Abstract
Antiretroviral therapy (ART) is associated with improved kidney function; however, the nucleotide reverse transcriptase inhibitor (NRTI) tenofovir disoproxil fumarate (TDF) has been associated with decreased kidney function and proteinuria.
Methods
We examined changes in urine protein:creatinine (UPCR) and albumin:creatinine (UACR) ratios in 245 ART-naïve participants in A5202 randomized in a substudy to blinded NRTI (abacavir/lamivudine, ABC/3TC, n=124 or TDF/emtricitabine, TDF/FTC, n=121) with open-label protease inhibitor (PI) atazanavir/ritonavir (ATV/r) or non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV).
Results
At baseline, 18% of participants had clinically significant proteinuria (UPCR ≥ 200 mg/g) and 11% had clinically significant albuminuria (UACR ≥ 30 mg/g). The prevalence of clinically significant proteinuria and albuminuria decreased from baseline to week 96 in all treatment groups. In intention-to-treat analyses, there was a significant effect of NRTI component on fold-change in UPCR (p=0.011) and UACR (p=0.018) from baseline to week 96, with greater improvements in participants randomized to ABC/3TC. There was no significant effect of NNRTI/PI component on fold-change in UPCR (p=0.23) or UACR (p=0.88), and no significant interactions between NRTI and NNRTI/PI components.
Conclusion
In this pre-specified secondary analysis, ART initiation was associated with improvements in proteinuria and albuminuria, with significantly greater improvements in participants randomized to ABC/3TC versus TDF/FTC. These are the first data from a randomized trial to suggest that initiation of TDF/FTC may not be associated with the same degree of improvement in proteinuria and albuminuria that have been reported with other regimens. Future studies should consider the long-term clinical significance of these findings.
Introduction
HIV-infected individuals are at increased risk for chronic kidney disease (CKD), as indicated by a decrease in estimated glomerular filtration rate (eGFR) or an increase in urinary protein excretion, and the presence of CKD is associated with both AIDS and non-AIDS events.1-5 Higher urinary protein excretion, ranging from overt proteinuria to microalbuminuria, has been associated with increased risk of mortality in HIV-infected individuals, independent of eGFR.2-5 Although multiple studies have demonstrated improvements in eGFR or proteinuria with antiretroviral therapy (ART),6-8 in observational studies cumulative exposure to the nucleotide reverse transcriptase inhibitor (NRTI) tenofovir disoproxil fumarate (TDF) and the ritonavir-boosted protease inhibitors (PI) atazanavir/ritonavir (ATV/r) and lopinavir/r have also been associated with low eGFR, and in the case of TDF, with increases in proteinuria.9-11 Randomized trials comparing TDF/emtricitabine (TDF/FTC) and abacavir/lamivudine (ABC/3TC) in ART-naïve adults have not demonstrated a higher risk of developing low eGFR among participants randomized to initiate ART containing TDF/FTC, and in the ASSERT study, the change in albuminuria was also similar between treatment arms over 48 weeks of therapy. 12,13,14 We evaluated the effects of NRTI and NNRTI/PI components on changes in proteinuria and albuminuria over 96 weeks among ART-naïve participants in the metabolic sub-study of a randomized HIV treatment trial.
Methods
AIDS Clinical Trials Group (AGTG) Study A5202 randomized ART-naïve participants to a blinded NRTI component (ABC/3TC or TDF/FTC) and to an open-label PI or NNRTI component (ATV/r or efavirenz, EFV).14 Intention to enroll in the metabolic substudy A5224s was a stratification factor for randomization in the parent trial. A pre-specified secondary objective of A5224s was to compare the change in proteinuria and albuminuria in participants randomized to TDF/FTC versus ABC/3TC, and in participants randomized to open-label ATV/r versus EFV.15
ACTG Studies A5202 and A5224s were supported by the National Institutes of Health. Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline provided the study medications. The decision to publish the manuscript was solely that of the academic authors.
Study Participants
Inclusion criteria for the parent trial A5202 (n=1858) included age ≥ 16 years, HIV-1 RNA > 1000 copies/mL, and Cockcroft-Gault creatinine clearance > 60 mL/min. The protocol did not initially exclude participants with hepatitis B virus (HBV) co-infection but was later amended to exclude participants with a positive HBV surface antigen within six months of study entry.14 Additional exclusion criteria for the metabolic sub-study A5224s (n=269) included endocrine disease (diabetes mellitus and Cushing’s syndrome), uncontrolled thyroid disease or hypogonadism, and use of glucocorticoids, anabolic steroids, growth hormone, or osteoporosis medications. The institutional review board at each participating site approved the study protocol, and all participants provided written informed consent. The study is registered with clinicaltrials.gov, number NCT00118898.
Study Measurements and Definitions
In A5224s, fasting urine and serum samples were collected at study entry (baseline), week 24, week 48, and week 96; additional specimens were collected at 48-week intervals until 96 weeks after the last subject was enrolled in A5202. Urine protein, albumin, and creatinine were measured centrally at Quest Diagnostics. Urine protein and creatinine were measured using colorimetric assays (Pyragallol red and Jaffe, respectively), and urine albumin was measured using an immunoturbidimetric assay for calculation of the urine protein:creatinine (UPCR) and albumin:creatinine (UACR) ratios; all assays were performed on a Beckman Coulter/Olympus platform. Clinically significant proteinuria and albuminuria were defined based on clinical guidelines as a UPCR ≥ 200 mg/g or a UACR ≥ 30 mg/g.16 Dipstick urinalysis and fasting serum creatinine and glucose were measured locally. For the purposes of this analysis, the CKD Epidemiology Consortium (CKD-EPI) equation was used to re-calculate eGFR from serum creatinine, in order to provide a more accurate estimate of this important potential confounder. The CKD-EPI eGFR equation has demonstrated the best overall performance in HIV-positive adults, as compared to a gold standard measure of GFR. 17, 18 Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated from fasting insulin and glucose. HBV and hepatitis C virus (HCV) co-infections were defined by positive HBV surface antigen or HCV antibody, respectively.
