Abstract
Surveillance of dengue virus (DENV) in Aedes (Stegomyia) aegypti (L.) females is of potential interest because human DENV infections are commonly asymptomatic, which decreases the effectiveness of dengue case surveillance to provide early warning of building outbreaks. Our primary aim was to examine if mosquito-based virological measures – monthly percentages of examined Ae. aegypti females infected with DENV or examined homes from which ≥1 DENV-infected Ae. aegypti female was collected – are correlated with reported dengue cases in the same or subsequent months within study neighborhoods in Mérida City, México. The study encompassed ~30 neighborhoods in the southern and eastern parts of the city. Mosquitoes were collected monthly over a 15-month period within study homes (average of 145 homes examined per month); this produced ~5,800 Ae. aegypti females subsequently examined for DENV RNA. Although monthly dengue case numbers in the study neighborhoods varied more than 100-fold during the study period, we did not find statistically significant positive correlations between monthly data for mosquito-based DENV surveillance measures and reported dengue cases in the same or subsequent months. Monthly average temperature, rainfall, and indoor abundance of Ae. aegypti females were positively correlated (P ≤ 0.001) with dengue case numbers in subsequent months with lag times of 3–5, 2, and 1–2 months, respectively. However, because dengue outbreak risk is strongly influenced by serotype-specific susceptibility of the human population to DENV, the value of weather conditions and entomological indices to predict outbreaks is very limited. Potential ways to improve the sensitivity of mosquito-based DENV surveillance are discussed.
Keywords: Aedes aegypti, dengue virus, virological surveillance, México
Introduction
Numerous previous studies have documented correlations between weather conditions (rainfall or temperature-related variables) or entomological indices for the dengue virus (DENV) vector Aedes (Stegomyia) aegypti (L.) (abundance measures based on surveys for immatures or trap catches) and the numbers of dengue cases in subsequent weeks or months (reviewed by Eisen and Moore 2013). Such linkages between weather or mosquito abundance and dengue disease are tenuous, however, because they are also strongly influenced by the constantly changing serotype-specific susceptibility of the human population to DENV (Scott and Morrison 2009). The lack of confidence in weather conditions and entomological indices as predictors of DENV transmission intensity, together with problems related to asymptomatic infections in humans and delays in diagnostic testing and reporting of dengue cases, raises the question of whether virological surveillance of DENV in mosquito vectors may be a useful complement to human-based surveillance. DENV is readily detected from field-collected Ae. aegypti females (reviewed by García-Rejón et al. 2008). The infected females can be linked to the spatial locations where they were collected, and very likely have far smaller spatial activity spaces compared with DENV-infected humans (Harrington et al. 2005, Vazquez-Prokopec et al. 2010).
As part of a study conducted in Mérida City, México over a 15-month period (May 2009– July 2010) to determine whether insecticide-treated curtains can be used to protect homes from intrusion by Ae. aegypti and intradomicillary DENV transmission (Loroño-Pino et al. 2013), we performed monthly indoor collections of Ae. aegypti females (typically in >125 homes/month) and determined their DENV infection status. Herein, we examine if mosquito-based virological measures (monthly percentages of examined Ae. aegypti females infected with DENV or examined homes from which ≥1 DENV-infected Ae. aegypti female was collected) are correlated with reported dengue cases in the same or subsequent months in our Mérida City study neighborhoods.
Materials and Methods
The Mérida City study area, the selection of study homes, and the methods used to collect mosquitoes and detect DENV RNA in Ae. aegypti females were described previously (Loroño-Pino et al. 2013). Study homes distributed across ~30 neighborhoods (Colonias) in the southern and eastern parts of Mérida City were followed over a 15-month period from May 2009–July 2010. The study homes represent ~3% of the total number of homes in these neighborhoods. Individual neighborhoods included both intervention homes with insecticide-treated curtains and control homes with similar but non-treated curtains. Monthly data presented herein for Ae. aegypti females and their DENV infection status are based on homes designated as control or intervention homes for the portion of the study period that preceded curtain installation (May–August 2009), whereas only the control homes were included for the months after curtains were installed (September 2009–July 2010). Weather data (rainfall and mean temperature) for the period from January 2009 – December 2010 were obtained from a weather station at the Mérida City airport operated by the Comision Nacional del Agua.
