Abstract
Rationale
Glia, including astrocytes and microglia, can profoundly modulate neuronal function and behavior; however, very little is known about the signaling molecules that govern neuronal-glial communication and in turn affect behavior. Morphine treatment activates microglia and astrocytes in the nucleus accumbens (NAcc) to induce the synthesis of cytokines and chemokines and this has important implications for addictive behavior. Blocking morphine-induced glial activation using the non-specific glial inhibitor, ibudilast, has no effect on the initial rewarding properties of morphine, but completely prevents the relapse of drug-seeking behavior months later.
Objectives
We sought to determine the cellular source of these cytokines and chemokines in the NAcc in response to morphine, and the cell type specific expression pattern of their receptors to determine whether neurons have the capacity to respond to these immune signals directly.
Methods
We used fluorescence-activated cell sorting (FACS) of neurons (Thy1+), astrocytes (GLT1+), and microglia (CD11b+) from the NAcc for the analysis of cell type specific gene expression following morphine or saline treatment.
Results
The results indicate that microglia and neurons each produce a subset of chemokines in response to morphine, and that neurons have the capacity to respond directly to a select group of these chemokines via their receptors. In addition, we provide evidence that microglia are capable of responding directly to dopamine release in the NAcc.
Conclusions
Future studies will examine the mechanism(s) by which neurons respond to these immune signals produced by microglia in an effort to understand their effect on addictive behaviors.
Keywords: microglia, astrocytes, chemokines, opioid, dopamine
INTRODUCTION
Drug addiction is caused by the dysregulation of neural circuits underlying reward that eventually leads to overwhelming compulsive drug-seeking behavior. Thus one of the primary goals of addiction research is to understand the factors which initiate or maintain the dysregulation of neural circuits in the presence of drugs of abuse. We have recently determined that morphine induces the robust expression of select cytokines and chemokines in the Nucleus Accumbens (NAcc) of rats, and this neuroimmune response that occurs at the time of morphine treatment results in an increased risk of relapse to drug-seeking behavior even after months of abstinence from the drug (Schwarz et al. 2011). The cytokines and chemokines that are up-regulated following morphine administration include CCL4, CCL17, their receptor CCR4, CCL25, IL-10, and several others (Schwarz et al. 2011). Microglia and astrocytes are the powerful immune competent and neuromodulatory cells in the brain, and blocking their activation at the time of morphine treatment completely prevents the production of these cytokines and chemokines in the brain, and thereby prevents the increased risk of drug-seeking behavior in a model of conditioned place preference (CPP) (Schwarz et al. 2011). Given the compelling evidence suggesting these molecules may have important neuromodulatory actions particularly within the circuits underlying reward and addiction, we hypothesized that neurons are capable of responding to these classic immune molecules. However, many of these particular chemokines have not been studied within the brain, and it is unclear whether neurons do in fact have the capacity to respond to any or all of these immune molecules.
We utilized Fluorescence Activated Cell Sorting (FACS) to examine the cell-type specific gene expression of the cytokines, chemokines and receptors previously identified as necessary for increasing the risk of relapse to drug-seeking behavior. The goal of these experiments was to determine the source of these cytokines and chemokines in the brain and the expression of their cognate receptor. These results lend novel insight into the role of cell-to-cell communication within the brain and the mechanisms by which neurons, microglia, and astrocytes can communicate to produce long-term changes in the neural circuitry underlying reward and addiction.
MATERIALS AND METHODS
Subjects
Sprague–Dawley rats (49–52 days) were obtained from Harlan (Indianapolis, IN) and housed in polypropylene cages with ad libitum access to food and water. The colony was maintained at 22°C on a 12:12-hour light-dark cycle (lights on at 0700 EST). All experiments were performed in males.
Drugs and Treatment Paradigm
Morphine sulfate was obtained from the National Institute on Drug Abuse Drug Inventory Supply (Rockville, MD). Morphine sulfate was prepared and is reported as free base concentrations. Approximately one week after the male rats arrived in our facility they were treated with either morphine (4 mg/kg) or its vehicle, saline (1 ml/kg), subcutaneously and sacrificed 20 minutes later. This time point was selected because we previously determined that morphine elicits the robust expression of chemokines and cytokines within the NAcc that peaks at 15–20 minutes and decreases within 60 minutes after morphine administration (Schwarz et al., 2011). Each rat was briefly perfused with approximately 50 ml of cold saline prior to decapitation and brain collection in order to clear blood and peripheral leukocytes (e.g., perivascular macrophages). The NAcc and/or the hippocampus (HP) from each rat were dissected on ice. We knew from previous experiments that the HP would yield a sufficient number of cells for flow cytometry and cell-type specific gene expression (Williamson et al., 2011), and thus it was used as an important staining control for the cells of the NAcc, which we feared would yield only a small number of cells. Ultimately, both brain regions produced a sufficient number of all cell types for the flow cytometry analysis and subsequent cell-type specific gene expression; however, these data remain in the Results for comparison.
Microglial Isolation
Isolated NAcc and/or HP samples were diced into small pieces using a sterile razor and transferred into a 2 ml sterile tube with 1 ml of HBSS (Hank’s Buffered Saline Solution, without CaCl2 and MgCL2) (Invitrogen, Carlsbad, CA), and spun at 300×g at room temperature (RT) for 2 min. Tissue was prepared to a single cell suspension using Miltenyi’s Neural Tissue Dissociation Kit (P) (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. After a final wash in HBSS containing CaCl2 and MgCl2, tissue was incubated with anti-Myelin microbeads (Miltenyi Biotec) in MACS® buffer (PBS containing 0.5% BSA and 2 mM EDTA, pH 7.2) for 15 minutes at 4°C. Tissue was then washed with 5 ml of MACS buffer and centrifuged at 300×g at 4°C for 10 min. The tissue was resuspended in 500 µl MACS buffer and passed through a 70 µm nylon filter onto LD columns (Miltenyi Biotec) exposed to a strong magnetic field. The flow through (de-myelinated cells) and subsequent washes were collected into a 5 ml polypropylene tube and centrifuged at 300×g at 4°C for 10 min. The de-myelinated cells were immediately stained for flow cytometric analysis and sorting as follows.