Statistical Analysis
A pre-specified secondary objective of A5224s was to compare changes in UPCR and UACR from baseline to week 96 between pooled, randomized NRTI components (TDF/FTC versus ABC/3TC, with NRTI/PI components combined) and between NNRTI and PI components (ATV/r versus EFV, with NRTIs combined). Primary analyses were performed using intent-to-treat principles based on randomized treatment assignment in which all available data were used and modifications to randomized treatment and missing values were ignored.
In February 2008, after a median follow-up of 97 weeks, the Data Safety and Monitoring Board (DSMB) recommended unblinding the NRTI component for A5202 participants with screening HIV-RNA ≥ 100,000 copies/mL; participants receiving ABC/3TC were permitted to modify their regimen.14 To account for changes in therapy, supplemental as-treated analyses were performed in which values were censored after a change in the randomized NRTI component (when comparing NRTI components) or NNRTI/PI component (when comparing NNRTI/PI components). Post hoc sensitivity analyses included only participants who had achieved virologic suppression (HIV-RNA < 50 copies/mL) at week 96.
Because UPCR and UACR were not normally distributed, data were log10 transformed prior to analysis. For ease of interpretation, the exponential of the estimated mean change in the transformed data was used to obtain the estimated mean fold change; a mean fold-change = 1 would correspond to no change in mean UPCR or UACR from baseline, while a mean fold-change < 1 would correspond to a decrease in mean UPCR or UACR from baseline. The unadjusted analysis of the effects of NRTI component and NNRTI/PI component on changes in UPCR or UACR were evaluated separately using two-sample t-tests. Linear regression was used to initially test for interactions between NRTI and NNRTI/PI components. P-values below 0.05 (< 0.10 for interactions) were considered statistically significant. To assess baseline characteristics independently associated with changes in UPCR or UACR, multivariable linear regression models were constructed using backwards selection. Baseline characteristics considered for inclusion were age, sex, race/ethnicity, HIV-1 RNA, CD4 cell count (CD4), eGFR, presence of dipstick proteinuria, systolic and diastolic blood pressure, HBV or HCV co-infection, HOMA-IR, and body mass index (BMI). All baseline variables with univariate p-value <0.20 were considered for inclusion in the models, and variables with a p-value <0.05 were retained; NRTI component and NNRTI/PI component were retained regardless of p-value. Analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Among 269 participants enrolled in the A5224s substudy, 245 participants with baseline UPCR and/or UACR contributed data to this analysis. Compared to excluded participants, included participants tended to be older and were less likely to be white, although these differences were not significant (p=0.14 and 0.07, respectively). Included and excluded participants were not significantly different with respect to other baseline characteristics (all p > 0.3). 181 participants with available data for UPCR and/or UACR at the 96-week visit were included in the primary intention to treat analyses. These participants had marginally higher mean body weight and diastolic blood pressure (p=0.066 and 0.052, respectively), but were not significantly different with respect to baseline characteristics from the 64 participants who did not contribute to the primary analysis (all other p>0.12).
Baseline characteristics of included participants were well balanced across the treatment groups (Table 1). The mean age of included participants was 39 years, 85% were male, and 35% were of non-Hispanic black race. Only 19% had an eGFR < 90mL/min/1.73m2, including two participants who met entry criteria based on creatinine clearance > 60mL/min but who had CKD-EPI eGFR < 60 mL/min/1.73m2. Dipstick proteinuria ≥ trace was detected in 29% of participants and was balanced across the treatment groups. The median UPCR (95 mg/g) and UACR (4.75 mg/g) were within the normal range (<200mg/g and <30mg/g, respectively).
Table 1. Baseline Characteristics of 245 Included A5224s Study Participants.
| Variable | TDF/FTC
|
ABC/3TC
|
Total | ||
|---|---|---|---|---|---|
| EFV | ATV/r | EFV | ATV/r | ||
| n=65 | n=56 | n=64 | n=60 | n=245 | |
| Age, years | 40 (10) | 38 (10) | 39 (10) | 38 (10) | 39 (10) |
| Men | 55 (84%) | 48 (86%) | 51 (80%) | 54 (90%) | 208 (85%) |
| Race or ethnicity | |||||
| Black, non-Hispanic | 21 (32%) | 20 (36%) | 19 (30%) | 26 (43%) | 86 (35%) |
| White, non-Hispanic | 34 (52%) | 21 (38%) | 29 (45%) | 25 (42%) | 109 (44%) |
| Hispanic | 8 (12%) | 12 (21%) | 14 (22%) | 8 (13%) | 42 (17%) |
| Other | 2 (3%) | 3 (5%) | 2 (3%) | 1 (2%) | 8 (3%) |
| Weight, kg | 77 (16) | 80 (18) | 77 (15) | 78 (15) | 78 (16) |
| Body mass index, kg/m2 | 24.8 (4.1) | 26.1 (5.6) | 25.6 (4.8) | 25.5 (4.5) | 25.5 (4.7) |
| CD4 cell count, cells/μL | 248 (156) | 220 (149) | 230 (173) | 233 (182) | 233 (165) |
| HIV-RNA, log copies/mL* | 4.6 (0.7) | 4.7 (0.7) | 4.6 (0.6) | 4.7 (0.7) | 4.6 (0.7) |
| <100,000 copies/mL | 52 (80%) | 44 (79%) | 53 (83%) | 45 (75%) | 194 (79%) |
| Hepatitis B co-infection | 4 (6%) | 0 (0%) | 3 (5%) | 1 (2%) | 8 (3%) |
| Hepatitis C co-infection | 5 (8%) | 2 (4%) | 8 (13%) | 7 (12%) | 22 (9%) |
| Systolic blood pressure, mmHg | 120 (16) | 120 (9) | 123 (14) | 120 (13) | 121 (13) |
| Diastolic blood pressure, mmHg | 75 (9) | 75 (7) | 77 (10) | 76 (11) | 76 (9) |
| HOMA-IR | 1.2 (1.7) | 1.6 (2.2) | 1.6 (1.5) | 1.5 (1.5) | 1.5 (1.7) |
| eGFR, mL/min/1.732 | 107.1 (15.0) | 106.5 (21.4) | 106.1 (19.5) | 110.0 (16.4) | 107.4 (18.1) |
| < 90** | 9 (14%) | 15 (27%) | 15 (24%) | 7 (12%) | 46 (19%) |
| Dipstick proteinuria | 16 (25%) | 18 (33%) | 21 (33%) | 16 (27%) | 71 (29%) |
| Urine protein:creatinine, mg/g | (n=63) | (n=52) | (n=64) | (n=55) | (n=234) |
| Median (IQR) | 87 (67, 132) | 86 (61, 156) | 98 (70.50, 143.50) | 101 (75, 170) | 95 (67, 146) |
| ≥ 200mg/g | 10 (16%) | 10 (19%) | 11 (17%) | 12 (22%) | 43 (18%) |
| Urine albumin:creatinine, mg/g | (n=64) | (n=54) | (n=64) | (n=60) | (n=242) |
| Median (IQR) | 4.60 (3.10, 9.85) | 4.65 (3.10, 11.60) | 4.90 (3.10, 11.35) | 4.80 (3.20, 14.05) | 4.75 (3.20, 10.00) |
| ≥ 30mg/g | 6 (9%) | 6 (11%) | 5 (8%) | 10 (17%) | 27 (11%) |
Urine protein: creatinine and albumin: creatinine ratios are presented as median (interquartile range, IQR). All other data are presented as mean (standard deviation) or as number (percent). TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ABC/3TC, abacavir/lamivudine; EFV, efavirenz; ATV/r, atazanavir/ritonavir. Hepatitis B co-infection, surface antigen positive; Hepatitis C co-infection, antibody positive; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate as calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation; urine dipstick proteinuria, any non-negative protein result on baseline urinalysis.
