Abstract
Objectives
To evaluate the safety and tolerability of latrepirdine in Huntington disease (HD) and explore its effects on cognition, behavior, and motor symptoms.
Design
Double-blind, randomized, placebo-controlled trial.
Setting
Multicenter outpatient trial.
Participants
Ninety-one participants with mild to moderate HD enrolled at 17 US and UK centers from July 18, 2007, through July 16, 2008.
Intervention
Latrepirdine, 20 mg 3 times daily (n=46), or matching placebo (n=45) for a 90-day treatment period.
Main Outcome Measures
The primary outcome variable was tolerability, defined as the ability to complete the study at the assigned drug dosage. Secondary outcome variables included score changes from baseline to day 90 on the Unified Huntington's Disease Rating Scale (UHDRS), the Mini-Mental State Examination (MMSE), and the Alzheimer Disease Assessment Scale–cognitive subscale (ADAS-cog).
Results
Latrepirdine was well tolerated (87% of the patients given latrepirdine completed the study vs 82% in the placebo group), and adverse event rates were comparable in the 2 groups (70% in the latrepirdine group and 80% in the placebo group). Treatment with latrepirdine resulted in improved mean MMSE scores compared with stable performance in the placebo group (treatment effect, 0.97 points; 95% confidence interval, 0.10-1.85; P=.03). No significant treatment effects were seen on the UHDRS or the ADAS-cog.
Conclusions
Short-term administration of latrepirdine is well tolerated in patients with HD and may have a beneficial effect on cognition. Further investigation of latrepirdine is warranted in this population with HD.
Huntington disease (HD) is a hereditary neurodegenerative disorder that affects movement, behavior, and cognition and leads to death within 20 years of disease onset. Cognitive impairment occurs early in the disease and deteriorates as HD progresses, contributing to loss of ability to work and perform activities of daily living. No effective treatments are currently available to alter the course of the disease or to improve cognition because tetrabenazine, the only approved therapy for HD, treats only chorea and other motor symptoms.
Abnormal mitochondrial depolarization, which can lead to collapse of the mitochondrial membrane and ultimately neuronal apoptosis, has been implicated in the pathophysiologic progression of HD and other neurodegenerative diseases.1,2 Latrepirdine is a synthetic molecule that stabilizes mitochondrial membranes3,4 and increases neurite outgrowth to an extent comparable to the maximally effective dose of brain-derived neurotrophic factor in cultured neurons.4 Improvement in mitochondrial function may prevent apoptosis and also improve the efficiency of dynamic cellular activities, such as firing of action potentials and the maintenance or formation of synapses. Latrepirdine, 20 mg 3 times daily, has been studied in a randomized, double-blind, placebo-controlled trial in patients with mild to moderate Alzheimer disease (AD) and demonstrated significant improvement compared with placebo in cognitive, behavioral, and functional scores at 6 and 12 months.5 An earlier open-label, dose-escalation study in patients with HD suggested dosages up to 20 mg 3 times daily would be tolerated in this population.6 We studied the safety and tolerability of latrepirdine, 20 mg 3 times daily, and explored its effects on symptoms in patients with mild to moderate HD.
METHODS
STUDY ORGANIZATION
This multicenter clinical trial was managed through the Huntington Study Group (HSG) and sponsored by Medivation Inc (San Francisco, California), which supplied study drug and matching placebo. The protocol and consent forms were approved by the University of Rochester institutional review board and the institutional review board at each participating site. The Steering Committee and an independent Data and Safety Monitoring Board monitored the safety, data integrity, and conduct of the trial.
STUDY PARTICIPANTS
Participants were men and women 29 years or older with a clinical diagnosis of HD and either a positive family history or a CAG repeat length of 36 or greater. Eligible patients were ambulatory, were living in the community, did not require skilled nursing care, and had a Unified Huntington's Disease Rating Scale (UHDRS) total functional capacity score of 5 or higher at baseline. Because some animal studies raised a potential concern about increased risk of seizures and clinical experience in this population was limited, all participants agreed to comply with general seizure precautions. Participants taking psychotropic medications were required to be taking a stable dosage for at least 30 days before the baseline visit. Participants agreed to use 2 contraception methods; women who were pregnant or nursing were excluded. Other exclusion criteria were a history of seizures, cardiovascular disease, QTc intervals greater than 450 milliseconds, recent investigational drug exposure, unstable medical or psychiatric illness, active peptic ulcer disease, bladder obstruction, human immunodeficiency virus, hepatitis B or C, or significant laboratory abnormalities. The use of cholinesterase inhibitors, N-methyl-D-aspartate antagonists, all forms of dextromethorphan (including hydrobromide and hydrochloride formulations), nonselective antihistamines, lithium, or clonidine was prohibited.