Fluorescence Staining and Cell Sorting
De-myelinated NAcc and HP cells were washed once in 2 ml PBS supplemented with 0.5% BSA and 2 mM EDTA (wash buffer), followed by centrifugation at 350×g for 5 minutes. The wash buffer was removed by aspiration and the pellet dispersed by vortex. Cells were incubated with 5 µl Rat Fc receptor block (CD32; BD Pharmingen) for 5 minutes at 4°C. Next, cells were incubated for 20 minutes at 4°C in the dark with 100 µl of antibody mixture, including APC-conjugated mouse anti-rat CD11b/c (BD Pharmingen), diluted 1:1000; FITC-conjugated mouse anti-rat Thy-1 (CD90; Biolegend); diluted 1:250; anti-rabbit GLT-1 (Novus Biologicals) diluted 1:100. Cells were washed in buffer before being incubated in 100 µl secondary antibody, PE-conjugated donkey anti-rabbit IgG (eBioscience), diluted 1:500, for 15 minutes at 4°C in the dark. The cells were washed and spun down at 350×g for 5 minutes prior to being sorted using a MoFlo High-speed Sorter (Beckman Coulter).
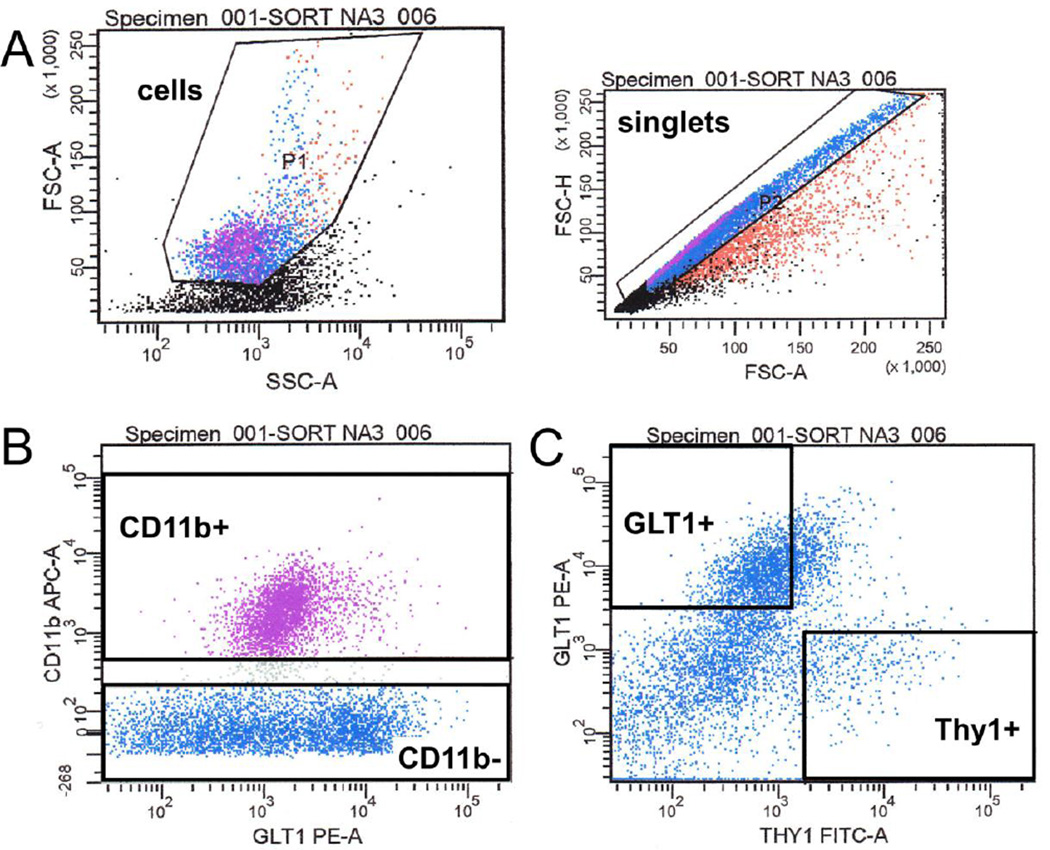
For each NAcc and HP sample, cellular debris was excluded based on the forward and side scatter of light passing by the particle, while any doublets were excluded from the analysis based on the area of the forward scatter height and area (Figure 1A). From there, cells were gated as CD11b+ (CD11b+ microglia) or CD11b− (Figure 1B), and the CD11b− cells were further sub-divided as either Thy1+/GLT− (Thy1+ neurons) or GLT1+/Thy1− (GLT1+ astrocytes) (Figure 1C). These three populations (CD11b+ microglia, Thy1+ neurons, and GLT1+ astrocytes) were collected by FACS sorting for analysis of transcript expression in purified populations. It should be noted that Thy1 was effective at isolating neurons as opposed to astrocytes or microglia, but that Thy1 may only be expressed on a certain subset of neurons (Feng et al., 2000) and thus our analysis may not include all neuronal populations from a given brain region. Similarly, GLT1 may not be expressed on all astrocyte populations within a given brain region.
Figure 1. Representative plots of Fluorescence Activated Cell Sorting of cells from the NAcc.
A) Population (P) 1 represents cells and P2 represents singlets that were sorted based on cell size, as determined by the area (A) and the height (H) of Light Forward Scatter (FSC), and cellular complexity/granularity, based on Light Side Scatter (SSC). Each dot represents one event (either a cell or debris) that has passed through the cytometer’s detector. B) Cells were then sorted based on CD11b fluorescent intensity (CD11b APC-A) as either CD11b+ or CD11b−. Positive and negative gates were drawn based on the intensity of an unstained control sample. Next, CD11b− cells were further resolved according to GLT1 or Thy1 expression. Again, gates were drawn based on the fluorescent intensity of an unstained control sample for GLT1 PE-A and Thy1 FITC-A, respectively, and identified either GLT1+ (and Thy1−) or Thy1+ (and GLT1−) cell populations. Red events = P1= cells, purple events = Cd11b+ cells, blue events = CD11b− cells.
One set of cells isolated from the NAcc were stained with an antibody for the D1 receptor (Novus Biologicals) diluted 1:100 and the APC-conjugated mouse anti-rat CD11b/c diluted 1:1000, followed by secondary antibody, FITC-conjugated donkey anti-goat (abcam). Cells were washed in MACS buffer, spun down at 350×g for 5 minutes, then fixed in 200 µl 1.5% paraformaldehyde prior to analysis using a FACSCanto II flow cytometer (BD Biosciences). The mean fluorescence intensity (MFI) of D1r expression on gated D1r+ microglia (D1r+ and CD11b+) was analyzed using FlowJo software (Treestar, Inc.).