HIV-1 RNA was measured at screening.
Two participants who met entry criteria based on Cockcroft-Gault creatinine clearance > 60mL/min but who had a CKD-EPI eGFR < 60 mL/min/1.73m2. Baseline data were missing for ≤ 3 participants for the following characteristics: Hepatitis B and C co-infection, eGFR, dipstick proteinuria, and urine albumin:creatinine ratio. Baseline data for urine protein:creatinine ratio were missing for 11 participants.
Effect of NRTI assignment on changes in UPCR and UACR
The effects of treatment assignment on the change in UPCR and UACR are summarized in Table 2. There were no significant interactions between the NRTI and NNRTI/PI components (p=0.33 for analysis of change in UPCR, p=0.91 for UACR). In the primary intention-to-treat analyses, there was significantly greater fold-change in both UPCR and UACR in those randomized to ABC/3TC versus TDF/FTC (Figure 1, panels A and C). The mean fold-change in UPCR from baseline to week 96 among 84 participants randomized to ABC/3TC was 0.80 (95% CI 0.70, 0.91), corresponding to a significant decrease in UPCR from baseline. Among 83 participants randomized to TDF/FTC, the mean fold-change in UPCR was 1.02 (95% CI 0.89, 1.17), corresponding to no significant change in UPCR from baseline to week 96. Overall, there was a 21.7% difference (Δ) in mean fold-change UPCR with ABC/3TC compared to TDF/FTC (p=0.011), corresponding to a significantly greater decrease in UPCR from baseline to week 96 in participants randomized to ABC/3TC. Results were similar in as-treated analysis in which values were censored after a change in the randomized NRTI component.
Table 2. Treatment assignment and differences in fold-change urine protein:creatinine and albumin:creatinine ratios.
| NRTI effect | Urine protein:creatinine ratio, mg/g
|
Urine albumin:creatinine ratio, mg/g
|
||
|---|---|---|---|---|
| n | Δ, ABC/3TC versus TDF/FTC | n | Δ, ABC/3TC versus TDF/FTC | |
| Intention-to-treat, Week 0 to 96 | 167 | -21.7% (95% CI -35.1%, -5.5%); p=0.011 | 178 | -28.8% (95% CI -46.2%, -5.8%); p=0.018 |
| As-treated, Week 0 to 96 | 140 | -20.7% (95% CI -35.4%, -2.6%); p=0.027 | 149 | -24.8% (95% CI -44.1%, 1.2%); p=0.060 |
| Stratified analysis, Week 0 to 96 | ||||
| ATV/r arm | 79 | -28.9% (95% CI -45.8%, -6.6%) | 88 | -27.7% (95% CI -51.6%, 8.1%) |
| EFV arm | 88 | -14.4% (95% CI -33.9%, 10.8%) | 90 | -29.9% (95% CI -52.9%, 4.1%) |
| Sensitivity analysis, Week 0 to 96 | ||||
| HIV-RNA<50copies/mL* | 144 | - 18.1% (95% CI -33.4%, 0.7%); p=0.058 | 154 | -26.5% (-46.0%, -0.1%); p=0.049 |
| Intention to treat, Week 0 to 48 | 191 | -16.5% (95% CI -29.9%, -0.6%); p=0.043 | 194 | -4.4% (95% CI -27.3%, 25.6%); p=0.74 |
| NNRTI/PI effect | n | Δ, ATV/r versusEFV | n | Δ, ATV/r versusEFV |
| Intention-to-treat, Week 0 to 96 | 167 | -11.0% (95% CI -26.5%, 7.7%); p=0.23 | 178 | -2.2% (95% CI -26.4%, 30.0%); p=0.88 |
| As-treated, Week 0 to 96 | 143 | -9.8 (95% CI -26.7%, 10.9%); p=0.32 | 154 | -11.6% (95% CI -34.5%, 19.4%); p=0.42 |
| Stratified analysis, Week 0 to 96 | ||||
| TDF/FTC | 83 | -2.2% (95% CI -25.1%, 27.7%) | 86 | -2.7% (95% CI -35.2%, 46.0%) |
| ABC/3TC | 84 | -18.7% (95% CI-37.6%, 6.0%) | 92 | 0.5% (95% CI -32.1%, 48.7%) |
| Sensitivity analysis, Week 0 to 96 | ||||
| HIV-RNA<50copies/mL* | 144 | -6.4% (-24.0%, 15.4%); p=0.54 | 154 | 0.8% (-26.2%, 37.6%); p=0.96 |
| Intention to treat, Week 0 to 48 | 191 | -11.1% (-25.5%, 5.9%); p=0.19 | 194 | -7.6% (-29.6%, 21.5%); p=0.57 |
ABC/3TC, abacavir/lamivudine; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ATV/r, atazanavir/ritonavir; EFV, efavirenz. In the as-treated analysis, participants were censored after a change in the assigned NRTI (for the analysis of NRTI effect) or NNRTI/PI (for the analysis of NNRTI/PI effect). Because of the limited sample size within strata, the results of the stratified analyses are provided for descriptive purposes only.
