Here, the authors sought to determine if ADC values and perfusion measurements provided prognostic information in 25 treatment-naïve immunocompetent patients with brain lymphoma. The studied parameters were obtained from the enhancing tumor regions. The results of this investigation reinforce the validity of ADC values as a prognostic biomarker and provide evidence of low tumor CBV as a novel risk factor for adverse prognosis in immunocompetent patients with primary brain lymphoma.
Abstract
BACKGROUND AND PURPOSE:
ADC derived from DWI has been shown to correlate with PFS and OS in immunocompetent patients with PCNSL. The purpose of our study was to confirm the validity of ADC measurements as a prognostic biomarker and to determine whether rCBV measurements derived from DSC perfusion MR imaging provide prognostic information.
MATERIALS AND METHODS:
Pretherapy baseline DWI and DSC perfusion MR imaging in 25 patients with PCNSL was analyzed before methotrexate-based induction chemotherapy. Contrast-enhancing tumor was segmented and coregistered with ADC and rCBV maps, and mean and minimum values were measured. Patients were separated into high or low ADC groups on the basis of previously published threshold values of ADCmin < 384 × 10−6 mm2/s. High and low rCBV groups were defined on the basis of receiver operating curve analysis. High and low ADC and rCBV groups were analyzed independently and in combination. Multivariate Cox survival analysis was performed.
RESULTS:
Patients with ADCmin values < 384 × 10−6 mm2/s or rCBVmean values < 1.43 had worse PFS and OS. The patient cohort with combined low ADCmin–low rCBVmean had the worst prognosis. No other variables besides ADC and rCBV significantly affected survival.
CONCLUSIONS:
Our study reinforces the validity of ADC values as a prognostic biomarker and provides the first evidence of low tumor rCBV as a novel risk factor for adverse prognosis in immunocompetent patients with PCNSL.
PCNSL, an extra-nodal variant of non-Hodgkin lymphoma confined to the CNS,1 is an aggressive primary brain cancer with rising incidence and variable response to therapy and clinical outcome.2–4 Unlike systemic lymphomas, relatively little is known about the pathobiology of PCNSL.5 Immune deficiency is the only established risk factor for developing PCNSL with uniformly devastating clinical outcome.6 In immunocompetent individuals, however, the disease etiology remains unknown and prognosis can be highly variable.7 Several markers of adverse prognosis have been suggested such as age older than 60 years, low performance scores, high lactate dehydrogenase level, high CSF protein level, enhancing lesions involving the brain stem,8,9 and no initial response to steroids.10 However, noninvasive biomarkers of prognosis and tumor biology that can be measured serially and quantitatively are needed to identify high-risk subgroups at initial diagnosis for formulation of a personalized therapeutic strategy, to assess response to therapy, and to detect tumor recurrence without delay.
Physiology-based MR imaging modalities, such as DWI and DSC perfusion MR imaging, are used clinically to characterize tumor biology beyond structural abnormalities.11 A recently published report suggests a significant correlation between DWI-derived ADC values and clinical outcomes of immunocompetent patients with PCNSL treated with high-dose methotrexate. Specifically, pretherapeutic minimum ADC values <384 × 10−6 mm2/s within enhancing tumor were predictive of shorter progression-free and overall survival.12 Although there are no published data on DSC perfusion MR imaging and prognosis in patients with PCNSL, there are several studies that have demonstrated the value of rCBV as a predictor of grade and prognosis in gliomas.13–16
The purpose of our study was to confirm the validity of ADC measurement as a prognostic biomarker and to determine whether rCBV measurements derived from DSC perfusion MR imaging provide prognostic information.
Materials and Methods
Patient Population
Twenty-five patients treated at our institution between June 2001 and July 2009 were selected for this retrospective study on the basis of the following criteria: histopathologic diagnosis of B-cell PCNSL as defined by the World Health Organization; negative immunodeficiency virus status; and absence of disease outside the CNS based on CT scans of the chest, abdomen, and pelvis. All patients had a pathologic diagnosis of large B-cell CNS lymphoma and received identical methotrexate-based induction chemotherapy treatment. Of 121 patients who met the inclusion criteria for this investigation, 25 had both diffusion and perfusion MR imaging available for analysis before their diagnostic biopsy. Of the 25, 15 were women and 10 were men. The average performance score at pretherapy baseline was 69.2 with a range of 50–100.