STUDY DESIGN AND RANDOMIZATION PROCEDURE
Participants received study drug (latrepirdine at a dosage of 20 mg or placebo 3 times daily) for 90 days followed by a 14-day safety observation period (total trial duration, 104 days). Eligible participants were randomized in a 1:1 allocation to the 2 treatment arms, and the permuted-block randomization was stratified by site. A nonmasked programmer from the University of Rochester, independent of the other study staff, generated the masked randomization code. Sites enrolled participants via a secure Web page that provided the randomized study drug kit numbers. Participants, investigators, HSG study staff, and sponsor were masked to study group assignment.
STUDY INTERVENTION
Participants initiated use of the study drug with a single 10-mg dose on day 1 followed by a dosage of 10 mg 3 times daily for 6 days, then titrated up to 20 mg 3 times daily for the remainder of the 90-day treatment period. The study drug was formulated as a tablet and encapsulated to maintain masking. Latrepirdine and matching placebo were supplied by KP Pharmaceutical Technology Inc (Bloomington, Indiana) and manufactured by QS Pharma (Boothwyn, Pennsylvania).
STUDY PROCEDURES
All participants gave written documentation of informed consent before any study-related procedure. Screening occurred within 21 days of the baseline visit, and participants were enrolled after an assessment of eligibility criteria. Before receiving study drug on day 1, physical examination, vital sign measurement, laboratory testing, electrocardiography (ECG), pregnancy test if indicated, the UHDRS,7 the Mini-Mental State Examination (MMSE),8 and the Alzheimer Disease Assessment Scale–cognitive subscale (ADAS-cog)9 were performed. Safety assessments (including assessment of adverse events, physical examination, vital sign measurements, ECG, urinalysis, and serum chemical, hematologic, and coagulation tests) were conducted in person on days 15, 30, 60, 90, and 104 and by telephone on days 2, 45, and 75. Efficacy assessments were performed on days 30 (UHDRS only), 60, and 90 (UHDRS, MMSE, and ADAS-cog). The final visit occurred 2 weeks after completion of the treatment period (day 104) and included the UHDRS and safety assessments.
OUTCOME MEASURES
The primary outcome measure was the ability of a participant to complete the 90-day treatment period on the assigned dosage of study drug (tolerability). Safety outcomes were assessed by inquiring about adverse events at each visit and telephone contact, as well as examining changes in laboratory values, vital signs, or ECGs. Adverse events, including serious adverse events, were reviewed throughout the trial by the medical monitor, the Steering Committee, the sponsor, and the Data and Safety Monitoring Board.
Efficacy outcome measures included changes from baseline to day 90 in the UHDRS, MMSE, and ADAS-cog scores. The UHDRS is a comprehensive scale used to assess motor function, cognition, behavior, and functional capacity. Outcomes include the total motor score, behavioral frequency score, behavioral frequency × severity score, functional assessment, independence scale, and total functional capacity. For motor and behavioral assessments, higher scores indicate worsening; for the functional outcomes, lower scores indicate worsening. Cognitive performance in the UHDRS is assessed by means of the verbal fluency test, the symbol digit modalities test, and the Stroop interference test. The MMSE is a common, validated screening instrument that is used as a general measure of cognitive function; lower scores indicate greater impairment. The ADAS-cog used in this study contains the standard 11-item scale developed for use in AD plus 3 additional items added to increase evaluation of executive function.10 Higher scores on the ADAS-cog indicate greater impairment.
STATISTICAL ANALYSIS
The primary tolerability outcome and occurrences of individual adverse events, abnormal laboratory test results, and abnormal ECG results were compared between the latrepirdine and placebo groups by means of either χ2 tests or Fisher exact tests, as appropriate. Analyses of safety outcomes included all participants who received at least 1 dose of the study drug.
The analyses of the efficacy outcomes were exploratory. Changes from baseline to day 90 for the UHDRS, MMSE, and ADAS-cog outcomes were compared between the treatment groups by means of repeated-measures analysis of covariance models that included treatment group, time, the interaction between treatment group and time, and the baseline value of the outcome variable. The analyses of the efficacy outcomes were performed with a modified intention-to-treat paradigm and included all participants who received at least 1 dose of study drug and had at least 1 postbaseline evaluation.