Data on monthly numbers of laboratory-confirmed dengue cases occurring in Mérida City, and the subset of these cases occurring within our study neighborhoods, during 2009–2010 were obtained through a collaborative agreement with Servicios de Salud de Yucatán. The monthly numbers of dengue cases for the specific study neighborhoods and overall for Mérida City were strongly correlated (Spearman rank correlation; ρ = 0.812, N = 24, P < 0.001); we therefore use data only for the specific study neighborhoods in the statistical analyses. The study was approved by the Institutional Review Board of Colorado State University and the Bioethics Committee of Universidad Autónoma de Yucatán.
Because of the skewed distribution for the number of reported dengue cases per month, the Spearman rank correlation coefficient (computed using the JMP statistical package; Sall et al. 2005) was used to examine correlations between dengue cases and the other measured variables (percentage of examined Ae. aegypti females infected with DENV, percentage of examined homes from which at least one DENV-infected Ae. aegypti female was collected, geometric mean number of Ae. aegypti per home, total rainfall, and average daily temperature). The Poisson approximation of the binomial distribution was used to evaluate the power to detect the presence of DENV in Ae. aegypti females for the monthly numbers of mosquitoes examined during the study period.
Results
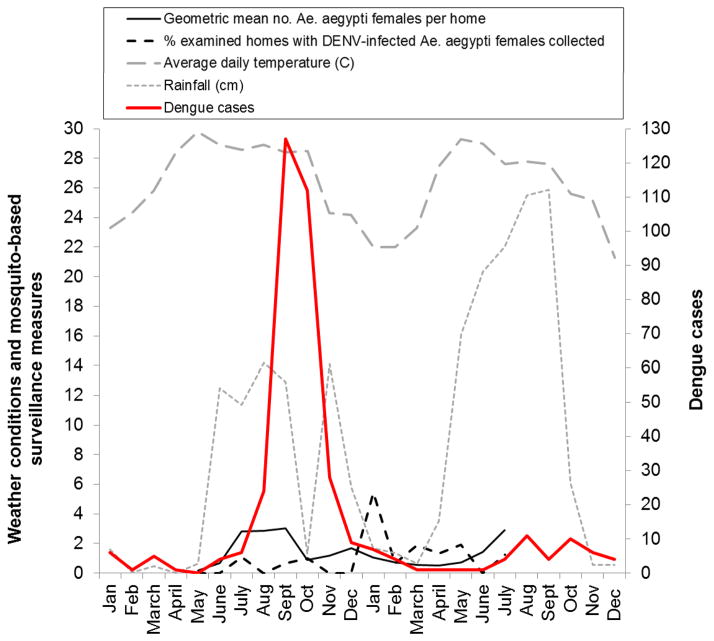
Monthly average daily temperatures in Mérida City ranged from 21–25 ºC from November to March but exceeded 27 ºC from April to September/October, and most rainfall occurred from May to September (Figure 1, Table 1). Consistent with our previous study from Mérida City (García-Rejón et al. 2008), the abundance of Ae. aegypti females indoors was low during the cooler and drier part of the year, started to increase in May/June, and peaked during July–September (Table 1, Figure 1). In contrast to our previous study, which focused on collection of mosquitoes specifically from the homes of dengue patients and found DENV-infected Ae. aegypti females from July–December but not from January–June (García-Rejón et al. 2008), we unexpectedly recorded lower percentages of examined homes with DENV-infected Ae. aegypti females collected from July–December 2009 (0–1.1%) compared with January–May 2010 (0.7–5.4%) (Table 1). The temporal distribution of reported dengue cases exhibited distinct seasonality, with most cases occurring from August/September to November/December (Figure 1, Table 1).
Figure 1.
Temporal curves for monthly numbers during 2009–2010 of reported dengue cases in the Mérida City study neighborhoods, weather conditions (total monthly rainfall and monthly average daily temperature), an entomological index (monthly geometric mean number of Ae. aegypti females collected per home) and a mosquito-based DENV surveillance measure (monthly percentage of examined homes from which at least one DENV-infected Ae. aegypti female was collected).
Table 1.