The induction chemotherapy (cycle = 28 days, 4 cycles) comprised a 3-drug regimen: methotrexate, 8 g/m2, days 1 and 15; temozolomide, 150–200 mg/m2/day, days 7–11; and rituximab, 375 mg/m2, every week. In patients who achieved a complete response, defined as complete resolution of contrast-enhancing lesions on follow-up MR imaging, to induction therapy, 2–3 additional cycles of methotrexate (8 g/m2) were administered every 14–21 days as consolidation therapy. Patients who achieved a partial response, defined as an interval decrease in the contrast-enhancing lesion, or who exhibited disease progression, defined by an increase in contrast-enhancing lesion volume or development of new enhancing lesions on follow-up MR imaging, were offered high-dose chemotherapy including methotrexate or whole-brain irradiation as salvage therapy. All patients with PCNSL included in this study underwent restaging with follow-up MR imaging within 5 cycles of methotrexate chemotherapy.2 The clinical performance score, the Karnofsky Performance Score, was assessed at pretherapy baseline evaluation.
MR Imaging Protocol
The imaging protocols consisted of preoperative MR imaging performed on a 1.5T clinical scanner (Signa Horizon; GE Healthcare, Milwaukee, Wisconsin). The MR imaging protocol was as follows: 3-plane localizer, sagittal T1-weighted spin-echo (TR/TE, 600/17 ms), axial 3D T2-weighted fast spin-echo (TR/TE, 3000/102 ms), axial FLAIR (TR/TE/TI, 10,000/148/2200 ms), axial DWI (TR/TE, 10,000/99 ms; section thickness/intersection gap, 5/0 mm; matrix size, 256 × 256; FOV, 24 cm; 3 orthogonal diffusion gradient direction; b-values, 0 and 1000 s/mm2) acquired in the transverse plane covering the whole brain, DSC perfusion MR imaging, contrast-enhanced 3D spoiled gradient-recalled T1-weighted imaging (TR/TE, 34/8 ms; section thickness/intersection gap, 1.5/0 mm), and axial T1-weighted postcontrast spin-echo imaging (TR/TE, 500/20 ms).
The standard DSC perfusion MR imaging protocol (TR/TE, 1250/54; flip angle 35°) at our institution was used to acquire a series of gradient-echo echo-planar images immediately before, during, and after a bolus injection of gadolinium diethylene triamine pentaacetic acid (Magnevist; Bayer Healthcare, Wayne, New Jersey). Eight axial 4-mm-thick sections were used to cover the entire tumor volume, as determined with T2-weighted FLAIR images. The first 10 echo-planar acquisitions were performed before gadolinium diethylene triamine pentaacetic acid (0.1 mmol/Kg body weight) was injected intravenously by using an MR imaging–compatible power injector (Spectris Solaris; MedRad, Indianola, Pennsylvania) at a rate of 4–5 mL/s through a 18- or 20-ga angiocatherer and followed immediately by a 20-mL continuous saline flush. A multisection image set was obtained every 1.25 seconds before, during, and after the first pass of contrast agent until images were obtained at 60 time points.
MR Image Processing
The contrast-enhanced 3D spoiled gradient-recalled images and raw diffusion data were transferred to a commercially available workstation, aligned to the same axial location, and processed by using FuncTool software and the Advantage workstation (GE Healthcare). ADC maps were calculated on a voxel-by-voxel basis from the diffusion imaging sets. ADC measurements of the contrast-enhancing tumor volume were measured. The average ADCmin values in units of 10−6 mm2/s for all ROIs were calculated. The investigator responsible for production of the region of interest was blinded to pathologic and clinical outcome (F.E.V.). All ROIs were approved by an attending neuroradiologist certified by the American Board of Radiology with a Certificate of Added Qualification in neuroradiology (S.C.).