RESULTS
PARTICIPANT ENROLLMENT
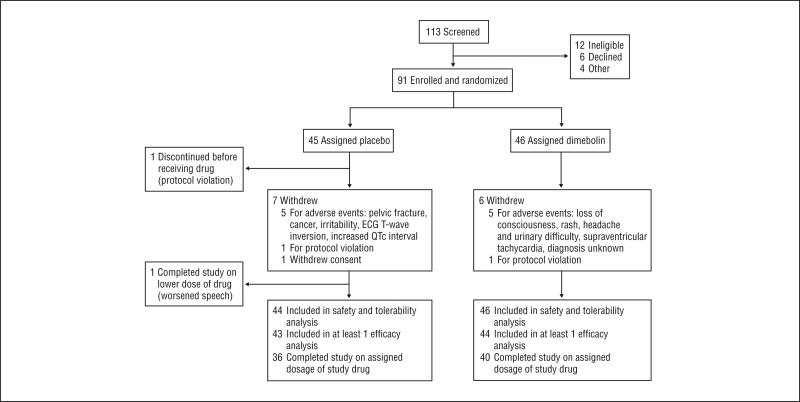
From July 18, 2007, through July 16, 2008, 113 potential participants were identified and evaluated. Of these, 91 were eligible and were enrolled and randomized to either the latrepirdine or placebo group (Figure 1). One participant (assigned to the placebo group) was withdrawn from the study after randomization but before receiving the study drug because of ineligibility. Demographic characteristics were similar between groups at baseline, although the placebo group had a somewhat higher percentage of women (60%) than the latrepirdine group (43%) (Table 1). Ninety-six percent of patients treated with latrepirdine and 89% of those treated with placebo were taking at least 1 concomitant medication. The use of any psychotropic medication was 74% in the latrepirdine group and 68% in the placebo group.
Figure 1.
Participant flow through the study. ECG indicates electrocardiography.
Table 1.
Baseline Demographic and Clinical Characteristics
| No. (%) of Study Participantsa |
||
|---|---|---|
| Characteristic | Latrepirdine (n=46) | Placebo (n=45) |
| Age, mean (SD), y | 53.7 (10.9) | 52.7 (10.2) |
| Female | 20 (43) | 27 (60) |
| Ethnicity | ||
| Black | 2 (4) | 3 (7) |
| White | 42.0 (91) | 40 (89) |
| Education, y | ||
| ≤12 | 13 (28) | 16 (36) |
| 13-16 | 17 (37) | 20 (44) |
| ≥17 | 16 (35) | 9 (20) |
| History of depression | 31 (67) | 26 (58) |
| Age at diagnosis, mean (SD), y | 49.3 (10.6) | 48.5 (10.4) |
| UHDRS scores, mean (SD) | ||
| Total motor | 40.2 (15.7) | 38.9 (16.7) |
| Behavioral frequency | 5.2 (5.0) | 5.2 (4.5) |
| Behavioral frequency × severity | 10.5 (12.0) | 9.4 (9.2) |
| Total functional capacity | 8.1 (2.5) | 8.2 (2.2) |
| Independence scale | 77.7 (10.5) | 77.8 (12.8) |
| Functional assessment | 19.2 (3.8) | 19.7 (4.3) |
| Verbal fluency | 20.7 (10.2) | 17.6 (10.4) |
| Symbol digit modalities test | 22.4 (10.9) | 20.6 (10.6) |
| Stroop color naming | 42.3 (11.9) | 41.9 (12.5) |
| Stroop word reading | 56.7 (19.2) | 54.1 (19.1) |
| Stroop interference | 24.5 (8.7) | 23.4 (9.3) |
| MMSE score, mean (SD) | 25.1 (3.2) | 25.6 (2.9) |
| ADAS-cog total score, mean (SD) | 20.3 (10.1) | 19.9 (8.4) |
Abbreviations: ADAS-cog, Alzheimer Disease Assessment Scale–cognitive subscale; MMSE, Mini-Mental State Examination; UHDRS, Unified Huntington's Disease Rating Scale.
Data are presented as number (percentage) of study participants unless otherwise indicated. Percentages do not total 100% owing to rounding.