Analyses in this table were planned a priori, with the exception of the sensitivity analyses including only participants who achieved HIV-RNA < 50 copies/mL at week 96.
Figure 1.
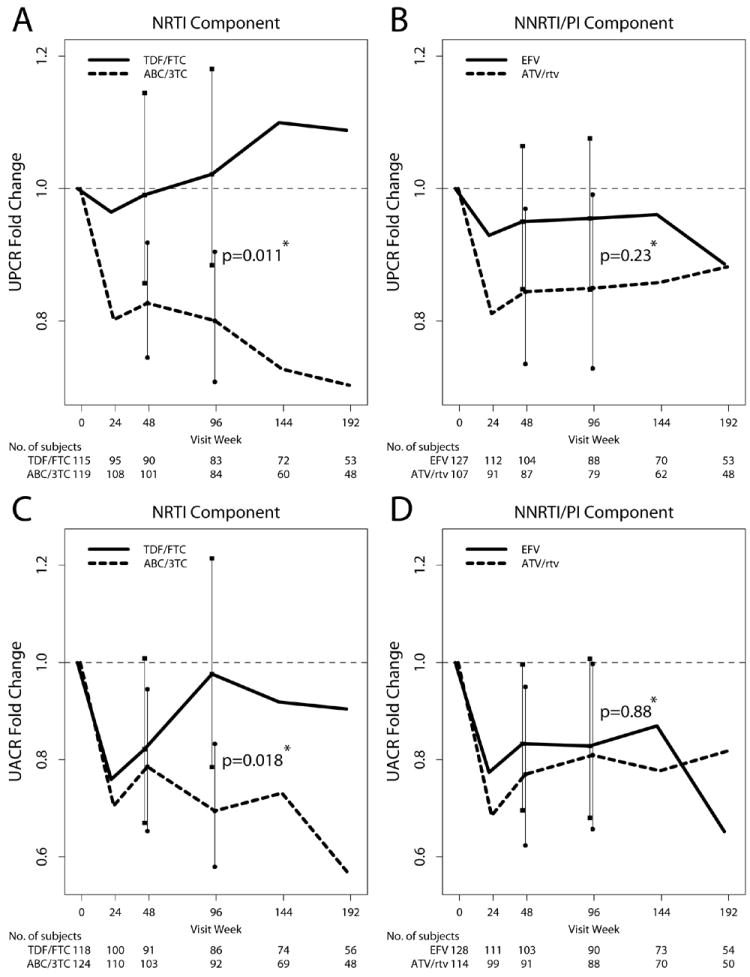
Mean fold-change (95% confidence interval) in urine protein:creatinine ratio (panels A and B) and urine albumin: creatinine ratio (panels C and D) among 245 participants randomized to initiate antiretroviral therapy in A5224s. Mean fold-change in each ratio is compared between participants randomized to abacavir/ lamivudine versus TDF/ emtricitabine (A and C) and between participants randomized to open-label EFV versus ATV/ ritonavir (B and D). Data are presented through week 192 for descriptive purposes; comparative statistics were performed at week 48 and week 96. *two-sample t-test.
In the primary intention-to-treat analysis for UACR, the mean fold-change from baseline to week 96 among 92 participants randomized to ABC/3TC was 0.69 (95% CI 0.57, 0.84), corresponding to a significant decrease in UACR from baseline. Among 86 participants randomized to TDF/FTC, the mean fold-change in UACR was 0.98 (95% CI 0.80, 1.19), corresponding to no significant change in UACR from baseline to week 96. Overall, there was a 28.8% difference in mean fold-change UACR with ABC/3TC compared to TDF/FTC (p=0.018), corresponding to a significantly greater decrease in UACR from baseline to week 96 in participants randomized to ABC/3TC. Results for the change in UACR were similar, although no longer statistically significant, in as-treated analysis in which values were censored after a change in the randomized NRTI component.
Although there were no significant interactions between the NRTI and NNRTI/PI components, pre-specified intention-to-treat analyses of NRTI effect were performed within each NNRTI/PI arm for descriptive purposes. The results were qualitatively similar to the results of the primary intention-to-treat analyses, with greater mean fold-changes in UPCR and UACR in participants randomized to ABC/3TC versus TDF/FTC, regardless of the NNRTI/PI component (Table 2).
There was no significant difference in mean fold-change UPCR or UACR between participants with and without virologic suppression (HIV-RNA < 50 copies/mL) at 96 weeks (data not shown; p>0.8 for both). The proportion of included participants who achieved virologic suppression did not differ significantly between those randomized to ABC/3TC (78/93, 83.9%) versus TDF/FTC (79/88, 89.8%; Fisher’s exact p=0.28). In post hoc sensitivity analyses, we evaluated the effect of treatment assignment on the changes in UPCR and UACR including only those participants who achieved virologic suppression at 96 weeks. In these analyses, the difference in mean fold-change UPCR was similar in magnitude and direction to the primary analysis, but was not statistically significant. The difference in mean fold-change UACR was marginally significant and similar in magnitude and direction to the primary analysis.
In a separate intention-to-treat analysis at week 48, the effect of NRTI component on mean fold-change in UPCR was significant but smaller in magnitude than at week 96 (p=0.043). The initial decline in UACR from baseline to week 24 appears to be similar in participants randomized to ABC/3TC versus TDF/FTC (Figure 1, panel C), and there was no statistically significant effect of NRTI component on the change in UACR at week 48 (p=0.74).