The raw data of T2*-weighted DSC perfusion MR images were transferred to the same workstation as mentioned above. rCBV maps were derived by using the methods published previously.17 Contrast-enhanced 3D spoiled gradient-recalled images with ROIs of enhancing tumor were aligned with rCBV maps by using the aforementioned image-processing software. In addition, one 50-mm2 region of interest was manually drawn around the enhancing region with the higher CBV for each transaxial section. A 50-mm2 region of interest within the contralateral normal-appearing white matter for each transaxial section was used to standardize the CBV measurements (rCBVmean and rCBVmin).
Statistical Analysis
The ADCmin group was stratified into low ADCmin (< 384) and high ADCmin (≥384) subgroups. The rCBVmean group was stratified into low rCBVmean (<1.43) and high rCBVmean (≥1.43) subgroups by using a receiver operating characteristic analysis. Similar analysis for the rCBVmin yielded a low rCBVmin subgroup (<0.56) and a high rCBVmin subgroup (≥0.56).
Using the Wilcoxon rank sum test, we performed multiple-comparison analysis among the 4 groups (high ADC/high rCBV, high ADC/low rCBV, low ADC/high rCBV, and low ADC/low rCBV). Univariate analyses were performed comparing patient age at diagnosis, CEL volume, edema volume, ADC values, and rCBV values.
Two clinical end points were measured in months: OS and PFS, defined as the time from initiation of therapy to death and to the first recurrence, respectively. A single covariate survival analysis was performed by using a logrank test. A Cox proportional hazards model was used for multivariable survival analysis. A P value <.05 was considered to indicate a significant difference between groups.
Results
Anatomic Tumor Burden at Pretherapy Baseline Does Not Predict Clinical Outcome
The CEL volume varied from 5.0 to 39.45 cm3 (mean, 17.43 ± 12.35 cm3), and the peritumoral edema volume ranged from 19.6 to 218.88 cm3 (95.21 ± 54.65 cm3). Cox multivariate survival analysis showed that CEL volume, edema volume, age, and Karnofsky Performance Score did not influence PFS or OS (P > .05). The Table summarizes patient demographics and MR imaging variables at pretherapy baseline.
Patient demographics and imaging characteristics
| Patient No. | Age (yr)/Sex | ADCmin × 1012 | ADC Group | CEL (vol · cm3) | Edema (vol · cm3) | rCBVmean | rCBVmean Group | rCBVmin | rCBVmin Group |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 80/M | 520 | High | 11.37 | 108.63 | 1.02 | Low | 0.01 | Low |
| 2 | 60/M | 618 | High | 6.26 | 29.37 | 1.43 | Low | 0.40 | Low |
| 3 | 70/F | 575 | High | 12.62 | 96.00 | 1.38 | Low | 0.33 | Low |
| 4 | 58/M | 133 | Low | 49.10 | 153.20 | 1.00 | Low | 0.50 | Low |
| 5 | 74/F | 213 | Low | 43.40 | 99.30 | 1.42 | Low | 0.26 | Low |
| 6 | 59/F | 594 | High | 20.04 | 72.43 | 1.17 | Low | 0.26 | Low |
| 7 | 53/F | 236 | Low | 6.90 | 62.60 | 1.15 | Low | 0.25 | Low |
| 8 | 64/F | 631 | High | 6.39 | 41.62 | 1.16 | Low | 0.52 | Low |
| 9 | 71/F | 279 | Low | 28.89 | 152.69 | 1.01 | Low | 0.27 | Low |
| 10 | 58/F | 660 | High | 18.80 | 193.90 | 1.17 | Low | 0.34 | Low |
