg. 2003;98:32–36. doi: 10.3171/jns.2003.98.1.0032. [DOI] [PubMed] [Google Scholar]
- 81.Farin A, Marshall LF. Lessons from epidemiologic studies in clinical trials of traumatic brain injury. Acta. Neurochir. 2004;(Suppl. 89):101–107. doi: 10.1007/978-3-7091-0603-7_14. [DOI] [PubMed] [Google Scholar]
- 82.Menon DK. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 2009;37:S129–S135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- 83.Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall ED. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8:152–167. doi: 10.1007/s13311-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mustafa AG, Singh IN, Wang J, Carrico KM, Hall ED. Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. J. Neurochem. 2010;114:271–280. doi: 10.1111/j.1471-4159.2010.06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mustafa AG, Wang JA, Carrico KM, Hall ED. Pharmacological inhibition of lipid peroxidation attenuates calpain-mediated cytoskeletal degradation after traumatic brain injury. J. Neurochem. 2011;117:579–588. doi: 10.1111/j.1471-4159.2011.07228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reiter RJ, Carneiro RC, Oh CS. Melatonin in relation to cellular antioxidative defense mechanisms. Horm. Metab. Res. 1997;29:363–372. doi: 10.1055/s-2007-979057. [DOI] [PubMed] [Google Scholar]
- 88.Longoni B, Salgo MG, Pryor WA, Marchiafava PL. Effects of melatonin on lipid peroxidation induced by oxygen radicals. Life Sci. 1998;62:853–859. doi: 10.1016/s0024-3205(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 89.Zhang H, Squadrito GL, Pryor WA. The reaction of melatonin with peroxynitrite: formation of melatonin radical cation and absence of stable nitrated products. Biochem. Biophys. Res. Commun. 1998;251:83–87. doi: 10.1006/bbrc.1998.9426. [DOI] [PubMed] [Google Scholar]
- 90.Zhang H, Squadrito GL, Uppu R, Pryor WA. Reaction of peroxynitrite with melatonin: a mechanistic study. Chem. Res. Toxicol. 1999;12:526–534. doi: 10.1021/tx980243t. [DOI] [PubMed] [Google Scholar]
- 91.Fujimoto T, Nakamura T, Ikeda T, Takagi K. Potent protective effects ofmelatonin on experimental spinal cord injury. Spine (Phila Pa 1976) 2000;25:769–775. doi: 10.1097/00007632-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 92.Kaptanoglu E, Tuncel M, Palaoglu S, Konan A, Demirpence E, Kilinc K. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J. Neurosurg. 2000;93:77–84. doi: 10.3171/spi.2000.93.1.0077. [DOI] [PubMed] [Google Scholar]
- 93.Gul S, Celik SE, Kalayci M, Tasyurekli M, Cokar N, Bilge T. Dose-dependent neuroprotective effects of melatonin on experimental spinal cord injury in rats. Surg. Neurol. 2005;64:355–361. doi: 10.1016/j.surneu.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 94.Samantaray S, Das A, Thakore NP, Matzelle DD, Reiter RJ, Ray SK, Banik NL. Therapeutic potential of melatonin in traumatic central nervous system injury. J. Pineal. Res. 2009;47:134–142. doi: 10.1111/j.1600-079X.2009.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Genovese T, Mazzon E, Crisafulli C, Esposito E, Di Paola R, Muia C, Di Bella P, Bramanti P, Cuzzocrea S. Effects of combination of melatonin and dexamethasone on secondary injury in an experimental mice model of spinal cord trauma. J. Pineal. Res. 2007;43:140–153. doi: 10.1111/j.1600-079X.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 96.Park K, Lee Y, Park S, Lee S, Hong Y, Kil Lee S. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J. Pineal. Res. 2010;48:270–281. doi: 10.1111/j.1600-079X.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 97.Ozdemir D, Tugyan K, Uysal N, Sonmez U, Sonmez A, Acikgoz O, Ozdemir N, Duman M, Ozkan H. Protective effect of melatonin against head trauma-induced hippocampal damage and spatial memory deficits in immature rats. Neurosci. Lett. 2005;385:234–239. doi: 10.1016/j.neulet.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 98.Ozdemir D, Uysal N, Gonenc S, Acikgoz O, Sonmez A, Topcu A, Ozdemir N, Duman M, Semin I, Ozkan H. Effect ofmelatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol. Res. 2005;54:631–637. [PubMed] [Google Scholar]
- 99.Beni SM, Kohen R, Reiter RJ, Tan DX, Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-kappaB and AP-1. FASEB J. 2004;18:149–151. doi: 10.1096/fj.03-0323fje. [DOI] [PubMed] [Google Scholar]
- 100.Kelso ML, Scheff NN, Scheff SW, Pauly JR. Melatonin and minocycline for combinatorial therapy to improve functional and histopathological deficits following traumatic brain injury. Neurosci. Lett. 2011;488:60–64. doi: 10.1016/j.neulet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3 rd ed. USA: Oxford University Press; 2008. [Google Scholar]
- 102.Vaishnav RA, Singh IN, Miller DM, Hall ED. Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. J. Neurotrauma. 2010;27:1311–1320. doi: 10.1089/neu.2009.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Althaus JS, Oien TT, Fici GJ, Scherch HM, Sethy VH, VonVoigtlander PF. Structure activity relationships of peroxynitrite scavengers an approach to nitric oxide neurotoxicity. Res. Commun. Chem. Pathol. Pharmacol. 1994;83:243–254. [PubMed] [Google Scholar]