Quantitative Real-time PCR of Sorted Cells
RNA was isolated from Thy-1+ cells, GLT1+ cells, and CD11b+ cells using the TRIzol method and DNASE-treated. Complimentary DNA (cDNA) was synthesized from 150 ng of isolated RNA using the Quantitect Reverse Transcription Kit (Qiagen, Valencia, CA). Gene expression was measured using quantitative real-time PCR using 1 µl each of the forward and reverse primers (Table 1), 6.5 µl of the QuantiFast SYBR Green qPCR Master Mix from Qiagen (Valencia, CA), 3.5 µl of nuclease free water, and 1 µl of cDNA from each sample. Each sample was run in duplicate.
Table 1.
| Gene | NCBI Reference Sequence |
Forward Primer 5’ → 3’ |
Reverse Primer 5’ → 3’ |
|---|---|---|---|
| GAPDH | NM_017008.4 | GTTTGTGATGGGTGTGAACC | TCTTCTGAGTGGCAGTGATG |
| NR1 | NM_001270610.1 | GGAGTTCACAGTCAATGGTG | CTTGATGAGCAGGTCTATGC |
| GFAP | NM_017009.2 | AGGGACAATCTCACACAGG | GACTCAACCTTCCTCTCCA |
| Iba1 | D82069.1 | GAATGATGCTGGGCAAGAGA | CAGTTGGCTTCTGGTGTTC |
| D1r | NM_012546.2 | ACAGATGCATTGTTGATGAC | TGCTAGTACAAATGGAGAGG |
| D2r | M36831.1 | TTAACATCGTCTCTCTTCCA | GCAGGTATAGTGATGTTACA |
| D3r | M69194.1 | CATTGTGCTTGGAGCCTTCA | AAGGCTTTGCGGAACTCCAC |
| D4r | NM_012944.1 | GTCACCTGGCTGGGCTATGT | CACTCTGCACACAGGCTTGG |
| MOR | NM_013071 | AGGGGCACCTCCGTTTCTT | TGCAGTTGACACTGTGCTTTC |
| DOR | NM_012617.1 | ACGCTGGTGGACATCAATCG | CCAGGAAGGCGTAGAGAACC |
| KOR | NM_017167.2 | CTCCCAGTGCTTGCCTACTC | AGGGATGGCTGGAGAGATGT |
| TLR4 | NM_019178.1 | CAGAGGAAGAACAAGAAGC | CCAGATGAACTGTAGCATTC |
| CCL4 | NM_053858.1 | CGGAAGATTCATCGGAACTT | TCAACTCCAAGTCATTCAC |
| CCL17 | NM_057151.1 | CGTGGTTCAGGACCTCAGTG | TCACAGCCTTTGGTTT |
| CCR4 | NM_133532.2 | ACATCGCCCAACTCTTCAGA | CAAAGCGTCACGGAAGTCAT |
| CCL25 | NM_001037203.1 | TTGACTGTTGATGGTTACT | ATGTCTTCTTCCTAACAAGC |
| CCR9 | NM_172329.1 | AGGGTTGAAAAGATGGCTCA | GTGTCTGTGTGAGAGTATCA |
| Arc | NM_019361.1 | ACAGAGGATGAGACTGAGGCAC | TATTCAGGCTGGGTCCTGTCAC |
| CX3CL1 | NM_134455.1 | TCCAGGGCTGTCCCCGCAAA | ACAGGCAGGCAAGCAGGCAG |
| CX3CR1 | NM_133534.1 | TTCCTCTTCTGGACGCCTTA | TAAACGCCACTGTCTCCG |
| IL-10 | NM_012854.2 | TAAGGGTTACTTGGGTTGCC | TATCCAGAGGGTCTTCAGC |
| IL10ra | NM_057193.2 | TGAGAGCACCTACTACGAA | GGAATACTGACTGTTGTCCA |
qRT-PCR Analysis
Threshold amplification cycle number (Ct) was determined for each reaction within the linear phase of the amplification plot. The mean Ct value was calculated for each sample and the relative gene expression of each sample was determined using the 2−ΔΔCT method.
Statistical Analysis
Relative gene expression across groups was compared across treatment groups (morphine v. saline) using a Student’s t-test to examine the effect of morphine treatment on the expression of chemokines and cytokines within each cell type (Thy1+ neurons, GLT1+ astrocytes, and CD11b+ microglia) (α-level < 0.05). Data in graphical form represent the mean ± SEM.
RESULTS
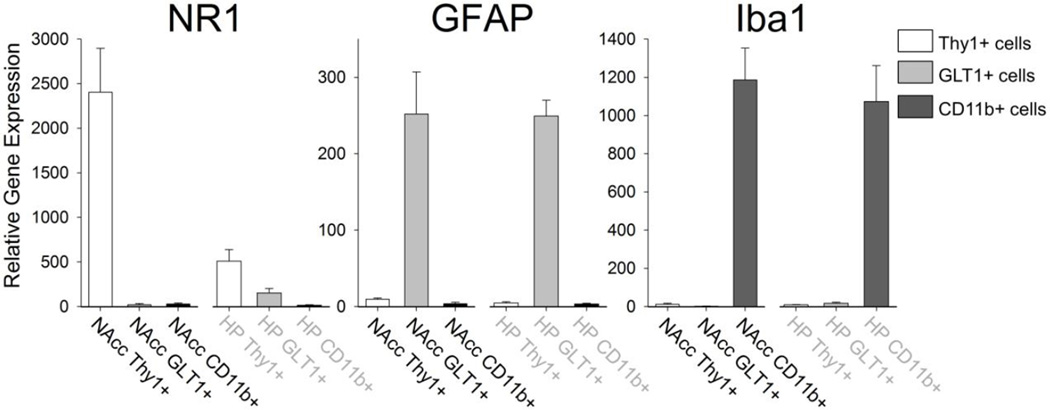
Sorted cell populations were consistently >98% pure based on surface expression of CD11b, Thy1 and GLT1. However, we also confirmed the purity of each population based on gene expression of known cell type specific genes: the glutamatergic NMDA receptor subunit 1 (NR1) found predominantly on neurons, the glial fibrillary acidic protein (GFAP) found predominantly on astrocytes, and the ionized calcium binding adaptor molecule 1 (Iba1) found predominantly on microglia (Figure 2). These data confirm that the sorted Thy1+ cells were neurons and expressed the highest levels of NR1, the sorted GLT1+ cells were astrocytes and expressed the highest levels of GFAP, and the sorted CD11b+ cells were microglia and expressed the highest levels of Iba1.
Figure 2. Confirmation of sorted cells using cell-type specific gene expression.