| 11 | 76/M | 640 | High | 5.00 | 26.10 | 2.82 | High | 0.18 | Low |
| 12 | 88/M | 580 | High | 14.70 | 112.60 | 2.76 | High | 1.19 | High |
| 13 | 84/M | 620 | High | 14.18 | 89.76 | 2.62 | High | 0.50 | Low |
| 14 | 57/F | 528 | High | 13.90 | 30.50 | 1.61 | High | 0.89 | High |
| 15 | 64/M | 260 | Low | 39.45 | 218.88 | 1.67 | High | 0.02 | Low |
| 16 | 64/F | 718 | High | 11.94 | 50.49 | 4.39 | High | 1.19 | High |
| 17 | 87/F | 586 | High | 4.06 | 91.71 | 2.37 | High | 0.55 | Low |
| 18 | 68/F | 1010 | High | 8.40 | 19.60 | 1.80 | High | 1.16 | High |
| 19 | 74/M | 684 | High | 16.86 | 130.90 | 1.79 | High | 0.22 | Low |
| 20 | 30/M | 505 | High | 29.00 | 186.10 | 4.21 | High | 1.29 | High |
| 21 | 70/F | 233 | Low | 22.60 | 102.20 | 1.90 | High | 0.02 | Low |
| 22 | 83/F | 409 | High | 8.85 | 94.84 | 1.97 | High | 0.38 | Low |
| 23 | 68/M | 562 | High | 22.60 | 115.20 | 1.60 | High | 0.95 | High |
| 24 | 60/F | 737 | High | 3.06 | 45.38 | 1.50 | High | 0.34 | Low |
| 25 | 59/F | 318 | Low | 17.48 | 56.36 | 1.72 | High | 0.59 | High |
Lower Pretherapy Baseline ADCmin Measurements Predict Shorter Survival
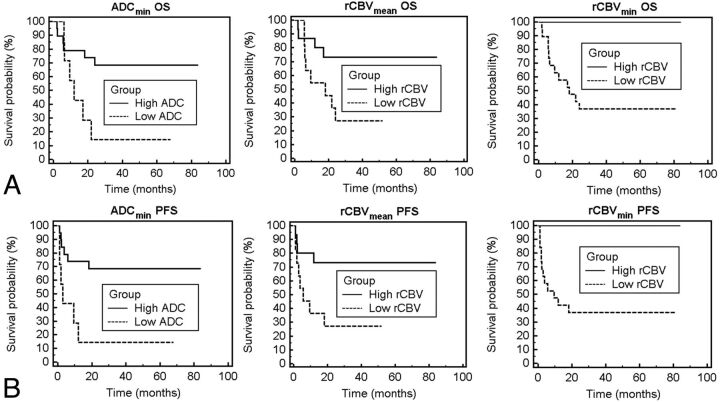
Within the patient cohort, we identified a wide range (133–1010 × 10−6 mm2/s) of intertumoral variability in pretherapy ADCmin values. Seven of the 25 patients included in this study had an ADCmin < 384 within enhancing regions and were assigned to the low ADCmin subgroup. The remaining 18 patients (ADCmin ≥ 384) were designated as the high ADCmin subgroup. As shown in Fig 1, patients in the low ADCmin subgroup had a greatly increased risk of both progression (P < .01 logrank test, hazard ratio = 0.24; Fig 1D) and death (P = 0.02, hazard ratio = 0.29; Fig 1A) compared with the high ADCmin group. Mean PFS and mean OS were significantly shorter in the low ADCmin group versus the high ADCmin group (P = .04 and P = .05, respectively).
Fig 1.
Overall survival and progression-free survival probability based on ADC or rCBV measurements. A and D, Patient outcome as a function of ADCmin stratification into low and high groups. Kaplan-Meier analysis (A) of OS for patients stratified into the low group (ADCmin < 384, dashed line) with a mean survival of 12.1 months versus those stratified into the high group (ADCmin ≥ 384, solid line) with a mean survival time of 20.1 months (P = .02, logrank test). Kaplan-Meier plot (D) of PFS stratified into the same low group (ADCmin < 384, dashed line) with a mean progression time of 13.8 months versus those stratified into the high group (ADCmin ≥ 384, solid line) with a mean progression time of 38.9 months (P < .01, logrank test). B and E, Patient outcome as a function of rCBVmean shows a statistically significant difference in OS and PFS between low (rCBVmean < 1.43) and high (rCBVmean ≥ 1.43) groups (P = .03, logrank test for OS; P = .03, logrank test for PFS). C and F, Patient outcome as a function of rCBVmin shows a statistically significant difference in OS and PFS between low (rCBVmin < 0.56) and high (rCBVmin ≥ 0.56) groups (P = .01, logrank test for OS; P < .01, logrank test for PFS).