TOLERABILITY
Latrepirdine and placebo were both generally well tolerated, with 40 of the 46 participants who took latrepirdine (87%) completing the study with the 60-mg/d dosage of drug vs 36 of the 44 participants in the placebo group (82%); study completion rates did not differ between groups. Of the 6 participants who took latrepirdine and did not complete the study, 5 discontinued the study because of adverse events (loss of consciousness, rash, headache and urinary difficulty, supraventricular tachycardia, diagnosis unknown) and 1 was withdrawn because of a protocol violation (participant was taking an excluded medication). Of the 7 participants who took placebo and did not complete the study, 5 discontinued the study because of adverse events (pelvic fracture, cancer, irritability, ECG T-wave inversion, increased QTc interval), 1 was withdrawn because of a protocol violation (participant was taking excluded medication), and 1 withdrew consent. In addition, 1 participant in the placebo group completed the study at a reduced dosage (10 mg 3 times daily) because of worsened speech.
SAFETY
Overall, 70% of participants who took latrepirdine and 80% of those who took placebo reported an adverse event. Thirty-three percent of participants who took latrepirdine and 43% of those who took placebo reported an adverse event of moderate or severe intensity. The most common adverse events are listed by treatment group in Table 2. Falling was the most common event, reported in 9% of the latrepirdine group and 16% of the placebo group. No clinically relevant differences in laboratory test results, ECG results, or vital sign measurements were noted between the treatment groups.
Table 2.
Adverse Events Reported by More Than 5% of Participants in Either Treatment Group
| No. (%) of Study Participantsa |
|||
|---|---|---|---|
| Adverse Events | Latrepirdine (n=46) | Placebo (n=44) | P Value |
| Falls | 4 (9) | 7 (16) | .35 |
| Headache | 7 (15) | 3 (7) | .32 |
| Dizziness | 3 (7) | 6 (14) | .31 |
| Nausea | 3 (7) | 5 (11) | .48 |
| Chorea | 3 (7) | 3 (7) | >.99 |
| Depression | 2 (4) | 3 (7) | .67 |
| Nasopharyngitis | 2 (4) | 3 (7) | .67 |
| Somnolence | 3 (7) | 1 (2) | .62 |
| Irritability | 1 (2) | 3 (7) | .36 |
| Increased electrocardiogram QTc interval | 0 | 3 (7) | .11 |
| Diarrhea | 0 | 3 (7) | .11 |
Percentages do not total 100% owing to rounding.
One serious adverse event occurred in the latrepirdine group (loss of consciousness), and 3 serious adverse events occurred in 2 participants in the placebo group (pelvic fracture in 1; myocardial infarction and breast cancer in another). The loss of consciousness was transient and did not recur, and the cause of the event remains unclear despite subsequent cardiac and neurologic evaluation. One participant in the latrepirdine group had a cluster of signs and symptoms, specifically fatigue, irritability, weight loss, and confusion, with associated neutrophilia, thrombocytosis, anemia, and elevated aspartate aminotransferase and alanine amino-transferase levels. The syndrome resolved without definitive intervention, and its cause and relation to study drug remain unclear.
COGNITIVE OUTCOMES
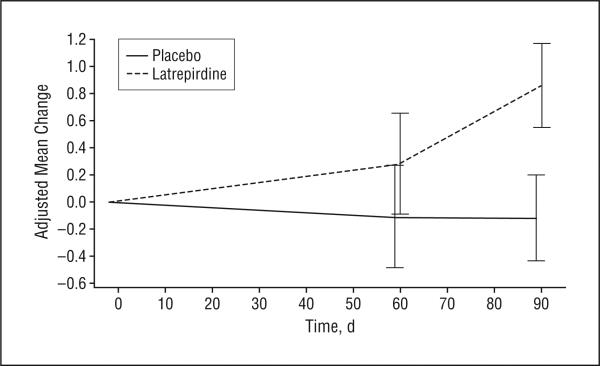
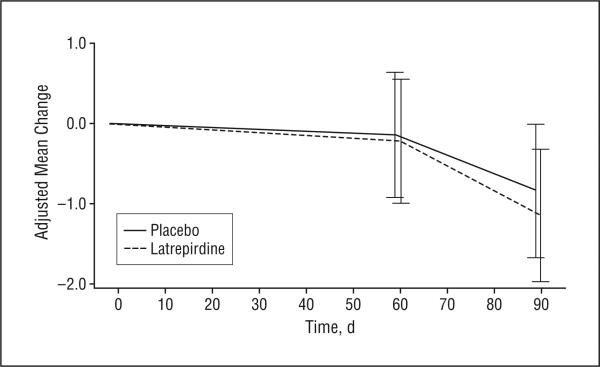
The number of participants included in the analysis of efficacy outcomes ranged from 81 (ADAS-cog) to 87 (UHDRS) (Table 3). During the 90-day treatment period, treatment with latrepirdine resulted in mean improvement on the MMSE compared with placebo (treatment effect, 0.97 points; 95% confidence interval, 0.10-1.85; P=.03). The treatment effect of 0.97 points largely reflected improvement in the latrepirdine group compared with stable performance in the placebo group (Figure 2). No apparent effects of latrepirdine were seen on the UHDRS cognitive tests (verbal fluency, symbol digit modalities test, and Stroop interference) or the ADAS-cog (Figure 3).