Cells sorted from either the nucleus accumbens (NAcc) or the hippocampus (HP) were analyzed for the expression of either the NMDA receptor subtype (NR1), glial fibrilary acidic protein (GFAP), or the ionized calcium binding adaptor molecule 1 (Iba1). Thy1+ cells expressed the highest levels of the NMDA receptor, confirming they were neurons. GLT1+ cells expressed the highest levels of GFAP, confirming they were astrocytes. CD11b+ cells expressed the highest levels of Iba1, confirming they were microglia.
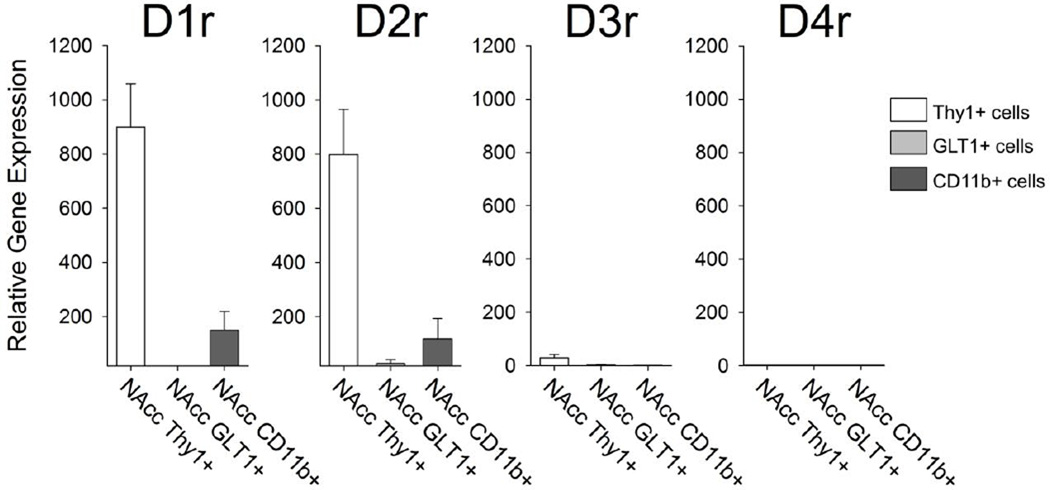
Next, we analyzed the expression profile of the dopamine receptors including D1, D2, D3, and D4 in the cells sorted from the NAcc. We found that D1 and D2 were highly expressed on the Thy1+ neurons sorted from the NAcc (Figure 3). In contrast, the D1 and D2 receptors were expressed at relatively low levels in Thy1+ cells collected from the hippocampus (data not shown). We were initially surprised to find a small amount of both D1 and D2 receptor expression in CD11b+ microglia isolated from the NAcc (Figure 3), and no expression of either D1 or D2 receptor on CD11b+ microglia isolated from the hippocampus (data not shown). We found extremely low levels of the D3 receptor only on the Thy1+ neurons collected from the NAcc, and no expression of D4r on any cell type collected from the NAcc (Figure 3). These data are consistent with other reports in the literature, indicating low or undetectable levels of D3 and D4 respectively within the rodent NAcc (Centonze et al. 2003, Noain et al. 2006). In a separate experiment, we performed flow cytometric analysis of D1 receptor staining on cells isolated from the NAcc, to confirm the PCR data indicating that CD11b+ microglia express the D1 receptor. On average 7.8% ± 3.2 of CD11b+ microglia were gated as positively expressing the D1 receptor. The median fluorescence intensity of D1r antigen staining on these cells was about 488 ± 63 (MFI CD11b+/D1r− = 185 ± 6; Figure 4). These data indicate that a notable percentage of microglia express the D1 receptor in the NAcc, and this is presumably the case for the D2 receptor as well, although we did not assess this directly.
Figure 3. Cell-type specific expression of dopamine receptors in cells sorted from the Nucleus Accumbens.
mRNA expression of the dopamine receptor subtypes D1r, D2r, D3r, and D4r was measured in Thy1+ neurons, GLT1+ astrocytes and CD11b+ microglia isolated from the NAcc. Thy1+ neurons exhibited the highest levels of the D1 and the D2 receptor, and low but detectable levels of the D3 receptor. CD11b+ microglia also exhibited low levels of D1 and D2 receptor mRNA.
Figure 4. A subset of the CD11b+ microglia from the NAcc expressed D1 receptors.
A) Representative plots of cells isolated from the NAcc and sorted based on their size as determined by the area (A) and the height (H) of Light Forward Scatter (FSC), and cellular complexity/granularity based on Light Side Scatter (SSC). Each dot represents one event (either a cell or debris). B) Microglia were sorted based on fluorescent CD11b staining (y-axis). C) CD11b+ cells were further sorted based on D1r staining (y-axis). In this sample, approximately 7.66% of CD11b+ cells (x-axis) were also positive for D1r staining. D) Staining controls for the D1r antibody, including the primary antibody alone (no secondary) and the secondary antibody alone (no primary), indicate no non-specific D1 receptor staining on CD11b+ cells.
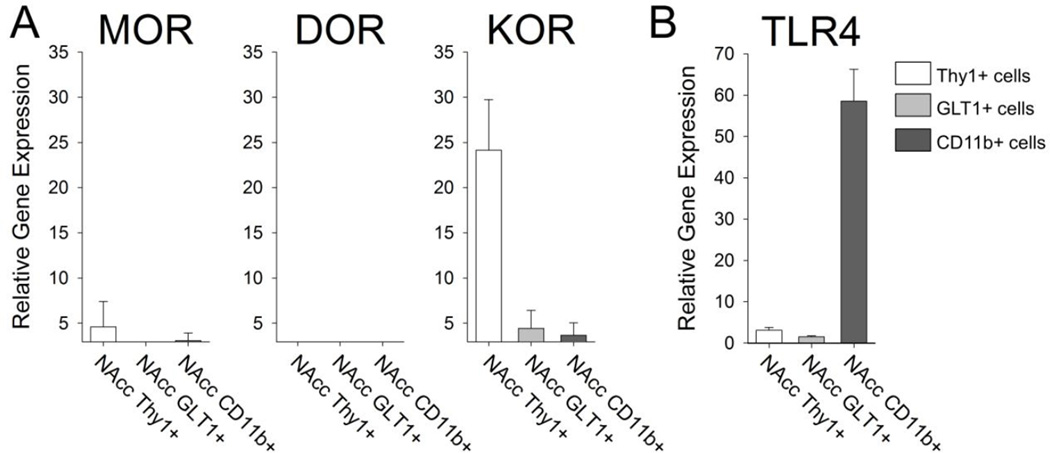
Next, we examined the expression pattern of the opioid receptors to which morphine can bind including the μ opioid receptor (MOR), the κ opioid receptor (KOR), and the δ opioid receptor (DOR). We found that the MOR was expressed at relatively low, almost undetectable levels in all three cell types sorted from the NAcc (Figure 5A). The DOR was undetectable in all cell types sorted from the NAcc, and the KOR was expressed at significant levels in the Thy1+ neurons isolated from the NAcc (Figure 5A).