Effect of NNRTI/PI assignment on changes in UPCR and UACR
There were no significant effects of open-label NNRTI/PI component on the change in UPCR or UACR from baseline to week 96 (Figure 1, panels B and D). The mean fold-change in UPCR from baseline to week 96 among 79 participants randomized to ATV/r (0.85, 95% CI 0.74, 0.98) was not significantly different than that observed among 88 participants randomized to EFV (0.96, 95% CI 0.84, 1.09; p=0.23). The mean fold-change in UACR among 88 participants randomized to ATV/r (0.81, 95% CI 0.66, 0.99) was not significantly different than that observed among 90 participants randomized to EFV (0.83, 95% CI 0.68, 1.01; p=0.88).
Adjusted analyses
In the multivariable intention-to-treat analyses, there remained a significantly greater fold-change in UPCR (Table 3) and UACR (Table 4) at week 96 in participants randomized to ABC/3TC versus TDF/FTC. After adjusting for NRTI and NNRTI/PI components, higher eGFR and HOMA-IR were associated with a decrease in UPCR from baseline to week 96; higher baseline CD4 count and absence of dipstick proteinuria were associated with an increase in UPCR. In the multivariable analysis for change in UACR, screening HIV-RNA ≥ 100,000 copies/mL and higher baseline eGFR and HOMA-IR were associated with a decrease in UACR from baseline to week 96; higher baseline CD4 and absence of dipstick proteinuria were associated with an increase in UACR. Traditional CKD risk factors, including older age, black race, and hepatitis co-infection, were not significantly associated with changes in UPCR or UACR from baseline to week 96.
Table 3. Baseline characteristics associated with change in log10-transformed urine protein:creatinine ratio, Week 0 to Week 96.
| Variable | Univariate Analyses
|
Multivariate Analyses
|
||
|---|---|---|---|---|
| Estimated mean fold-change (95%CI) | p-value | Estimated mean fold-change (95%CI) | p-value | |
| Assigned to ABC/3TC (vs TDF/FTC) | 0.78 (0.65, 0.95) | 0.011 | 0.76 (0.69, 1.01) | 0.001 |
| Assigned to ATV/r (vs EFV) | 0.89 (0.74, 1.08) | 0.23 | 0.94 (0.80, 1.12) | 0.50 |
| Age (per 1 year) | 1.00 (0.99, 1.01) | 0.78 | ||
| Male sex | 0.95 (0.72, 1.25) | 0.71 | ||
| Race or ethnicity | ||||
| White, non-Hispanic | Reference | 0.26 | ||
| Black, non-Hispanic | 0.84 (0.68, 1.05) | |||
| Hispanic | 0.87 (0.67, 1.14) | |||
| Body mass index (per 1 kg/m2) | 1.00 (0.98, 1.02) | 0.87 | ||
| CD4 cell count (per 50 cells/μL) | 1.06 (1.02, 1.09) | <0.001 | 1.06 (1.03, 1.09) | <0.001 |
| HIV-RNA (per 1 log10 copies/mL) | 0.86 (0.75, 0.98) | 0.028 | ||
| HIV-RNA ≥ 100,000 copies/mL* | 0.84 (0.69, 1.02) | 0.074 | ||
| Hepatitis B or C co-infection | 0.77 (0.57, 1.06) | 0.11 | ||
| Systolic blood pressure (per 10 mmHg) | 0.98 (0.91, 1.06) | 0.58 | ||
| Diastolic blood pressure (per 10 mmHg) | 1.01 (0.91, 1.12) | 0.88 | ||
| HOMA-IR (per unit higher) | 0.94 (0.89, 0.98) | 0.010 | 0.94 (0.90, 0.98) | 0.006 |
| eGFR (per 10 mL/min/1.732) | 0.96 (0.91, 1.01) | 0.092 | 0.94 (0.89, 0.98) | 0.004 |
| Negative dipstick protein | 1.57 (1.29, 1.92) | <0.001 | 1.43 (1.19, 1.72) | <0.001 |
ABC/3TC, abacavir/ lamivudine; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ATV/r, atazanavir/ritonavir; EFT, efavirenz. Hepatitis B co-infection, surface antigen positive; Hepatitis C co-infection, antibody positive; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate as calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.
HIV-1 RNA was measured at screening. The estimated mean change in log10-transformed urine protein:creatinine ratio was exponentiated to obtain the estimated mean fold change; values < 1 correspond to a decrease in mean urine protein:creatinine ratio from baseline. Covariates with univariate p-value <0.20 were considered for inclusion in the multivariate model, and variables with a p-value <0.05 were retained; treatment assignment variables were retained regardless of p-value.
Table 4. Baseline characteristics associated with change in log10-transformed urine albumin:creatinine ratio, Week 0 to Week 96.
| Variable | Univariate Analyses
|
Multivariate Analyses
|
||
|---|---|---|---|---|
| Estimated mean fold-change (95%CI) | p-value | Estimated mean fold-change (95%CI) | p-value | |
| Assigned to ABC/3TC (vs TDF/FTC) | 0.71 (0.54, 0.94) | 0.018 | 0.69 (0.54, 0.88) | 0.003 |
| Assigned to ATV/r (vs EFV) | 0.98 (0.74, 1.30) | 0.88 | 1.04 (0.81, 1.34) | 0.74 |
| Age (per 1 year) | 1.01 (1.00, 1.03) | 0.10 | ||
| Male | 0.87 (0.58, 1.29) | 0.48 | ||
| Race or ethnicity | ||||
| White, non-Hispanic | Reference | 0.37 | ||
| Black, non-Hispanic | 0.79 (0.57, 1.10) | |||
| Hispanic | 0.94 (0.63, 1.40) | |||
| Body mass index (per 1 kg/m2) | 1.01 (0.98, 1.04) | 0.56 | ||
| CD4 cell count (per 50 cells/μL) | 1.08 (1.03, 1.12) | 0.001 | 1.07 (1.03, 1.11) | 0.001 |
| HIV-RNA (per 1 log10 copies/mL) | 0.78 (0.64, 0.96) | 0.017 | ||
| HIV-RNA ≥ 100,000 copies/mL* | 0.67 (0.51, 0.90) | 0.007 | 0.72 (0.56, 0.93) | 0.013 |
| Hepatitis B or C co-infection | 0.76 (0.48, 1.20) | 0.24 | ||
| Systolic blood pressure (per 10 mmHg) | 0.96 (0.86, 1.07) | 0.46 | ||
| Diastolic blood pressure (per 10 mmHg) | 1.03 (0.88, 1.20) | 0.74 | ||
| HOMA-IR (per unit higher) | 0.93 (0.86, 1.00) | 0.064 | 0.92 (0.86, 0.98) | 0.014 |
| eGFR (per 10 mL/min/1.732) | 0.90 (0.83, 0.97) | 0.005 | 0.89 (0.83, 0.95) | 0.001 |
| Negative dipstick protein | 1.85 (1.38, 2.48) | <0.001 | 1.67 (1.26, 2.20) | <0.001 |
ABC/3TC, abacavir/ lamivudine; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ATV/r, atazanavir/ritonavir; EFT, efavirenz. Hepatitis B co-infection, surface antigen positive; Hepatitis C co-infection, antibody positive; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate as calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.