Lower Pretherapy Baseline rCBVmean and rCBVmin Measurements Predict Adverse Outcome
Ten of the 25 patients in the study with rCBVmean values <1.43 were assigned to the low rCBVmean subgroup, and the remaining 15 patients with rCBVmean ≥ 1.43 were designated as the high rCBVmean subgroup. Patients in the low rCBVmean subgroup had increased risk for both rapid progression (P = .03 logrank test, hazard ratio = 0.28; Fig 1E) and death (P = .03 logrank test, hazard ratio = 0.30; Fig 1B).
Eighteen of the 25 patients had rCBVmin values < 0.56 and were designated the low rCBVmin subgroup, and the remaining 7 patients (rCBVmin ≥ 0.56) were assigned to the high rCBVmin subgroup. When patient outcome was stratified by rCBVmin, the low subgroup had increased risk for rapid progression (P < .01, logrank test; Fig 1F) and death (P = .01, logrank test; Fig 1C).
Combined ADC-rCBV Groups Are Predictive of Outcomes in Which the Low ADC–low rCBV Group Has the Worst Prognosis
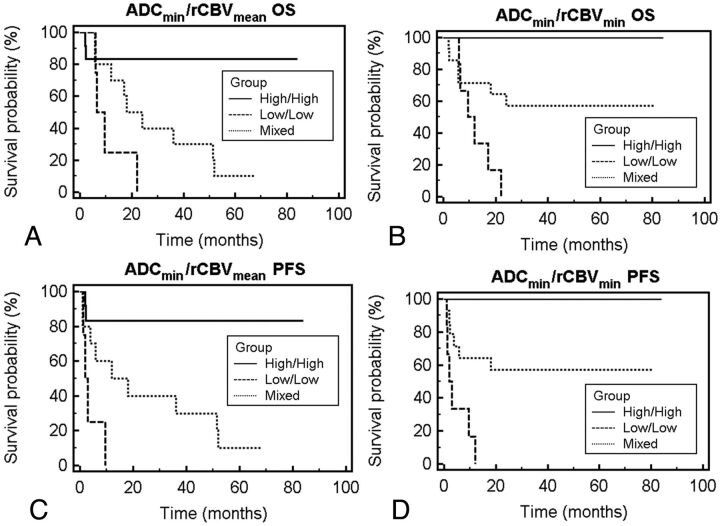
To evaluate the potential additive effects of ADC and rCBV stratification methods, we grouped patient cohorts by using a combined stratification method. Figure 2 illustrates survival difference among different combinations of the ADC-rCBV cohort, in which patients in the low ADC–low rCBV group had the greatest risk for both rapid disease progression (P < .01, logrank test) and death (P < .01, logrank test).
Fig 2.
Overall survival and progression-free survival probability based on combined ADC and rCBV measurements. A and C, Patient outcome as a function of combined stratification by using ADCmin and rCBVmean. Patients stratified into the low-low group (ADCmin < 384, rCBVmean < 1.43; dashed line), high-high group (ADCmin ≥ 384, rCBVmean ≥ 1.43; solid line), and mixed (low ADCmin and high rCBVmean or high ADCmin and low rCBVmean; dotted line). Kaplan-Meier analysis (A) of OS shows decreased median survival for the low-low group of 7.9 months and a median survival of 36.0 months for the mixed group (P < .01, logrank test). Kaplan-Meier analysis (C) of PFS shows a median progression for the low-low group of 2.5 months and a median progression of 36 months for the mixed group (P < .01, logrank test). C and D, Patient outcome as a function of combined stratification by using ADCmin and rCBVmin: patients stratified into the low-low group (ADCmin < 384, rCBVmin < 0.56; dashed line), high-high group (ADCmin ≥ 384, rCBVmin ≥ 0.56; solid line), and mixed (low ADCmin and high rCBVmin or high ADCmin and low rCBVmin; dotted line). Kaplan-Meier analysis shows statistically significant differences between groups in overall survival and progression (P < .01, logrank test for OS; P < .01, logrank test for PFS).
The PFS outcomes (Fig 2C, -D) were observed to cluster into nonbimodal cohorts, while the overall survival outcomes (Fig 2A, -B) were observed to cluster into bimodal cohorts for both categorization strategies.