Figure 5. Cell-type specific expression of opioid receptors and the Toll-like receptor 4 in cells sorted from the Nucleus Accumbens.
A) mRNA expression of the opioid receptor subtypes MOR (μ opioid receptor), KOR (κ opioid receptor), and DOR (δ opioid receptor) was measured in Thy1+ neurons, GLT1+ astrocytes and CD11b+ microglia isolated from the NAcc. Thy1+ neurons expressed low levels of the MOR and high levels of the KOR. B) mRNA expression of TLR4 revealed strong expression of this innate immune receptor on CD11b+ microglia.
We also examined the expression of Toll-like receptor 4 (TLR4), the innate immune receptor to which morphine can bind via its adaptor protein, MD2 (Hutchinson et al. 2012). We found that TLR4 is expressed almost exclusively on CD11b+ microglia isolated from the NAcc (Figure 5B). These data confirm previous data from our lab, which detected TLR4 protein exclusively on microglia (Schwarz and Bilbo, 2013).
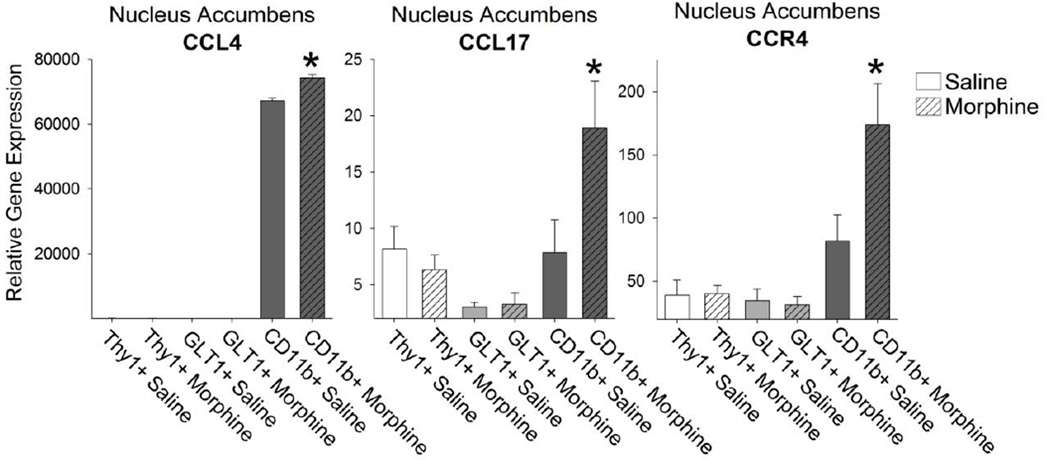
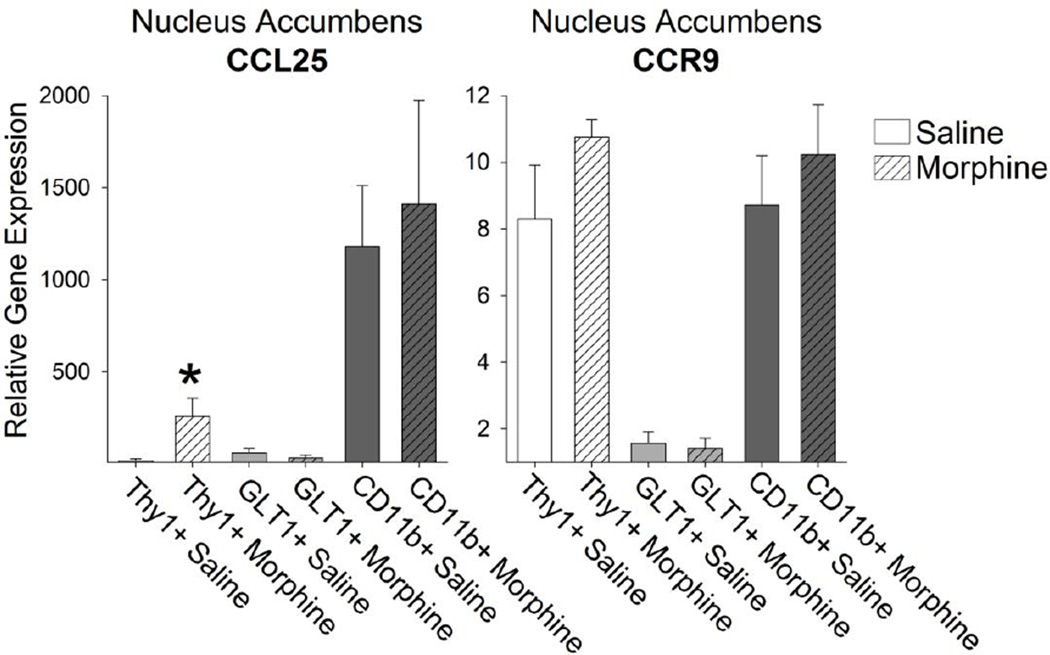
Next, we isolated the Thy1+ neurons, GLT1+ astrocytes, and CD11b+ microglia from the NAcc of rats treated in vivo with either saline or morphine and collected 20 minutes later. We found no significant difference in the number of Thy1+ neurons (saline = 3675 ± 823, morphine = 3738 ± 1029, p = 0.96), GLT1+ astrocytes (saline = 5175 ± 704, morphine = 5066 ± 710, p = 0.92), or CD11b+ microglia (saline 6630 ± 760, morphine = 7733 ± 747, p = 0.32) collected from saline or morphine treated rats. We then examined the cell-type specific expression pattern of immune molecules known to be rapidly up-regulated by morphine treatment (Schwarz et al. 2011). Of these, chemokine ligands (CCL) 4 and 17 are significantly up-regulated following morphine treatment in CD11b+ microglia (Figure 6; t14= −.24; p = 0.04). The receptor for these two ligands, CCR4, is also found predominantly on microglia (Figure 6).Another important chemokine, CCL25, is also up-regulated within the NAcc following morphine administration. We found that CCL25 is expressed constitutively in CD11b+ microglia isolated from the NAcc; however, CCL25 expression was only increased in response to morphine in Thy1+ neurons (Figure 7; t12= −2.54; p = 0.02). Interestingly, the CCL25 receptor, CCR9, was expressed on Thy1+ neurons and CD11b+ microglia (Figure 7), which makes the interpretation of CCL25 mechanism of action complicated but interesting.