- 104.Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- 105.Hall ED, Kupina NC, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann. N. Y. Acad. Sci. 1999;890:462–468. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- 106.Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J. Neurochem. 2008;104:708–718. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 107.Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J. Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- 108.Galvani S, Coatrieux C, Elbaz M, Grazide MH, Thiers JC, Parini A, Uchida K, Kamar N, Rostaing L, Baltas M, Salvayre R, Negre-Salvayre A. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic. Biol. Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 109.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carroll RT, Galatsis P, Borosky S, Kopec KK, Kumar V, Althaus JS, Hall ED. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol. 2000;13:294–300. doi: 10.1021/tx990159t. [DOI] [PubMed] [Google Scholar]
- 111.Krishna MC, DeGraff W, Hankovszky OH, Sar CP, Kalai T, Jeko J, Russo A, Mitchell JB, Hideg K. Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J. Med. Chem. 1998;41:3477–3492. doi: 10.1021/jm9802160. [DOI] [PubMed] [Google Scholar]
- 112.Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- 113.Zhang R, Shohami E, Beit-Yannai E, Bass R, Trembovler V, Samuni A. Mechanism of brain protection by nitroxide radicals in experimental model of closed-head injury. Free Radic. Biol. Med. 1998;24:332–340. doi: 10.1016/s0891-5849(97)00267-0. [DOI] [PubMed] [Google Scholar]
- 114.Xiong Y, Singh IN, Hall ED. Tempol protection of spinal cord mitochondria from peroxynitrite-induced oxidative damage. Free. Radic. Res. 2009;43:604–612. doi: 10.1080/10715760902977432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hillard VH, Peng H, Zhang Y, Das K, Murali R, Etlinger JD, Zeman RJ. Tempol, a nitroxide antioxidant, improves locomotor and histological outcomes after spinal cord contusion in rats. J. Neurotrauma. 2004;21:1405–1414. doi: 10.1089/neu.2004.21.1405. [DOI] [PubMed] [Google Scholar]
- 116.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid. Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 117.Kiziltepe U, Turan NN, Han U, Ulus AT, Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia–reperfusion injury. J. Vasc. Surg. 2004;40:138–145. doi: 10.1016/j.jvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 118.Yang YB, Piao YJ. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta. Pharmacol. Sin. 2003;24:703–710. [PubMed] [Google Scholar]
- 119.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Kocak A, Yologlu S, Turkoz Y. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta. Pharmacol. Sin. 2006;27:1317–1325. doi: 10.1111/j.1745-7254.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 120.Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 121.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell. Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 122.Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci. Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 123.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 124.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 125.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-alpha, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010;2010:238–321. doi: 10.1155/2010/238321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mao L, Wang H, Wang X, Liao H, Zhao X. Transcription factor nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J. Surg. Res. 2011;170:e105–e115. doi: 10.1016/j.jss.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 128.Wang X, De Rivero Vaccari JP, Wang H, Diaz P, German R, Marcillo A, Keane RW. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective following spinal cord injury. J. Neurotrauma. 2011 doi: 10.1089/neu.2011.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan W, Wang HD, Hu ZG, Wang QF, Yin HX. Activation of Nrf2-ARE pathway in brain after traumatic brain injury. Neurosci. Lett. 2008;431:150–154. doi: 10.1016/j.neulet.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 130.Hong Y, Yan W, Chen S, Sun CR, Zhang JM. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta. Pharmacol. Sin. 2010;31:1421–1430. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009;460:103–107. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen G, Fang Q, Zhang J, Zhou D, Wang Z. Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J. Neurosci. Res. 2011;89:515–523. doi: 10.1002/jnr.22577. [DOI] [PubMed] [Google Scholar]
- 133.Jin W, Kong J, Wang H, Wu J, Lu T, Jiang J, Ni H, Liang W. Protective effect of tert-butylhydroquinone on cerebral inflammatory response following traumatic brain injury in mice. Injury. 2011;42:714–718. doi: 10.1016/j.injury.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 134.Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 135.Thompson SN, Gibson TR, Thompson BM, Deng Y, Hall ED. Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury inmice. Exp. Neurol. 2006;201:253–265. doi: 10.1016/j.expneurol.2006.04.013. [DOI] [PubMed] [Google Scholar]