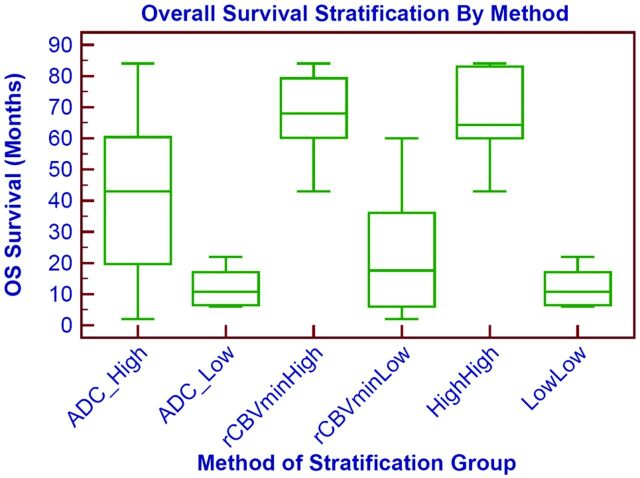
When we compared the combined variables with each individual variable, the overall survival is better stratified into high and low survival groups by using both ADC and rCBV than by using only 1 individual variable as shown in Fig 3. The high ADC–high rCBV group and the high ADC group have statistically different survival rates (P = .01); similarly the low ADC–low rCBV group and the low rCBV group have different survival rates (P = .03).
Fig 3.
Prognostic survival groups based on individual or combined imaging variables. Overall survival stratification based on ADCmin, rCBVmin, or combined ADCmin–rCBVmin is shown as a boxplot. The survival difference is most marked when comparing the combined low ADCmin–low rCBVmin (low-low) group and the high ADCmin–high rCBVmin (high-high). Similar results are obtained if rCBVmean is used instead of rCBVmin.
Discussion
Here, we confirm the validity of ADC measurement as a prognostic biomarker and present the first evidence of rCBV values as an additional imaging biomarker of clinical outcome in patients with PCNSL. In this study, we reproduced the previously reported finding of tumor ADC values derived from DWI as a powerful predictor of clinical outcomes in immunocompetent patients with PCNSL who are treated uniformly with methotrexate-based chemotherapy. We found that the lower the pretherapy baseline tumor ADC values, the shorter the PFS and OS in this group of patients with lymphoma. Both mean and minimum ADC values of tumor at pretherapy show a strong statistical correlation with clinical end points of PFS and OS. When the patients were separated into high-risk (low ADC) versus low-risk (high ADC) subgroups based on the previously published cutoff value of ADCmin < 384 × 10−6 mm2/s, the difference in clinical outcomes between the 2 groups was highly significant, in that the low-risk group had a greater than 2-fold increase in OS and an almost 3-fold increase in PFS compared with the high-risk group.
The exact biologic or molecular correlate of ADC measurement in PCNSL remains unclear. Previous studies have shown significant inverse correlation between tumor cell attenuation and ADC values in PCNSL, suggesting ADC as a surrogate marker of tumor proliferation. Summarizing from numerous published reports on DWI of gliomas in which ADC values have been closely linked to tumor cell attenuation and nuclear-to-cytoplasmic ratio,18–21 one can postulate that ADC values in PCNSL are imaging surrogates of tumor cell attenuation and that lower ADC values reflect higher cellular proliferation in PCNSL, hence correlating with adverse clinical outcome.
We found those patients with low tumor rCBV values (<1.43) at pretherapy baseline to have significantly shorter PFS and OS compared with the patients with high tumor rCBV values, similar to the ADC-based stratification. The biologic correlate of rCBV values in PCNSL remains conjectural, but one can postulate 2 possible explanations as to why low tumor rCBV values may be associated with adverse outcome in patients with PCNSL. First, rCBV values measure bulk vessel attenuation and thus reflect tumor angiogenesis. Second, rCBV values depend on patent vessels for delivery of intravenous gadolinium contrast agent. Therefore, low tumor rCBV values in PCNSL may signify a relative lack of tumor angiogenesis or a hypoxic microenvironment and decrease in patent vessels that are able to deliver intravenous methotrexate to the tumor bed. Our results are in agreement with the literature, in which PCNSL has been found to have hypoxic environments due to low perfusion22 and these hypoxic environments lead to hypoxic tumors that are resistant to treatment.23–25
From further stratification based on a combination of ADC-rCBV values, we found the strongest imaging prediction of clinical outcomes in those patients with combined low tumor ADC and low rCBV values. The high ADC–high rCBV group had very low mortality (no patients died within 5 years), while the low ADC–low rCBV group had 100% mortality within 2 years (Fig 2A, -B). This new stratification suggests that the prognostic value of each imaging variable is additive and synergistic, in that the combination of both variables best predicted the worst prognostic subgroup of patients with PCNSL.