Table 3.
Changes From Baseline to Day 90 in Efficacy Outcome Measures
| Outcome Measure | Mean (SD) |
Treatment Effect for Latrepirdine vs Placebo (95% Confidence Interval)a | P Value | |
|---|---|---|---|---|
| Latrepirdine | Placebo | |||
| UHDRS | ||||
| Verbal fluencyb | 3.78 (1.04) | 4.75 (1.06) | –1.98 (–4.94 to 0.98) | .19 |
| Symbol digit modalities testb | 1.06 (0.71) | –0.14 (0.72) | 1.20 (–0.82 to 3.22) | .24 |
| Stroop color namingb | 0.44 (1.16) | 0.58 (1.16) | –0.14 (–3.42 to 3.13) | .93 |
| Stroop word readingb | –1.36 (1.53) | –3.04 (1.54) | 1.68 (–2.64 to 6.00) | .44 |
| Stroop interferenceb | –0.63 (0.97) | 0.75 (0.98) | –1.38 (–4.13 to 1.38) | .32 |
| Behavioral frequencyc | –0.86 (0.54) | 0.04 (0.56) | –0.90 (–2.44 to 0.65) | .25 |
| Behavioral frequency × severityc | –1.47 (1.26) | 0.55 (1.29) | –2.02 (–5.60 to 1.57) | .27 |
| Total motorc | –0.17 (1.11) | 0.21 (1.13) | –0.39 (–3.54 to 2.76) | .81 |
| Total functional capacityb | –0.04 (0.15) | 0.01 (0.15) | –0.06 (–0.47 to 0.36) | .79 |
| Functional assessmentb | 0.01 (0.25) | 0.11 (0.26) | –0.10 (–0.82 to 0.62) | .79 |
| Independence scaleb | –0.48 (0.77) | –0.58 (0.78) | 0.10 (–2.09 to 2.29) | .93 |
| MMSEb | 0.86 (0.31) | –0.12 (0.31) | 0.97 (0.10 to 1.85) | .03d |
| ADAS-cogc | –1.15 (0.82) | –0.84 (0.83) | –0.31 (–2.64 to 2.02) | .79 |
Abbreviations: ADAS-cog, Alzheimer Disease Assessment Scale–cognitive subscale; MMSE, Mini-Mental State Examination; UHDRS, Unified Huntington's Disease Rating Scale.
Treatment effect is the difference (latrepirdine–placebo) between the adjusted group mean changes calculated from a repeated-measures analysis of covariance model.
Positive mean change indicates improvement.
Negative mean change indicates improvement.
P < .05.
Figure 2.
Change over time in Mini-Mental State Examination score by treatment group. The values plotted are adjusted group means derived from a repeated-measures analysis of covariance model. The bars indicate 1 SEM. Positive mean change indicates improvement.
Figure 3.
Change over time in Alzheimer Disease Assessment Scale–cognitive subscale score by treatment group. The values plotted are adjusted group means derived from a repeated-measures analysis of covariance model. The bars indicate 1 SEM. Negative mean change indicates improvement.
Because a substantial number of participants in both groups entered the study with maximum or near-maximum scores on the MMSE, a post hoc subgroup analysis was performed to evaluate the effect of latrepirdine in participants with a greater degree of cognitive impairment at baseline, defined as an MMSE score of 26 or lower (n=51). In this subgroup the difference between treatment groups at 90 days was 1.63 points (95% confidence interval, 0.44-2.82; P=.008), driven by a 1.9-point improvement in the latrepirdine group.
