Abstract
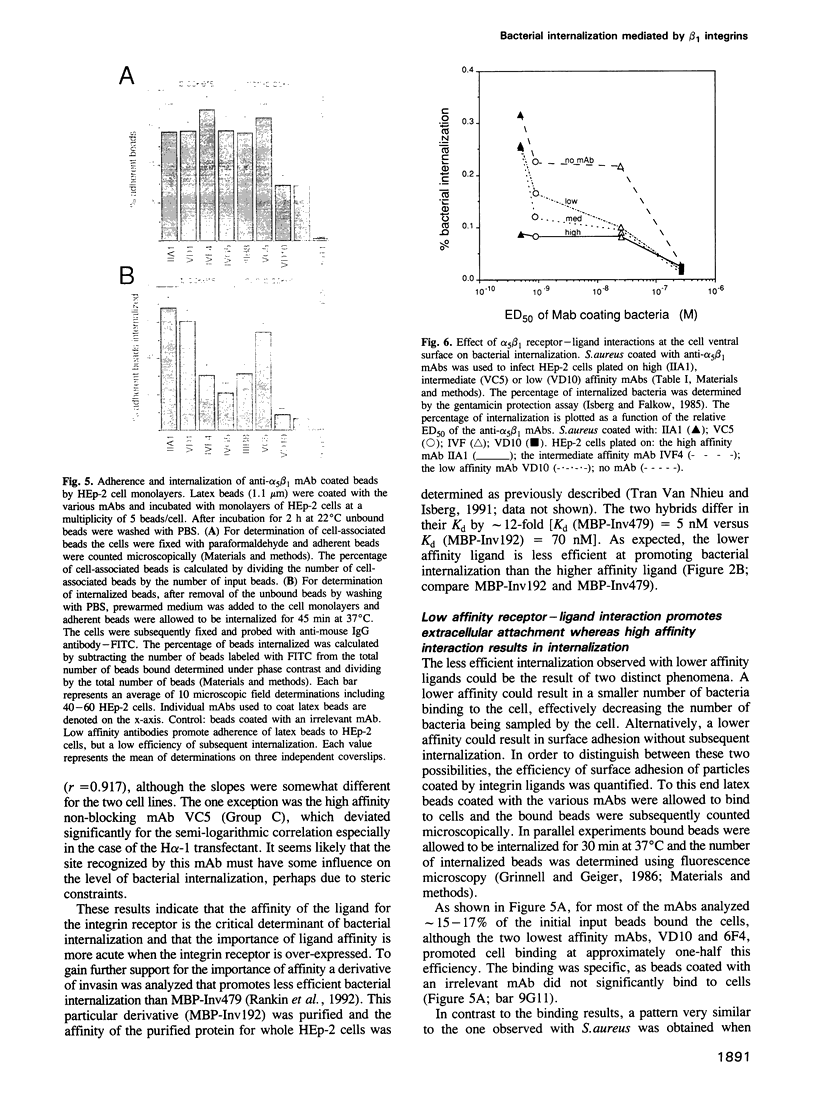
Binding of bacteria to beta 1 chain integrin receptors results in either bacterial adherence or uptake by cultured cells (Isberg, 1991). In this report we show that Staphylococcus aureus coated with high affinity ligands for the beta 1 chain integrin family can be internalized efficiently, whereas bacteria coated with low affinity ligands are poorly internalized. Overproduction of the alpha 5 beta 1 integrin increased the efficiency of bacterial internalization, indicating that the uptake efficiency is directly related to the level of expression of the receptor. By using latex beads or S. aureus coated with mAbs directed against the alpha 5 beta 1 integrin, a roughly semi-logarithmic correlation was observed between the affinity of the receptor-ligand interaction and the rate of bacterial internalization. Evidence is presented that high affinity binding of the bacterium allows the microorganism to compete efficiently with receptor-ligand interactions at the basolateral surface of the cell.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Argraves W. S., Pytela R., Suzuki S., Millán J. L., Pierschbacher M. D., Ruoslahti E. cDNA sequences from the alpha subunit of the fibronectin receptor predict a transmembrane domain and a short cytoplasmic peptide. J Biol Chem. 1986 Oct 5;261(28):12922–12924. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle D. L., Attie A. D. Microelisa reader quantitation of fixed, stained, solubilized cells in microtitre dishes. Biotechniques. 1988 May;6(5):418–419. [PubMed] [Google Scholar]
- Bretscher M. S. Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. EMBO J. 1992 Feb;11(2):405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J Cell Sci Suppl. 1987;8:231–250. doi: 10.1242/jcs.1987.supplement_8.13. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Van Leuven F., Carmeliet G., Van Den Berghe H., Cassiman J. J. Cultured human fibroblasts contain a large pool of precursor beta 1-integrin but lack an intracellular pool of mature subunit. Eur J Biochem. 1991 Jul 1;199(1):25–33. doi: 10.1111/j.1432-1033.1991.tb16087.x. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Flock J. I., Fröman G., Jönsson K., Guss B., Signäs C., Nilsson B., Raucci G., Hök M., Wadström T., Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987 Aug;6(8):2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. L., Starnbach M. N., Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992 Nov;6(21):3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Geiger B. Interaction of fibronectin-coated beads with attached and spread fibroblasts. Binding, phagocytosis, and cytoskeletal reorganization. Exp Cell Res. 1986 Feb;162(2):449–461. doi: 10.1016/0014-4827(86)90349-6. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol Biol Med. 1990 Feb;7(1):73–82. [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990 Jun;9(6):1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Genetic determinants of Shigella pathogenicity. Annu Rev Microbiol. 1988;42:127–150. doi: 10.1146/annurev.mi.42.100188.001015. [DOI] [PubMed] [Google Scholar]
- McAbee D. D., Grinnell F. Binding and phagocytosis of fibronectin-coated beads by BHK cells: receptor specificity and dynamics. J Cell Physiol. 1985 Aug;124(2):240–246. doi: 10.1002/jcp.1041240211. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann B., Porstmann T., Nugel E. Comparison of chromogens for the determination of horseradish peroxidase as a marker in enzyme immunoassay. J Clin Chem Clin Biochem. 1981 Jul;19(7):435–439. doi: 10.1515/cclm.1981.19.7.435. [DOI] [PubMed] [Google Scholar]
- Rankin S., Isberg R. R., Leong J. M. The integrin-binding domain of invasin is sufficient to allow bacterial entry into mammalian cells. Infect Immun. 1992 Sep;60(9):3909–3912. doi: 10.1128/iai.60.9.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Duronio V., Finlay B. B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992 Jun;60(6):2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. III. Comparative studies between Y. enterocolitica and Y. pseudotuberculosis. Microbiol Immunol. 1977;21(9):505–516. doi: 10.1111/j.1348-0421.1977.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Van Nhieu G. T., Isberg R. R. The Yersinia pseudotuberculosis invasin protein and human fibronectin bind to mutually exclusive sites on the alpha 5 beta 1 integrin receptor. J Biol Chem. 1991 Dec 25;266(36):24367–24375. [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]