Monthly numbers of reported dengue cases during 2009–2010 in relation to data for weather conditions, abundance of Ae. aegypti females, and surveillance for dengue virus (DENV) in Ae. aegypti females.
| Month and Year | No. of reported dengue cases | Weather conditions | No. of homes examined for mosquitoes | Abundance of Ae. aegypti females | Surveillance for DENV in Ae. aegypti females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Mérida City | Study neigh-borhoods within Mérida City | Total rainfall (mm) | Average daily temp. (ºC) | Total no. collecteda | Geometric mean per home | Total no. DENV-infected females collected | DENV infection prevalence in examined females (%)a,b | Total no. homes with DENV-infected females collected | Percentage of examined homes with DENV-infected females collected | ||
| Jan 2009 | 35 | 6 | 16 | 23.3 | 0 | ||||||
| Feb 2009 | 14 | 1 | 0.3 | 24.3 | 0 | ||||||
| March 2009 | 10 | 5 | 4.7 | 25.8 | 0 | ||||||
| April 2009 | 13 | 1 | 0 | 28.4 | 0 | ||||||
| May 2009 | 7 | 0 | 6.5 | 29.8 | 74 | 21 | 0.17 | 0 | 0 | 0 | 0 |
| June 2009 | 22 | 4 | 125 | 28.9 | 128 | 182 | 0.68 | 0 | 0 | 0 | 0 |
| July 2009 | 19 | 6 | 114 | 28.6 | 184 | 1,092 | 2.83 | 3 | 0.27 | 2 | 1.09 |
| Aug 2009 | 62 | 24 | 142 | 28.9 | 27 | 139 | 2.86 | 0 | 0 | 0 | 0 |
| Sept 2009 | 363 | 127 | 129 | 28.4 | 152 | 836 | 3.04 | 1 | 0.12 | 1 | 0.66 |
| Oct 2009 | 782 | 112 | 13 | 28.5 | 203 | 288 | 0.87 | 2 | 0.69 | 2 | 0.99 |
| Nov 2009 | 527 | 28 | 141 | 24.3 | 180 | 441 | 1.17 | 0 | 0 | 0 | 0 |
| Dec 2009 | 334 | 9 | 58 | 24.2 | 149 | 447 | 1.67 | 0 | 0 | 0 | 0 |
| Jan 2010 | 210 | 7 | 17 | 22.0 | 166 | 319 | 1.05 | 16 | 5.02 | 9 | 5.42 |
| Feb 2010 | 99 | 4 | 14 | 22.0 | 150 | 258 | 0.73 | 7 | 2.71 | 1 | 0.67 |
| March 2010 | 38 | 1 | 6.4 | 23.3 | 160 | 158 | 0.54 | 4 | 2.53 | 3 | 1.88 |
| April 2010 | 7 | 1 | 35 | 27.5 | 151 | 160 | 0.50 | 9 | 5.63 | 2 | 1.32 |
| May 2010 | 9 | 1 | 161 | 29.3 | 157 | 226 | 0.71 | 6 | 2.65 | 3 | 1.91 |
| June 2010 | 24 | 1 | 203 | 29.0 | 143 | 423 | 1.45 | 0 | 0 | 0 | 0 |
| July 2010 | 74 | 4 | 220 | 27.6 | 161 | 803 | 2.92 | 2 | 0.25 | 2 | 1.24 |
| Aug 2010 | 149 | 11 | 255 | 27.8 | 0 | ||||||
| Sept 2010 | 123 | 4 | 259 | 27.6 | 0 | ||||||
| Oct 2010 | 202 | 10 | 61 | 25.6 | 0 | ||||||
| Nov 2010 | 142 | 6 | 5.7 | 25.2 | 0 | ||||||
| Dec 2010 | 52 | 4 | 5.5 | 21.3 | 0 | ||||||
All collected Ae. aegypti females were examined for presence of DENV RNA.
Infection prevalence was calculated as: (total no. DENV-infected Ae. aegypti females/total no. collected Ae. aegypti females)*100.
The strongest positive correlations with the monthly number of dengue cases in the study neighborhoods were observed for monthly average daily temperature 3 months prior (ρ = 0.844, N = 21, P < 0.001), rainfall 2 months prior (ρ = 0.553, N = 22, P = 0.008), and the monthly geometric mean number of Ae. aegypti females collected indoors per home 1 month prior (ρ = 0.756, N = 15, P = 0.001) (Table 2). In striking contrast, we did not find statistically significant positive correlations between monthly data for the mosquito-based DENV surveillance measures and dengue cases in the study neighborhoods (Table 2). Instead, the recorded significant correlations with dengue cases – occurring for each of the two mosquito-based DENV surveillance measures 2 months prior (ρ ≥ −0.557, N =15, P ≤ 0.031 in both cases) – were negative (Table 2).
Table 2.