MOTOR, BEHAVIORAL, AND FUNCTIONAL OUTCOMES
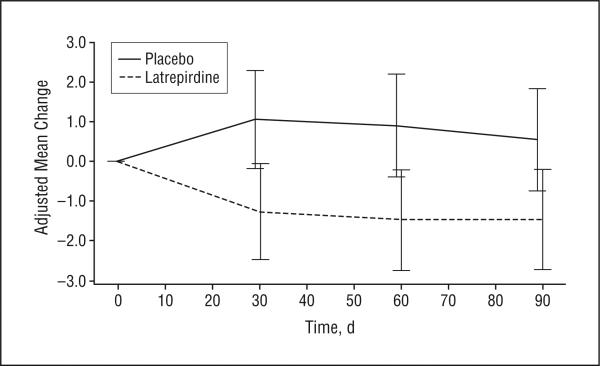
No apparent treatment effects were seen on the UHDRS motor or functional outcomes subscales. Behavioral outcomes were improved in the latrepirdine group (Figure 4), but the treatment group differences were not significant.
Figure 4.
Change over time in the Unified Huntington's Disease Rating Scale score for behavioral frequency × severity by treatment group. The values plotted are adjusted group means derived from a repeated-measures analysis of covariance model. The bars indicate 1 SEM. Negative mean change indicates improvement.
COMMENT
Latrepirdine is an investigational drug that may improve cognition in patients with AD.5 It has a mechanism of action distinct from other drugs, such as cholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists,currently approved to treat neurodegenerative cognitive disorders. Nonclinical data available to date suggest that the mechanism of action of latrepirdine is to enhance mitochondrial function in the setting of cellular stress.4 Mitochondrial dysfunction has been documented in several neurodegenerative diseases, such as AD, HD, and Parkinson disease.11,12 Given the mechanism of action of latrepirdine and the lack of approved therapies for cognitive impairment in HD, we elected to study latrepirdine in HD.
The current randomized, double-blind, placebo-controlled study was undertaken to determine the tolerability of latrepirdine in patients with HD. Our data show that latrepirdine, at a dosage of 20 mg 3 times daily, was well tolerated compared with placebo for patients with HD during a 90-day treatment period. Headache and somnolence were observed more commonly in those taking latrepirdine than in those taking placebo in this study. This finding is distinct from the experience in AD, for which the adverse events reported more commonly in patients with AD treated with latrepirdine compared with placebo were dry mouth, depressed mood or depression, and hyperhidrosis.5 The reasons for these differences are not known but may relate to differences in study populations, concomitant medications, or sample size. Both studies reported no increase in adverse events or serious adverse events in patients treated with latrepirdine compared with those treated with placebo. Because the treatment period in this study was 90 days, we cannot evaluate the risk for adverse effects that may emerge after longer exposure; however, data in AD5 suggest latrepirdine is well tolerated for up to 12 months.
Although the primary end points of this study were safety and tolerability, we had an a priori interest in the potential effects of latrepirdine on cognition and behavior, given its mechanism of action and its effects as previously demonstrated in patients with AD. We therefore included several measures of cognition and behavior in our assessment battery and used a modified form of the ADAS-cog to attempt to capture executive function more thoroughly. The study was not designed to detect a minimally clinically significant effect on any of the efficacy measures; however, we were better able to detect small treatment differences on the MMSE than the UHDRS or ADAS-cog because of reduced variability in the MMSE. The MMSE results suggest that latrepirdine may benefit cognition in HD, although these effects were not consistently supported by the other cognitive assessments.