HIV-1 RNA was measured at screening. The estimated mean change in log10-transformed urine albumin:creatinine ratio was exponentiated to obtain the estimated mean fold change; values < 1 correspond to a decrease in mean urine albumin:creatinine ratio from baseline. Covariates with univariate p-value <0.20 were considered for inclusion in the multivariate model, and variables with a p-value <0.05 were retained; treatment assignment variables were retained regardless of p-value.
Effect of treatment assignment in participants with significant proteinuria or albuminuria
At baseline, 18% of included participants had clinically significant proteinuria (UPCR ≥ 200 mg/g) and 11% had clinically significant albuminuria (UACR ≥ 30 mg/g) (Table 1). Only four participants had a baseline UPCR ≥ 1 g/g, consistent with overt proteinuria of more than 1 g/24 hours. These four participants and one additional participant had a baseline UACR ≥ 300 mg/g, signifying macroalbuminuria; by chance, all five of these participants with overt proteinuria and/or macroalbuminuria were randomized to open-label ATV/r. Overall, there was a decrease in the prevalence of clinically relevant proteinuria and albuminuria from baseline to week 96 in all treatment groups. Among participants with data available at baseline and at week 96, the prevalence of UPCR ≥ 200 mg/g decreased from 18.0% to 9.6%, and the prevalence of UACR ≥ 30 mg/g decreased from 11.2% to 5.1% (Exact McNemar’s test p=0.013 for both comparisons).
In 21 of 30 participants with baseline UPCR ≥ 200mg/g, the week 96 UPCR was < 200 mg/g. These 21 cases were balanced across the 4 treatment arms. New onset proteinuria ≥ 200 mg/g was observed in 7 of 137 participants with baseline UPCR < 200 mg/g. Five of these participants were randomized to TDF/FTC (2 with EFV and 3 with ATV/r) and 2 were randomized to ABC/3TC (one in each NNRTI/PI arm). In 14 of 20 participants with baseline UACR ≥ 30 mg/g, the week 96 UACR was normal. Five of these participants were randomized to TDF/FTC (4 with EFV and 1 with ATV/r) and 9 were randomized to ABC/3TC (3 with EFV and 6 with ATV/r). New onset albuminuria ≥ 30 mg/g was observed in 3 of 158 participants with normal baseline UACR, all of whom were randomized to TDF/FTC (2 with EFV and 1 with ATV/r). The numbers of participants with clinically significant proteinuria and albuminuria were too small to allow statistical comparisons.
Discussion
In this pre-specified secondary analysis of the metabolic substudy of a randomized clinical trial, we observed significantly greater improvements in proteinuria and albuminuria over 96 weeks among ART-naïve adults randomized to ART containing ABC/3TC versus TDF/FTC. This effect remained significant after adjusting for potential imbalances in randomization. Assignment to open-label ATV/r versus EFV was not associated with a significant difference in proteinuria or albuminuria over 96 weeks of treatment.
These results are consistent with previous clinical trials demonstrating improvements in markers of CKD with the initiation of ART,6-8 even with regimens that include TDF, and it is reassuring that the prevalence of clinically significant proteinuria and albuminuria decreased in all treatment arms. Although observational studies have demonstrated an association between cumulative exposure to TDF and increased incidence of proteinuria,10 the change in albuminuria over 48 weeks did not differ significantly between participants randomized to TDF/FTC versus ABC/3TC in the ASSERT study. 13 While we did not observe an increase in the prevalence or level of proteinuria or albuminuria over 96 weeks of therapy with TDF/FTC, the pattern of change in UACR suggests that an early improvement with ART initiation was followed by a rebound in albuminuria with longer exposure to TDF/FTC (Figure 1, panel C). This result is consistent with the previously reported association between cumulative exposure to TDF and increased incidence of proteinuria as assessed by dipstick urinalysis, which largely detects albuminuria.10 While it is possible that our results reflect an initial improvement in HIV-related kidney injury with virologic suppression, followed by the development of proximal tubular injury or dysfunction in the setting of TDF/FTC, the current study was not designed to evaluate potential mechanisms. Similarly, we cannot explain the apparent differences in pattern and magnitude of change in proteinuria and albuminuria, which may indicate different types of kidney injury. Future studies should include additional biomarkers that can help to distinguish between HIV-related and ART-related kidney injury. Finally, it is notable that clinically significant proteinuria and albuminuria were observed in fewer than 20% of participants at baseline. Nonetheless, even small elevations in proteinuria and albuminuria, such as those observed in this study, have been associated with clinically significant differences in cardiovascular disease and all-cause mortality.2-5
While recent studies have suggested that cumulative exposure to ATV/r is associated with decreased eGFR,9-11 we did not observe a differential effect of ATV/r versus EFV on changes in proteinuria or albuminuria, other indicators of CKD. It is possible that this lack of difference reflects a deleterious effect of EFV, consistent with a previously reported association between NNRTI use and higher albuminuria.19 Of note, by chance all subjects with high levels of proteinuria and albuminuria were randomized to the ATV/r arm. We have previously reported greater improvements in albuminuria in individuals with higher baseline levels.20 It is possible that imbalance in baseline proteinuria and albuminuria could have masked a true benefit of EFV over ATV/r. Nonetheless, the number of participants with high levels of proteinuria or albuminuria was small, and the effect of outliers should be minimized by the log transformation of UPCR and UACR data. Of note, the distribution of proteinuria and albuminuria was similar between the NRTI treatment arms, the primary focus of this analysis.