Correlations between dengue case numbers in the Mérida City study neighborhoods and data for weather conditions, abundance of Ae. aegypti females, or surveillance for dengue virus (DENV) in Ae. aegypti females in the same or subsequent months.
| Lag time | Correlation with numbers of dengue cases in the study neighborhoods in the same or subsequent months based on the monthly data presented in Table 1a
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total monthly rainfall (mm) | Monthly average daily temperature (ºC) | Geometric mean no. Ae. aegypti females per home | Percentage of examined Ae. aegypti females infected with DENV | Percentage of examined homes with DENV-infected Ae. aegypti females collected | ||||||||||||||||
|
| ||||||||||||||||||||
| Typeb | N | ρ | P | Typeb | N | ρ | P | Typeb | N | ρ | P | Typeb | N | ρ | P | Typeb | N | ρ | P | |
| + 0 mo | NA | 24 | 0.272 | 0.198 | NA | 24 | −0.137 | 0.525 | Pos | 15 | 0.666 | 0.007 | NA | 15 | −0.178 | 0.525 | NA | 15 | −0.199 | 0.477 |
| + 1 mo | Pos | 23 | 0.476 | 0.022 | NA | 23 | 0.268 | 0.215 | Pos | 15 | 0.756 | 0.001 | NA | 15 | −0.506 | 0.054 | NA | 15 | −0.402 | 0.138 |
| + 2 mo | Pos | 22 | 0.553 | 0.008 | Pos | 22 | 0.646 | 0.001 | NA | 15 | 0.512 | 0.051 | Neg | 15 | −0.641 | 0.010 | Neg | 15 | −0.557 | 0.031 |
| + 3 mo | NA | 21 | 0.364 | 0.105 | Pos | 21 | 0.844 | <0.001 | NA | 15 | 0.112 | 0.691 | NA | 15 | −0.386 | 0.156 | NA | 15 | −0.255 | 0.359 |
| + 4 mo | NA | 20 | 0.017 | 0.944 | Pos | 20 | 0.750 | <0.001 | NA | 15 | −0.170 | 0.545 | NA | 15 | −0.291 | 0.292 | NA | 15 | −0.258 | 0.354 |
| + 5 mo | NA | 19 | −0.280 | 0.245 | Pos | 19 | 0.508 | 0.027 | NA | 15 | −0.400 | 0.140 | NA | 15 | −0.184 | 0.511 | NA | 15 | −0.077 | 0.785 |
Based on Spearman’s rank correlation (ρ) along with the number of data points (N) and the p-value for the test of significance (P).
Pos – Positive correlation (P<0.05); Neg – Negative correlation (P<0.05); NA – non-significant correlation (P≥0.05).
Discussion
Surveillance of DENV in Ae. aegypti females is of potential interest as a means for early detection of dengue outbreaks because human DENV infections are commonly asymptomatic or “silent”(Gubler 1998, Kyle and Harris 2008), which decreases the effectiveness of dengue case surveillance to provide early warning of building outbreaks. Improved methods for detection of DENV RNA or antigen in mosquitoes (Bangs et al. 2007, Chao et al. 2007, Chen et al. 2010, Tan et al. 2011, Muller et al. 2012, Voge et al. 2013) provide new opportunities for mosquito-based DENV surveillance, and studies focusing entirely or in part on dengue patient premises have shown promise with regards to the potential for linking DENV infections in Ae. aegypti to human dengue cases in space and time (Pinheiro et al. 2005, Urdaneta et al. 2005, García-Rejón et al. 2008, Guedes et al. 2010, Yoon et al. 2012). Despite a substantial effort – including monthly indoor backpack aspiration mosquito collections from a large number of homes (typically >125 per month) scattered over ~30 neighborhoods in Mérida City, and with nearly 5,800 Ae. aegypti females examined for presence of DENV RNA – we disappointingly failed to reproduce the strong linkage between DENV-infected Ae. aegypti females and dengue cases seen in our previous study from Mérida City that focused specifically on dengue patient premises (Table 2; García-Rejón et al. 2008). Instead, our study yielded the paradoxical result of infected mosquitoes being collected primarily in the winter and spring of 2010, whereas dengue cases peaked in the preceding late summer and fall of 2009 in the study neighborhoods (Table 1). The only other comparable study of which we are aware that used a similar approach, i.e., collection of adult mosquitoes in randomly selected “sentinel blocks”, similarly failed to find a statistically significant positive association between DENV infection rates of pooled mosquitoes and DENV infection rates in humans (Mendez et al. 2006).
The number of homes examined monthly was sufficient to detect a positive, significant correlation between the geometric mean number of Ae. aegypti females collected per home and dengue cases in the following month, but it likely was not sufficient for collection of DENV-infected females. Moreover, the study homes were selected at random within the study neighborhoods in southern and eastern Mérida City, and we cannot rule out the possibility that a more strategic selection of homes would have produced a different result. The selection of homes – including their number and locations – to target in mosquito-based DENV surveillance programs to ensure success in detecting building dengue outbreaks under a range of different DENV transmission intensities would seem to be a fruitful area for further research.