The results of our study have potentially high clinical impact in the treatment and management of patients with PCNSL. Noninvasive pretherapeutic imaging biomarkers that can accurately categorize patients into different risk groups of methotrexate-based chemotherapy could greatly facilitate clinical decision-making, allowing the initiation of individualized second-line salvage therapies, which may result in improved clinical outcome.26–29 While ADC may signal a subtype of PCNSL that is more aggressive as demonstrated by cellularity,12 our rCBV measure may help predict which tumors would be less treatable by chemotherapy due to lower perfusion.
Our study had several limitations. The results of our study may be influenced by its modest sample size and retrospective nature. Second, due to the paucity of available tumor-tissue samples, we were unable to correlate the rCBV measurements observed to histopathologic standards of tumor angiogenesis and hypoxia.
Conclusions
Our study further supports the important role of ADC measurement as a prognostic biomarker in immunocompetent patients with PCNSL in which the lower ADC measurement at pretherapy baseline indicates shorter overall survival. In addition, we present the first evidence of rCBV measurement as a potential prognostic biomarker in patients with PCNSL, in which lower value is associated with adverse prognosis. The predictive power of adverse outcome is even greater when risk stratification is based on combined low ADC and low rCBV values, suggesting the additive and complementary role of diffusion and perfusion MR imaging as prognostic biomarkers in patients with PCNSL.
ABBREVIATIONS:
- CEL
contrast-enhancing lesion
- DSC
dynamic susceptibility-weighted contrast-enhanced
- min
minimum
- OS
overall survival
- PCNSL
primary central nervous system lymphoma
- PFS
progression-free survival
- rCBV
relative cerebral blood volume
Footnotes
Disclosures: Francisco Valles—RELATED: Grant: (Doris Duke Charitable Foundation Clinical Research Fellowships, Comments: The DDCF Clinical Research Fellowship program for medical students at UCSF is a 1-year mentored clinical research opportunity designed to span a broad range of research ranging from bench laboratory science to clinical and translational science, epidemiology, and outcomes research. We define “clinical and translational research” as research designed to address a question of clinical importance. The program is designed to be flexible and will be tailored to meet the needs of each student. James Rubenstein—RELATED: Grant: Dr Rubenstein is a Clinical Scholar of the Leukemia and Lymphoma Society and is a recipient of National Cancer Institute R01 CA139083–01A1.
This publication was supported by NIH/NCRR/OD UCSF-CTSI grant No. TL1RR024129. The study was funded in part by the Leukemia and Lymphoma Society, the UCSF Brain Tumor Specialized Programs of Research Excellence, and NIH R01CA1398301.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Paper previously presented in part at: 49th Annual Meeting of the American Society of Neuroradiology, June 4–June 9, 2011; Seattle, Washington.