Figure 6. The expression of CCL4 and CCL17 was elevated exclusively in CD11b+ microglia of the NAcc following morphine treatment.
mRNA expression of CCL4 and CCL17 was significantly elevated following morphine administration in CD11b+ microglia isolated from the NAcc (*p < 0.05 compared to CD11b+ Saline). The receptor for these chemokines, CCR4, was expressed predominantly on CD11b+ microglia and this expression was increased following morphine administration (*p < 0.05 compared to CD11b+ Saline).
Figure 7. The chemokine ligand CCL25 was expressed constitutively in CD11b+ microglia, whereas expression of the same chemokine is elevated significantly in Thy1+ neurons following morphine administration.
CCL25 was expressed constitutively in CD11b+ microglia and this expression was not changed following morphine administration. CCL25 expression was, however, increased significantly in Thy1+ neurons following morphine administration (*p < 0.05 compared to Thy1+ Saline). Analysis of the CCL25 receptor, CCR9, revealed expression of this receptor in both Thy1+ neurons and CD11b+ microglia.
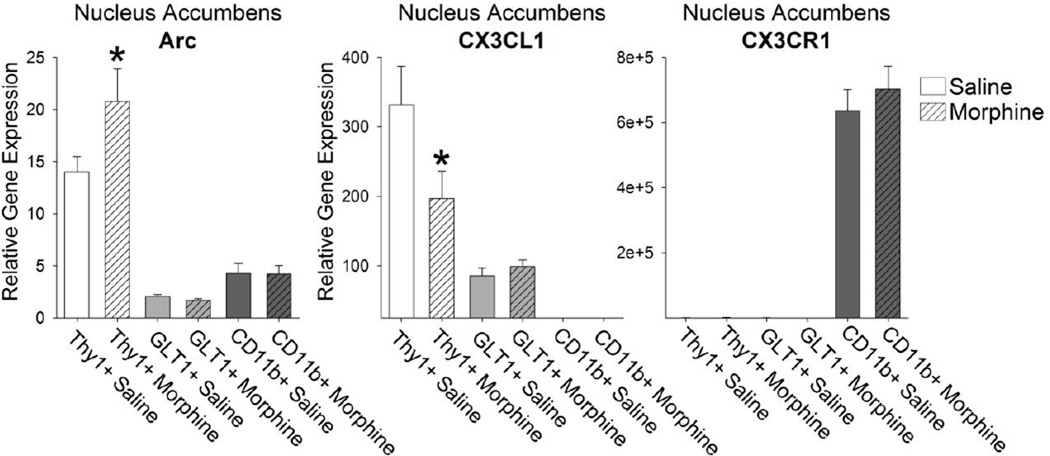
Next, we examined the activation of neurons sorted by FACS following morphine administration. We determined that activity-regulated cytoskeletal protein (Arc/Arg3.1), an immediate early gene expressed in neurons, was up-regulated in Thy1+ neurons following morphine administration (Figure 8; t14= −2.73; p = 0.01). We also examined the expression of the chemokine CX3CL1, also known as fractalkine, a chemokine that is expressed primarily in neurons. We found no expression in CD11b+ microglia, and limited expression in GLT1+ astrocytes, but significant expression in Thy1+ neurons that went down following morphine administration (Figure 8; t14= 2.21; p = 0.04). These data confirm previous reports from our lab indicating that morphine decreases the expression of CX3CL1 in the NAcc (Schwarz et al. 2011). The receptor for CX3CL1, CX3CR1, is expressed exclusively on microglia (Figure 8), as has been previously reported (Nishiyori et al., 1998; Wolf et al., 2013).
Figure 8. Thy1+ neurons in the NAcc were significantly activated and exhibit a significant decrease in the expression of fractalkine, CX3CL1, following morphine administration.
Thy1+ neurons expressed the highest levels of the immediate early gene, Arc. Arc expression in Thy1+ neurons was significantly increased following morphine administration (*p < 0.05 compared to Thy1+ Saline). Fractalkine, CX3CL1, was expressed predominantly and constitutively in Thy1+ neurons. Treatment of rats with morphine significantly decreased the expression of CX3CL1 in neurons (*p < 0.05 compared to Thy1+ Saline). The fractalkine receptor, CX3CR1, was found exclusively on CD11b+ microglia.
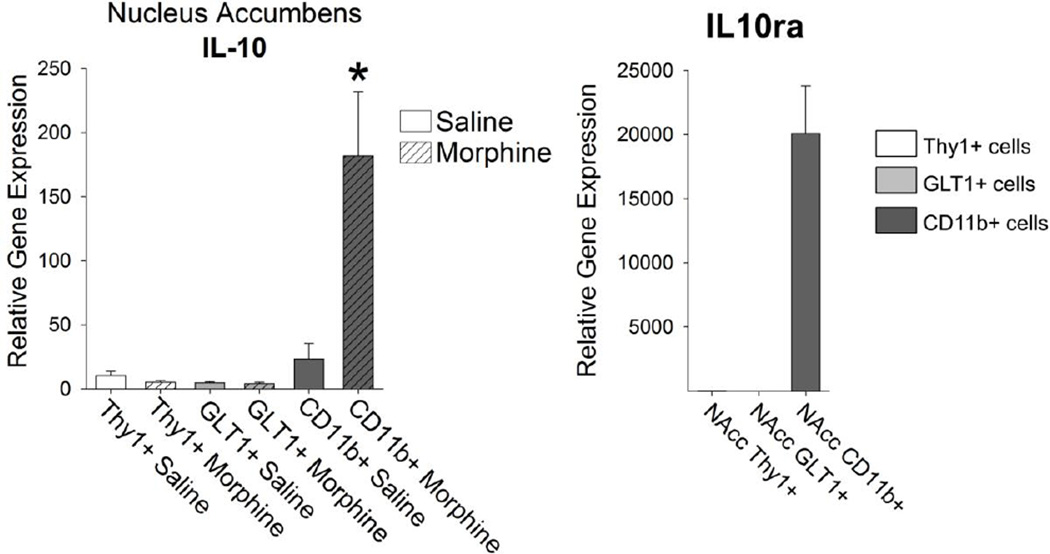
Finally, we examined the expression of the anti-inflammatory cytokine IL-10. We have previously reported that IL-10 is robustly up-regulated within the NAcc following morphine administration, and that its expression is negatively correlated to morphine-seeking behavior relapse liability (Schwarz et al. 2011). As expected, IL-10 was produced exclusively by microglia, and this expression was increased approximately 8-fold within 20 minutes after morphine administration (Figure 9; t14= −3.07; p = 0.008). Moreover, the IL-10 receptor α (IL10ra) is localized exclusively on microglia (Figure 9), suggesting an autocrine role for this cytokine in microglia.