Although this is the first study to demonstrate differential effects of TDF/FTC and ABC/3TC on proteinuria and albuminuria using data from a randomized trial, several additional limitations should be acknowledged. First, although UPCR and UACR measurements were centralized, our analysis was based on a single measurement of proteinuria and albuminuria at each time-point. It is reassuring that the observed effects of NRTI component were in the same direction, albeit less significant, at 48 weeks. Second, the study was not specifically powered for this secondary analysis, and missing data at week 96 further limited our sample size for the primary intention-to-treat analyses. Although there were no statistically significant differences with respect to baseline characteristics, it is also possible that participants with missing data at week 96 differed from participants who were included in the primary analysis. Third, our analysis was complicated by the unblinding of the NRTI component and switches from ABC/3TC to TDF/FTC in participants with high screening HIV-RNA following the DSMB report in February 2008. Results of the as-treated analyses with data censored after a change in NRTI were qualitatively similar, although the differences in albuminuria reduction were no longer statistically significant. Fourth, while it is reassuring that proteinuria and albuminuria did not worsen in participants randomized to TDF/FTC, our results are short term when viewed in the context of lifelong ART. By design, follow-up of study participants ended when the last enrolled participant reached week 96; as a result there is a decreasing sample size on visits after this time point. Particularly given the low baseline prevalence of clinically significant proteinuria/ albuminuria or decreased eGFR, these short-term results do not exclude the possibility of worsening proteinuria or albuminuria with more prolonged exposure. We did observe a more significant effect of NRTI assignment at week 96 than at week 48, which could suggest a cumulative effect of treatment; unfortunately the small sample size after week 96 did not allow us to explore this further. Fifth, although we did not detect a significant interaction between NRTI and NNRTI/PI component, the current analysis likely only had adequate power to detect large treatment interactions. Finally, our results may not be generalizable to patients with baseline creatinine clearance <60 mL/min or with uncontrolled diabetes, who were excluded from the current study and who are at greatest risk for adverse renal and cardiovascular outcomes. In contrast, more than one-third of participants were of self-reported black race, another important risk factor for CKD. There were no significant interactions between NRTI component and race, age, or blood pressure, suggesting that the observed NRTI effects were not significantly influenced by these traditional CKD risk factors.
Our data are consistent with previous studies demonstrating improvements in proteinuria or albuminuria following the initiation of ART.6-8 These are the first data from a randomized clinical trial to suggest that the initiation of TDF/FTC in ART-naïve individuals may not be associated with the same degree of improvements in proteinuria and albuminuria that are observed with ABC/3TC. Future studies should consider the long-term clinical significance of these differences, particularly among individuals with pre-existing CKD or CKD risk factors.
Acknowledgments
We thank the study participants for their generous donation of time and effort in the successful completion of this trial.
We also thank the following participating sites and site investigators: Sadia Shaik, M.D. and Ruben Lopez, M.D.- Harbor-UCLA Medical Center (Site 603) CTU Grant # AI0694241, UL1RR033176, CTSI Grant # UL1TR000124; Susan L. Koletar, MD and Diane Gochnour, RN- The Ohio State University Medical Center (Site 2301) CTU Grant # AI069474; Geyoul Kim, RN and Mark Rodriguez, RN- Washington University (Site 2101) CTU Grant # U01AI069495, GCRC Grant # UL1 RR024992; Elizabeth Lindsey, RN and Tamara James, BS – Alabama Therapeutics CRS (Site 5801) CTU Grant # U01 AI069452; Ann C. Collier, MD and Jeffrey Schouten, MD, JD University of Washington (Site 1401) CTU Grant # AI069434, UL1 RR025014; Jorge L. Santana Bagur, MD and Santiago Marrero,MD- Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant # 5 U0I AI069415-03; Jenifer Baer, RN, BSN and Carl Fichtenbaum, MD-University of Cincinnati (Site 2401) CTU Grant # AI069513; Patricia Walton, BSN, RN and Barbara Philpotts, BSN, RN- Case Western Reserve (Site 2501) CTU Grant #: AI69501; Princy Kumar, M.D. and Joseph Timpone, M.D.- Georgetown University (Site 1008) CTU Grant# AIDS Clinical Trials Group grant # 5U01AI069494; Donna Pittard, RN, BSN and David Curri, RN-University of North Carolina (Site 3201) CTU Grant # 5-U01 AI069423–03, UNC CFAR # P30 AI050410(-11), UNC CTRC # UL 1RR 025747; Julie Hoffman, R.N. and Edward Seefried, R.N.- San Diego Medical Center UC (Site 701) CTU Grant # AI69432; Susan Swindells, MBBS and Frances Van Meter, APRN-University of Nebraska (Site 1505) CTU Grant # AI 27661; Deborah McMahon, MD and Barbara Rutecki, MSN, MPH, CRNP- University of Pittsburgh (Site 1001) CTU Grant # 1 U01 AI069494-01; Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Indiana University (Site 2601) CTU Grant # 5U01AI025859, GCRC #: M01 RR00750; Ilene Wiggins, RN, and Eric Zimmerman, RN- Johns Hopkins University (Site 201) CTU Grant # AI27668, CTSA Grant # UL1 RR025005; Judith. Aberg, M.D. and Margarita Vasquez R.N.