References
- 1. Deckert M, Engert A, Bruck W, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia 2011;25:1797–807 [DOI] [PubMed] [Google Scholar]
- 2. Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer 2002;95:1504–10 [DOI] [PubMed] [Google Scholar]
- 3. Eby NL, Grufferman S, Flannelly CM, et al. Increasing incidence of primary brain lymphoma in the US. Cancer 1988;62:2461–65 [DOI] [PubMed] [Google Scholar]
- 4. Schlegel U, Schmidt-Wolf IG, Deckert M. Primary CNS lymphoma: Clinical presentation, pathological classification, molecular pathogenesis and treatment. J Neurol Sci 2000;181:1–12 [DOI] [PubMed] [Google Scholar]
- 5. Hochberg FB, Hochberg E. Primary CNS lymphoma. Nat Clin Pract Neurol 2007;3:24–35 [DOI] [PubMed] [Google Scholar]
- 6. Wolf T, Brodt HR, Fichtlscherer S, et al. Changing incidence and prognostic factors of survival in AIDS-related non-Hodgkin's lymphoma in the era of highly active antiretroviral therapy (HAART). Leuk Lymphoma 2005;46:207–15 [DOI] [PubMed] [Google Scholar]
- 7. Bayraktar S, Bayraktar UD, Ramos JC, et al. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol 2011;101:257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abrey LE, Batchelor TT, Ferreri AJ, et al. , for the International Primary CNS Lymphoma Collaborative Group. Report on an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034–43 [DOI] [PubMed] [Google Scholar]
- 9. Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the international extranodal lymphoma study group experience. J Clin Oncol 2003;21:266–72 [DOI] [PubMed] [Google Scholar]
- 10. Mathew BS, Carson KA, Grossman SA. Initial response to glucocorticoids. Cancer 2006;106:383–87 [DOI] [PubMed] [Google Scholar]
- 11. Cha S. Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 2006;27:475–87 [PMC free article] [PubMed] [Google Scholar]
- 12. Barajas RF, Jr, Rubenstein JL, Chang JS, et al. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 2009;31:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirai T, Murakami R, Nakamura H, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: Long-term follow-up study. AJNR Am J Neuroradiol 2008;29:1505–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitmore RG, Krejza J, Kapoor GS, et al. Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J Neurosurg 2007;107:600–09 [DOI] [PubMed] [Google Scholar]
- 15. Chaskis C, Stadnik T, Michotte A, et al. Prognostic value of perfusion-weighted imaging in brain glioma: a prospective study. Acta Neurochir (Wien). 2006;148:277–85, discussion 285 [DOI] [PubMed] [Google Scholar]
- 16. Caseiras GB, Chheang S, Babb J, et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol 2010;73:215–20 [DOI] [PubMed] [Google Scholar]
- 17. Cha S, Lupo JM, Chen MH, et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2007;28:1078–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo AC, Cummings TJ, Dash RC, et al. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 2002;224:177–83 [DOI] [PubMed] [Google Scholar]
- 19. Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol 2003;45:208–13 [DOI] [PubMed] [Google Scholar]
- 20. Khayal IS, Vandenberg SR, Smith KJ, et al. MRI apparent diffusion coefficient reflects histopathologic subtype, axonal disruption, and tumor fraction in diffuse-type grade II gliomas. Neuro Oncol 2011;13:1192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999;9:53–60 [DOI] [PubMed] [Google Scholar]
- 22. Kim JA, Kim SJ, Do IG, et al. Hypoxia-associated protein expression in primary central nervous system diffuse large b-cell lymphoma: does it predict prognosis? Leuk Lymphoma 2011;52:205–13 [DOI] [PubMed] [Google Scholar]
- 23. Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets 2009;9:381–90 [DOI] [PubMed] [Google Scholar]
- 24. Yetkin FZ, Mendelsohn D. Hypoxia imaging in brain tumors. Neuroimaging Clin N Am 2002;12:537–52 [DOI] [PubMed] [Google Scholar]
- 25. Knisely JP, Rockwell S. Importance of hypoxia in the biology and treatment of brain tumors. Neuroimaging Clin N Am 2002;12:525–36 [DOI] [PubMed] [Google Scholar]
- 26. Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350–56 [DOI] [PubMed] [Google Scholar]
- 27. Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 2008;26:2512–18 [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto Y, Horiike S, Fujimoto Y, et al. Effectiveness and limitation of gamma knife radiosurgery for relapsed central nervous system lymphoma: a retrospective analysis in one institution. Int J Hematol 2007;85:333–37 [DOI] [PubMed] [Google Scholar]
- 29. Ryken TC, Meeks SL, Pennington EC, et al. Initial clinical experience with frameless stereotactic radiosurgery: analysis of accuracy and feasibility. Int J Radiat Oncol Biol Phys 2001;51:1152–58 [DOI] [PubMed] [Google Scholar]