Greater numbers of Ae. aegypti females examined for DENV undoubtedly would have been beneficial, but there are indications that the sample size of examined females per se was not a critical issue in this study. First, we were able to detect a statistically significant, but unfortunately negative, correlation between monthly percentages of examined Ae. aegypti females infected with DENV or examined homes from which at least one DENV-infected Ae. aegypti female was collected and the number of dengue cases reported from the study neighborhoods 2 months later (Table 2). Second, a power analysis indicated that the number of examined females had greater than 90% power to detect the presence of DENV in Ae. aegypti females for each month during the study period except for May 2009 based on an overall DENV infection prevalence of 2%; for a DENV infection prevalence of 1%, the power ranged from approximately 80% to 99% except for August 2009 and May 2009 (75% and 19% power, respectively). Infection prevalence values for DENV in Ae. aegypti females in the 1–2% range were recorded in this study (Table 1) as well as previous studies from Mérida City (García-Rejón et al. 2008, 2011). Thus, our failure to document a positive correlation between mosquito-based DENV surveillance measures and dengue case numbers within the study neighborhoods likely resulted from an insufficient number of homes from which mosquitoes were collected rather than insufficient numbers of mosquitoes examined for DENV. Another potential criticism of the study is that it only covered a 15-month period. Although a study of extended duration certainly would have been beneficial, we point out that the study period during which mosquitoes were collected extended across times of the year with low and high DENV transmission intensity, and that the numbers of monthly reported dengue cases varied >100-fold for the study neighborhoods (range, 0–127) during our study period (Table 1).
Although the peaks for dengue cases in Mérida City occurred during the fall in both 2009 and 2010, substantial numbers of dengue cases were reported from January to April in both years, and we also collected DENV-infected Ae. aegypti females during all four of these months in 2010 (Table 1). This is intriguing because a study conducted in 1984 indicated that dengue cases were very rare in Mérida City during the cooler and drier part of the year from January to April (Loroño-Pino et al. 1993). Therefore, it would be interesting to examine the seasonal curves for dengue cases in Mérida City for selected years over the last decades to determine if DENV activity in the cooler and drier part of the year is a recently emerged phenomenon.
Acknowledgments
We thank Mildred López, Luis Flores, María Puc, Carlos Estrella, Roger Arana, Rosa Cetina, Lourdes Talavera, Jorge Morales, Elsy Rosado, Nubia Rivero, Yamili Chan, Fernando Nieves, Daniela Flores, Ana Uitz, Jesus Baak, Fernando Chan, Claudia Carrillo, Lucio Cabrera, Guadalupe Arevalo, Eric Diaz, Rita Pacho, Luis Solis, Chac Sosa, Shijani Aguilar, and Marilia Caamal of Universidad Autónoma de Yucatán for technical assistance with field and laboratory work; and the involved households for participating in the study and granting us permission to collect mosquitoes in their homes. The project was administered by the Fundación de la Universidad Autónoma de Yucatán, A. C. The study was supported by the Innovative Vector Control Consortium and by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (International Collaborations in Infectious Disease Research Program U01-AI-088647). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
References Cited
- Bangs MJ, Pudiantari R, Gionar YR. Persistence of dengue virus RNA in dried Aedes aegypti (Diptera: Culicidae) exposed to natural tropical conditions. J Med Entomol. 2007;44:163–167. doi: 10.1603/0022-2585(2007)44[163:podvri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chao DY, Davis BS, Chang GJJ. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J Clin Microbiol. 2007;45:584–589. doi: 10.1128/JCM.00842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Shu PY, Teng HJ, Su CL, Wu JW, Wang JH, Lin TH, Huang JH, Wu HS. Screening of dengue virus in field-caught Aedes aegypti and Aedes albopictus (Diptera: Culicidae) by one-step SYBR Green-based Reverse Transcriptase-Polymerase Chain Reaction assay during 2004–2007 in southern Taiwan. Vector-Borne Zoonotic Dis. 2010;10:1017–1025. doi: 10.1089/vbz.2008.0069. [DOI] [PubMed] [Google Scholar]