Figure 9. Anti-inflammatory IL-10 was expressed exclusively in CD11b+ microglia of the NAcc, and was increased significantly following morphine treatment.
The anti-inflammatory cytokine IL-10 is expressed robustly in CD11b+ microglia following morphine administration (*p < 0.05 compared to CD11b+ Saline). The IL-10 receptor α (IL10ra) is similarly expressed on CD11b+ microglia isolated from the NAcc.
DISCUSSION
This study is the first to examine and characterize cell-type specific responses to morphine within the NAcc. To date, the primary mechanism for examining gene expression in the brain has relied on examining gene expression in whole tissue, which includes a variety of cell types. While this technique is useful for understanding broad changes in gene expression, it provides little information regarding mechanism of action and little understanding of cell-to-cell communication. In addition, many reports often assume that the molecules examined and their receptors are expressed by neurons exclusively. By selectively isolating individual cell types from whole tissue, in this case Thy1+ neurons, GLT1+ astrocytes, and CD11b+ microglia, we were able to examine the cell-type expression profile of cytokines and chemokines and their receptors, all molecules that impact the relapse liability of drug-seeking behavior following repeated morphine exposure (Schwarz et al., 2011).
First, we determined that microglia within the NAcc, and not the HP, express the D1 and D2 dopamine receptors. Dopamine is a neurotransmitter implicated in various neural functions ranging from motor control, motivation, reward, and attention. Distinct populations of dopamine neurons predominantly located in the midbrain, project to different brain regions that express the dopamine receptor, such as the dorsal striatum, the nucleus accumbens (NAc), and the medial Prefrontal Cortex (mPFC). Drugs of abuse, including morphine, induce the release of dopamine from the ventral tegmental area (VTA) located in the midbrain into the NAcc, exposing postsynaptic cells (neurons and glia) in the NAcc to this neurotransmitter. Using FACS, we were able to confirm other reports indicating that microglia express the D1 and D2 receptors (Färber et al. 2005). Following our initial analysis of microglial-specific mRNA expression, we confirmed the protein expression of the D1 receptor on microglia using flow cytometry. Based on the mRNA data we obtained from isolated cell types, one might assume that microglia within the NAcc homogenously exhibit low levels of the D1 receptor; however, using flow cytometric analysis which allows us to assess protein expression levels on a per cell basis, we found instead that a small percentage of microglia in the NAcc express the D1 receptor. These data reveal that microglia exhibit regional specificity in the expression of certain receptors, similar to neurons, which may be the result of their location in the brain (NAcc as opposed to the HP) and the inputs these cells receive. Secondly, these data indicate that microglia are heterogeneous even within a given brain region; thus, only a percentage of microglia within the NAcc may be capable of responding to the release of DA into the NAcc following exposure to drugs of abuse. Fäber et al proposed that microglia that express the DA receptor may be attracted to areas of dopaminergic transmission during early brain development (Färber et al. 2005) where they are poised to respond to and modulate dopaminergic transmission. Future studies will investigate how these microglia are localized within the NAcc, and their relation to DA terminals.
Importantly, these data indicate that DA receptor expression on microglia may be one mechanism by which microglia can respond to the activity of surrounding neurons in response to drugs of abuse. Dopamine is a neurotransmitter that is located in both the central and peripheral nervous system and it can enhance the function of immune cells, inducing migration of T cells and macrophages; or DA can suppress immune cell function (see Basu and Dasgupta 2000; Sarkar et al., 2010). These data add to a novel facet of research focusing on the effects of dopamine on microglia and perivascular macrophages, and the role this interaction may play in the etiology of certain disorders including Alzheimer’s disease, Parkinson’s disease, schizophrenia, and drug abuse (Gaskill et al., 2012; Sarkar et al., 2010).
We were surprised to find that two of the three opioid receptors examined, the MOR and the DOR, were not strongly expressed on the cells isolated via FACS from the NAcc. The KOR was, however, significantly expressed on Thy1+ neurons. We have determined that all of these receptors are expressed in whole tissue samples extracted from the NAcc, which indicates that these receptors may be found on other cell types not sorted from the NAcc using the antibodies presented here. Specifically, there may be other neuronal cell types that were not collected using the Thy1 antibody, and it may be that these cell types express the MOR or the DOR. Thy1 is an immunoglobulin super-family member that is expressed by neurons in many parts of the nervous system, in addition to its well-characterized expression by T lymphocytes (Feng et al. 2000). Our data indicate that the Thy1+ neurons express dopamine receptors and the KOR, and this is certainly important in the interpretation of the chemokine expression obtained here. Similarly, we detected relatively low expression of the NR1 subunit, the D1 receptor, and the D2 receptor on Thy1+ neurons from the HP. While we might expect lower levels of dopamine receptor expression in the HP, particularly when compared to the NAcc, we were surprised to find such low levels of the NMDA receptor subunit given the importance of glutamatergic transmission in the HP. Again, we anticipate that there may be other neuronal cell types that were not collected from the HP using the Thy1 antibody that might express these receptors. However, future experiments will further examine more distinct neural cell types using a variety of specific neuronal antibodies.
Despite this, our results reveal novel information regarding the potential cell-to-cell communication within the NAcc at the time of morphine administration. We and others have examined the neuroimmune response to drugs of abuse, including morphine, and found that microglia and astrocytes within the NAcc are activated following morphine administration, as determined by up-regulation of CD11b and GFAP respectively (Schwarz et al., 2011; Schwarz and Bilbo, 2013). This activation of glia by morphine is critical for predicting the reinstatement of drug-seeking behavior in a model of conditioned place preference (CPP) (Schwarz et al., 2011; Schwarz and Bilbo, 2013). Blocking the activation of microglia and astrocytes specifically in the NAcc prevents the drug-induced reinstatement of drug-seeking behavior in a model of CPP (Schwarz et al., 2011). Many of the molecules produced by this glial activation include cytokines and chemokines that have rarely been explored in the brain, let alone in the context of addiction. Thus the goal of this paper was to further examine these molecules, their source of production, and their site of action (their receptor localization).