A significant finding on the MMSE was surprising, given that the MMSE is generally considered a relatively insensitive measure of cognitive function. However, there are several possible explanations for this unexpected discrepancy, such as variation in psychometric properties, the specific cognitive domains assessed by each outcome, and ability to detect clinically relevant short-term cognitive change in HD. For example, the MMSE provides a broader assessment of cognition than the other end points assessed. Although not developed specifically for the type of impairment commonly seen in patients with HD, it is highly stable during a 6-month to 12-month period with low variability in patients who were untreated (unpublished data, available on request). These characteristics could enhance our ability to detect an intervention that resulted in improvement over baseline compared with the other measures. The UHDRS cognitive tests were selected to track the long-term natural history of cognitive decline in HD rather than the effect of a short-term intervention. These tests are also limited by the relatively narrow range of cognitive function assessed. The ADAS-cog provides a broader assessment of cognitive function and was developed for use in short-term symptomatic studies; however, the individual domains were chosen to be sensitive to cognitive changes in AD and may not be as relevant to the types of impairment in HD. An effect of multiple comparisons cannot be excluded; however, the MMSE effect is comparable to that previously demonstrated in patients with AD. It is unknown whether MMSE scores would continue to improve beyond 90 days, although participants treated with latrepirdine demonstrated continued improvement in cognition during a 6-month to 12-month period in the AD trial on both the MMSE and the ADAS-cog.5 These exploratory results on the MMSE are worthy of further evaluation in a randomized trial of treatment efficacy because there are no therapeutics currently available for the disabling cognitive impairment of HD and because the findings observed in this trial on the MMSE are consistent with findings in the population with AD.5
Our analyses did not show a short-term symptomatic effect of latrepirdine on function or on the motor or behavioral symptoms of HD, although the trial was not designed, in terms of sample size, to detect important effects of latrepirdine on any efficacy outcome. The results for the UHDRS behavioral assessments showed qualitative improvement in the latrepirdine group relative to the placebo group. These results are also consistent with the significant effect of latrepirdine in improving behavioral symptoms observed in patients with AD.5
Taken together, our data suggest that latrepirdine, at a dosage of 20 mg 3 times daily, is well tolerated for 90 days in patients with HD and may have a beneficial effect on cognition. Future studies of latrepirdine are planned to further evaluate the effect of latrepirdine on the cognitive and behavioral symptoms of HD.
Acknowledgments
Funding/Support: This study was funded by a grant from Medivation Inc to the University of Rochester and in turn through subcontracts to the participating research sites.
Role of the Sponsors: The HSG designed and conducted the study; collected, analyzed, and interpreted the data; and prepared, reviewed, and approved the manuscript. The sponsor assisted in the study design, data analysis, and manuscript review. The HSG authors retained final approval of the manuscript.
Footnotes
Author Contributions: All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Kieburtz, McDermott, Hodgeman, Kayson, Rabinowitz, Ravina, Seely, and Feigin. Acquisition of data: McDermott, Voss, Corey-Bloom, Deuel, Dorsey, Factor, Geschwind, Hodgeman, Kayson, Noon-berg, Pourfar, Rabinowitz, Ravina, Sanchez-Ramos, Seely, Walker, and Feigin. Analysis and interpretation of data: Kieburtz, McDermott, Voss, Corey-Bloom, Deuel, Dorsey, Factor, Geschwind, Noonberg, Pourfar, Ravina, Sanchez-Ramos, Seely, Walker, and Feigin. Drafting of the manuscript: Kieburtz, McDermott, Voss, and Ravina. Critical revision of the manuscript for important intellectual content: Kieburtz, McDermott, Voss, Corey-Bloom, Deuel, Dorsey, Factor, Geschwind, Hodgeman, Kayson, Noon-berg, Rabinowitz, Ravina, Sanchez-Ramos, Seely, Walker, and Feigin. Statistical analysis: McDermott, Ravina, and Feigin. Obtained funding: Kieburtz and Feigin. Administrative, technical, and material support: Kieburtz, Hodgeman, Kayson, Noonberg, Rabinowitz, and Seely. Study supervision: Kieburtz, McDermott, Voss, Hodgeman, Kayson, Noonberg, Ravina, Seely, Walker, and Feigin.