- Eisen L, Moore CG. Aedes (Stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range. J Med Entomol. 2013;50:467–478. doi: 10.1603/me12245. [DOI] [PubMed] [Google Scholar]
- García-Rejón JE, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores L, Rosado-Paredes ED, Rivero-Cardenas N, Nájera-Vázquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P, Lozano-Fuentes S, Elizondo-Quiroga D, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes DRD, Cordeiro MT, Melo-Santos MAV, Magalhaes T, Marques E, Regis L, Furtado AF, Ayres CFJ. Patient-based dengue virus surveillance in Aedes aegypti from Recife, Brazil. J Vector Borne Dis. 2010;47:67–75. [PubMed] [Google Scholar]
- Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Loroño-Pino MA, Farfán-Ale JA, Rosado-Paredes EP, Kuno G, Gubler DJ. Epidemic dengue 4 in the Yucatán, México, 1984. Rev Inst Med Trop Sao Paulo. 1993;35:449–455. doi: 10.1590/s0036-46651993000500011. [DOI] [PubMed] [Google Scholar]
- Loroño-Pino MA, García-Rejón JE, Machain-Williams C, Gomez-Carro S, Nuñez-Ayala G, Nájera-Vázquez MR, Losoya A, Aguilar L, Saavedra-Rodriguez K, Lozano-Fuentes S, Beaty MK, Black WC, IV, Keefe TJ, Eisen L, Beaty BJ. Towards a Casa Segura: A consumer product study of the effect of insecticide-treated curtains on Aedes aegypti and dengue virus infections in the home. Am J Trop Med Hyg. 2013;89:385–397. doi: 10.4269/ajtmh.12-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez F, Barreto M, Arias JF, Rengifo G, Munoz J, Burbano ME, Parra B. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am J Trop Med Hyg. 2006;74:678–683. [PubMed] [Google Scholar]
- Muller DA, Frentiu FD, Rojas A, Moreira LA, O’Neill SL, Young PR. A portable approach for the surveillance of dengue virus-infected mosquitoes. J Virol Meth. 2012;183:90–93. doi: 10.1016/j.jviromet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Pinheiro VCS, Tadei WP, Barros PMSS, Vasconcelos PFC, Cruz ACR. Detection of dengue virus serotype 3 by reverse transcription-polymerase chain reaction in Aedes aegypti (Diptera, Culicidae) captured in Manaus, Amazonas. Mem Inst Oswaldo Cruz. 2005;100:833–839. doi: 10.1590/s0074-02762005000800003. [DOI] [PubMed] [Google Scholar]
- Sall J, Creighton L, Lehman A. JMP Start Statistics. 3. Brooks/Cole; Belmont, CA: 2005. [Google Scholar]
- Scott TW, Morrison AC. Vector dynamics and transmission of dengue virus: Implications for dengue surveillance and prevention strategies. Vector dynamics and dengue prevention. Curr Top Microbiol Immunol. 2009;338:115–128. doi: 10.1007/978-3-642-02215-9_9. [DOI] [PubMed] [Google Scholar]
- Tan CH, Wong PSJ, Li MZI, Vythilingam I, Ng LC. Evaluation of the Dengue NS1 Ag Strip for detection of dengue virus antigen in Aedes aegypti (Diptera: Culicidae) Vector-Borne Zoonotic Dis. 2011;11:789–792. doi: 10.1089/vbz.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdaneta L, Herrera F, Pernalete M, Zoghbi N, Rubio-Palis Y, Barrios R, Rivero J, Comach G, Jimenez M, Salcedo M. Detection of dengue viruses in field-caught Aedes aegypti (Diptera: Culicidae) in Maracay, Aragua state, Venezuela by type-specific polymerase chain reaction. Infect Genet Evol. 2005;5:177–184. doi: 10.1016/j.meegid.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA. Quantifying the spatial dimension of dengue virus epidemic spread within a topical urban environment. PLoS Negl Trop Dis. 2010;4:e920. doi: 10.1371/journal.pntd.0000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voge NV, Sánchez-Vargas I, Blair CD, Eisen L, Beaty BJ. Detection of dengue virus NS1 antigen in infected Aedes aegypti using a commercially available kit. Am J Trop Med Hyg. 2013;88:260–266. doi: 10.4269/ajtmh.2012.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJM, Fansiri T, Jones JW, Morrison AC, Jarman RG, Nisalak A, Mammen MP, Thammapalo S, Srikiatkhachorn A, Green S, Libraty DH, Gibbons RV, Endy T, Pimgate C, Scott TW. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6:e1730. doi: 10.1371/journal.pntd.0001730. [DOI] [PMC free article] [PubMed] [Google Scholar]