We found that chemokine ligands CCL4 and CCL17 were up-regulated following morphine treatment, specifically by microglia. Interestingly, the receptor for these chemokines, CCR4, was also expressed exclusively by microglia, suggesting an autocrine regulation of microglia by these particular molecules. In contrast, chemokine ligand, CCL25 was produced constitutively by microglia and selectively up-regulated by neurons following morphine administration. The receptor for this chemokine, CCR9, was localized to both neurons and microglia; thus, questions remain about how neural cells use this chemokine to signal within the brain. Chemokines are well characterized for their ability to induce chemotaxis in a variety of immune cells. It is unclear whether peripheral immune cells are similarly recruited into the brain, particularly the parenchyma, in response to morphine and subsequent chemokine induction, in the absence of infection or trauma. Conversely, we predict that chemokines may be playing more local neuromodulatory roles within the NAcc (e.g. neuron-glia crosstalk) and could induce local chemotaxis of axon terminals or dendritic spines. Similarly, cytokines (e.g. IL-17 and IL-1β) can modulate neuronal function by activating kinases and phosphorylating receptor subtypes, ultimately regulating synaptic function (Srinivasan et al., 2004; Huang et al., 2011; Meng et al., 2013). We predict that chemokines are likely doing the same in our model of addiction. Importantly, we do know that blocking the synthesis of these chemokines can prevent the relapse of drug-seeking behavior following drug re-exposure months later (Schwarz et al., 2011), suggesting that the effect of these chemokines on the neural circuits underlying addiction is profound.
We also found that the chemokine fractalkine is produced by neurons and this expression is decreased following morphine administration, an effect we have previously documented (Schwarz et al., 2011). The receptor for fractalkine, CX3CR1, was expressed exclusively by microglia. The exact mechanism of CX3CL1 is complicated, and data indicate that CX3CL1 can have both pro-and anti-inflammatory effects in the brain and the periphery. Under certain conditions of challenge such as in an inflammatory state of pain, CX3CL1 can be released from neurons and trigger the production of pro-inflammatory cytokines (see Wolf et al., 2013 for review). In contrast, many studies indicate that CX3CL1 produced constitutively by neurons can attenuate microglial pro-inflammatory cytokine and chemokine production; and thus a decrease in the production of CX3CL1 can induce the activation of microglia and the downstream production of cytokines and chemokines (Zujovic et al., 2000; Biber et al., 2007; Wolf et al., 2013). Future studies could examine the mechanism(s) by whether fractalkine is cleaved in response to morphine in order to further activate neurons (reviewed in Wolf et al., 2013). The data presented here are consistent with this latter interpretation, and provide an alternate mechanism by which neurons and microglia can communicate in the presence of a drug such as morphine.
In conclusion, we have determined multiple mechanisms by which neurons and, in particular, microglia can communicate with each other. First, a small percentage of microglia express the D1 and D2 receptor and are thus capable of responding to dopamine release into the NAcc. Secondly, microglia express the innate immune receptor, TLR4, which can be activated by morphine via its adaptor molecule MD2 (Wang et al. 2012, Hutchinson et al. 2010, Hutchinson et al. 2012). We have examined TLR4 expression in microglia previously (Schwarz and Bilbo. 2013), and confirmed those results here. Both of these results indicate mechanisms by which morphine can activate microglia to induce the synthesis of the chemokines listed above. We do know that microglia do not express any of the three opioid receptors examined here and thus opioids cannot activate microglia via activation of the opioid receptor directly. Third, microglia express the fractalkine receptor. When neurons are activated by morphine (as we determined here via Arc expression), they decrease the expression of fractalkine, which can in turn disinhibit microglia, stimulating the expression of the chemokines examined here. Finally, neurons also produce CCL25 in response to morphine treatment, which may influence microglial function and/or neuronal function as both cell types express the cognate receptor, CCR9. We also found that CCL4 and CCL17 are up-regulated by microglia, and the receptor for these chemokines, CCR4, is similarly expressed by microglia. The same was found for the anti-inflammatory cytokine IL-10. These final findings suggest mechanisms by which microglia modulate their own activity.
These data are important as they lend insight into the mechanism by which neurons and glia communicate with each other, particularly in the presence of a drug of abuse such as morphine. Given these data and others previously published, it is clear that the molecules produced by glia, in particular microglia, can significantly influence neuronal function and ultimately behavior. Using these same techniques, future studies should yield more information regarding the relationship between different cell types in the brain, the expression of critical molecules in a cell-type specific manner, and the receptor expression of the cognate receptor, all with the goal of understanding how glia and the molecules they produce can affect neural function and behavior.
Acknowledgements
The authors would like to acknowledge the technical assistance of Nancy Martin at the Duke Flow Cytometry Shared Resource. This work was supported by NIH grants R01DA025978 to SDB and F32DA030136 to JMS.
Footnotes
The authors declare no conflict of interest.
Bibliography
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmuno. 2000;102:113–124. doi: 10.1016/s0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Molecular and Cellular Neuroscience. 2005;29:128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging Neuronal Subsets in Transgenic Mice Expressing Multiple Spectral Variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J. Neuroinflamm. 2012;9:203–217. doi: 10.1186/1742-2094-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Smith DE, Ibañez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1β ar emediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31:18048–18059. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid Activation of Toll-Like Receptor 4 Contributes to Drug Reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhang Y, Lao L, Saito R, Li A, Bäckman CM, Berman BM, Ren K, Wei PK, Zhang RX. Spinal interleukin1 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inlammatory pain rat model. Pain. 2013;154:294–305. doi: 10.1016/j.pain.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Nami K, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M, Rubinstein M. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci. 2006;24:2429–2438. doi: 10.1111/j.1460-9568.2006.05148.x. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: An update. Brain, Behavior, and Immunity. 2010;24:525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Adolescent Morphine Exposure Affects Long-Term Microglial Function and Later-Life Relapse Liability in a Model of Addiction. The Journal of Neuroscience. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell-type specific interleukin-1β signaling in the CNS. J Neurosci. 2004;24:6482–6488. doi: 10.1523/JNEUROSCI.5712-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. The Journal of Neuroscience. 2012;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y, Yona S, Kim KW, Jung S. Microglia, seen from the CX3CR1 angle. Front Cell Neurosci. 2012;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Benavides J, Vigé X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]