List of Group Members: Steering Committee: Karl Kieburtz (principal investigator), Andrew Feigin (coprincipal investigator), Merit Cudkowicz, Karen Hodgeman (project manager), Elise Kayson, Michael P. McDermott, Karen Rabinowitz, Bernard Ravina (clinical monitor), Lynn Seely, Wendy Seitz (lay advocate), Tiffini S. Voss (clinical monitor), and Francis Walker. Participating Sites (investigators and coordinators): United States: University of California, San Diego: Jody Corey-Bloom, Stephanie Lessig, MD, and Jody Goldstein, MS; Wake Forest University: Francis Walker, MD, Michael S. Cartwright, MD, Vicki Hunt, RN, and Christine O'Neill; University of Rochester: E. Ray Dorsey, MD, MBA, Charlyne Hickey, MS, RNC, and Lisa M. Deuel, BA; Massachusetts General Hospital: Steven Hersch, MD, PhD, and Alex Bender, BA; Indiana University: Joanne Wojcieszek, MD, and Joanne Belden, LPN; Columbia University: Steven Frucht, MD, and Carol Moskowitz, MS, RNC; Emory University: Stewart Factor, DO, Joan Harrison, RN, and Cathleen Wood-Siverio, MS; North Shore–Long Island Jewish Health System: Michael Pourfar, MD, and Jean Ayan, RN; University of California, San Francisco: Michael D. Geschwind, MD, PhD, Mira Guzijan, MA, and Erika Mozer; University of South Florida: Juan Sanchez-Ramos, MD, PhD, Marcia McCall, MTS, and Kolleen Elliott, RN; The Johns Hopkins University: Adam Rosenblatt, MD, Abhijit Agarwal, MBBS, MPH, Claire Welsh, and Nadine Yoritomo; University of California, Davis: Vicki Wheelock, MD, and Teresa Tempkin, RNC, MSN; Ohio State University: Sandra Kostyk, MD, PhD, and Allison Seward, MS; Albany Medical College: Eric Molho, MD, Sharon Evans, LPN, and Constance Nickerson, LPN; University of Pennsylvania: Amy Colcher, MD, Lisa Altin, BS, and Mary Matthews, RN; Rush University Medical Center: Kathleen Shannon, MD, and Jeana Jaglin, RN; United Kingdom: Cambridge Centre for Brain Repair: Roger A. Barker, MBBS, MRCP, Benjamin Wright, MBBS, and Sarah Mason, BSC; Biostatistics/Coordination Center: Connie Orme, BA, Joseph Weber, BS, and Arthur Watts, BS; Medivation Inc (sponsor): Michele Bronson, PhD, David Hung, MD, Alexander McNees, MPH, Brian Selby, PhD, Michelle Stanley, Rob Tatum, MS, and Monica Sweany; Safety Monitoring Committee: Carl Leventhal, MD; Roger Porter, MD, LLC; and Jason Roy, PhD.
Additional Contributions: Statistical analysis was conducted by Arthur Watts, BS, and Michael P. McDermott, PhD. The HSG Coordination and Biostatistics Center at the University of Rochester independently collected and analyzed the data for this study. The investigators thank the patients and families who participated in this study.
Trial Registration clinicaltrials.gov Identifier: NCT00497159
Financial Disclosure: None of the HSG investigators has equity interest or has received personal remuneration from the sponsoring company since the initiation of the study. Through subcontract with the University of Rochester, Dr Kieburtz has received research grant support from the National Institutes of Health, the Michael J. Fox Foundation, Medivation Inc, NeuroSearch, and Pfizer Inc. He has served as a consultant to the Food and Drug Administration, National Institutes of Health, Abbott Laboratories, Biogen Idec, Ceregene, EMD Serono, FoldRx, Impax, Ipsen, Eli Lilly, Lundbeck, Merz, NeuroSearch, Novartis Pharmaceuticals Corporation, Orion Health, Prestwick Pharmaceuticals Inc, Schering-Plough Corporation, Solvay SA, Teva Pharmaceutical Industries Ltd, and UCB Pharma and has provided legal consultation to Pfizer Inc and the Welding Rod Litigation defendants. Drs Noonberg and Seely are employees of the sponsor. Drs Dorsey and Ravina and Ms Deuel have received research support from the sponsor for activities not reported in this article. Dr Feigin has received honoraria from the sponsor that were deposited in an academic fund.
REFERENCES
- 1.Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Arch Biochem Biophys. 2003;410(1):1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- 2.Tang TS, Slow E, Lupu V, et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci U S A. 2005;102(7):2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachurin S, Bukatina E, Lermontova N, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernales SW, Protter SA, Hung DT. Dimebon incudes neurite outgrowth and mitochondrial stabilization.. Proceedings of the Society for Neuroscience Annual Meeting; Washington, DC. November 15-19, 2008; Washington, DC: Society of Neuroscience; 2008. [Google Scholar]
- 5.Doody RS, Gavrilova SI, Sano M, et al. Dimebon Investigators. Effect of dime-bon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372(9634):207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 6.Medivation Health Safety Group. Safety study of the novel drug dimebon to treat patients with Huntington's disease. US National Institutes of Health Clinical Trials Web site. http://www.clinicaltrials.gov/ct2/show/NCT00387270. Accessed November 13, 2009.
- 7.Huntington Study Group. Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 10.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope: the Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13–S21. [PubMed] [Google Scholar]
- 11.Lin MT, Beal MF. Alzheimer's APP mangles mitochondria. Nat Med. 2006;12(11):1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 12.Beal F, Lang A. The proteasomal inhibition model of Parkinson's disease: “boon or bust”? Ann Neurol. 2006;60(2):158–161. doi: 10.1002/ana.20939. [DOI] [PubMed] [Google Scholar]