- New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant # AI27665, New grant number: AI069532 10; Martin McCarter and M. Graham Ray, R.N., M.S.N. - Colorado AIDS Clinical Trials Unit, (Site 6101) CTU Grant # AI69450, RR025780; Mamta Jain, MD and Tianna Petersen, MS- University of Texas Southwestern Medical Center (Site 3751) CTU Grant # 3U01AI046376–05S4; Emily Stumm, BS and Pablo Tebas MD- University of Pennsylvania, Philadelphia (Site 6201) CTU Grant # P30- AI0450008–11, CFAR Grant # UO1-AI069467-04; Mary Albrecht, MD and Neah Kim, NP- Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant # U01 AI069472-04; Paul Edward Sax, M.D. and Joanne Delaney RN- Brigham and Women’s Hospital (Site 107) CTU Grant # UOI AI 069472; Christine Hurley, RN and Roberto Corales, DO- AIDS Care (Site 1108) CTU Grant # U01AI069511–02 (as of 2/12/08), GCRC UL1 RR 024160; Keith Henry, MD and Bette Bordenave, RN- Hennepin County Medical Center (Site 1502) CTU Grant # N01 AI72626; Wendy Armstrong, MD and Ericka R. Patrick, RN, MSN, CCRC- Emory University HIV/AIDS Clinical Trials Unit (Site 5802) CTU Grant # UO1Al69418–01/CFAR Grant Number: P30Al050409; Jane Reid, RNC, MS and Mary Adams RN, MPh- University of Rochester (Site 1101) CTU Grant # U01AI069511–02 (as of 2/12/08), GCRC: UL1 RR 024160; Gene D. Morse, Pharm.D., FCCP, BCPS- SUNY- Buffalo, Erie County Medical Ctr. (Site 1102) CTU Grant # AI27658; Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN-Rush University Medical Center (Site 2702) CTU Grant # U01 AI069471; Nancy Hanks, RN, and Debra Ogata-Arakaki, RN, - University of Hawaii at Manoa, Leahi Hospital (Site 5201) CTU Grant # AI34853; Ardis Moe, MD and Maria Palmer, PA-C- UCLA Medical Center (Site 601) CTU Grant 1U01AI069424-01; Jeffery Meier, M.D. and Jack T. Stapleton, M.D. – University of Iowa Hospitals and Clinics (Site 1504) CTU Grant # UL1RR024979; Gary Matthew Cox, MD and Martha Silberman, RN-Duke University Medical Center Adult CRS (Site 1601) CTU Grant # 5U01 AI069 484-02 2705; Gerianne Casey, RN and William O’Brien, MD-University of Texas, Galveston (Site 6301) CTU Grant # AI32782; Valery Hughes, FNP and Todd Stroberg, RN- Cornell CRS (Site 7803, 7804) CTU Grant# U01 AI069419, CTSC # UL1 RR024996; Nyef El-Daher, MD -McCree McCuller Wellness Center at the Connection (Site 1107) CTU Grant #: U01AI069511–02 (as of 2/12/08), GCRC: UL1 RR 024160; Rebecca J. Basham, B.S. and Husamettin Erdem, M.D.-Vanderbilt Therapeutics CRS (Site 3652) CTU Grant # AI46339–01; MO1 RR 00095
Sources of Support: This work was supported by Award Numbers U01AI068636, AI068634, AI38855 from the National Institute of Allergy and Infectious Diseases, UL1 RR 025005 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health supported by National Institute of Mental Health (NIMH), and the National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
CMW has received research grants from Gilead Sciences, Inc. and honoraria from Bristol-Myers Squibb; SKG has received research grants from Merck & Co., Inc., Janssen Pharmaceutics, Inc., and Gilead Sciences, Inc., travel support from Gilead Sciences, Inc., and received advisory/consultancy/lecture fees from Bristol-Myers Squibb and Merck & Co., Inc.; CT has received payment for participation on a data monitoring committee for Janssen Therapeutics; ESD has received research grants from Bristol-Myers Squibb, Gilead Sciences, Inc., Merck & Co., Inc., and ViiV Healthcare and has received consultancy/advisory fees from Bristol-Myers Squibb, Gilead Sciences, Inc., Janssen Pharmaceuticals., Inc., Merck & Co., Inc., Abbvie, Inc., Teva, and ViiV Healthcare; PES has received research grants from Bristol-Myers Squibb, Gilead Sciences, Inc., and GlaxoSmithKline and is a consultant or scientific advisory board member for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Inc., Merck & Co., Inc., and Janssen Pharmaceutics, Inc.; BH is an employee and stockholder of ViiV Healthcare; KM is an employee and stockholder in Gilead Sciences, Inc.; GAM has served as a scientific advisor for Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Gilead Sciences, Inc. and has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, Abbott, Merck & Co., Inc., and Gilead Sciences and has served as the DSMB Chair for a Pfizer-sponsored study;
Footnotes
Data included in this manuscript were previously presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta GA, March 3-6, 2013
Conflicts of Interest:
all other authors, no conflicts.
References
- 1.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199–1206. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 2.Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyatt CM, Hoover DR, Shi Q, et al. Microalbuminuria is associated with all-cause and AIDS mortality in women with HIV infection. J Acquir Immune Defic Syndr. 2010;55:73–77. doi: 10.1097/QAI.0b013e3181cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt CM, Hoover DR, Shi Q, et al. Pre-existing albuminuria predicts AIDS and non-AIDS mortality in women initiating antiretroviral therapy. Antivir Ther. 2011;16:591–596. doi: 10.3851/IMP1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid A, Stohr W, Walker AS, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46:1271–1281. doi: 10.1086/533468. [DOI] [PubMed] [Google Scholar]
- 7.Peters PJ, Moore DM, Mermin J, et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int. 2008;74:925–929. doi: 10.1038/ki.2008.305. [DOI] [PubMed] [Google Scholar]
- 8.Kalayjian RC, Franceschini N, Gupta SK, et al. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008;22:481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 10.Scherzer R, Estrella M, Li Y, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012 doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207:1359–1369. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KY, Patel P, Fine D, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS. 2009;23:1547–1556. doi: 10.1097/QAD.0b013e32832cbcc2. [DOI] [PubMed] [Google Scholar]
- 13.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55:49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]
- 14.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foundation NK. NKF KDOQI Clinical Practice Guidelines. 2000 [Google Scholar]
- 17.Inker LA, Wyatt C, Creamer R, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. J Acquir Immune Defic Syndr. 2012;61:302–309. doi: 10.1097/QAI.0b013e31826a6c4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrouenraets SM, Fux CA, Wit FW, et al. A comparison of measured and estimated glomerular filtration rate in successfully treated HIV-patients with preserved renal function. Clin Nephrol. 2012;77:311–320. doi: 10.5414/cn107214. [DOI] [PubMed] [Google Scholar]
- 19.Szczech LA, Grunfeld C, Scherzer R, et al. Microalbuminuria in HIV infection. AIDS. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta SK, Parker RA, Robbins GK, Dube MP. The effects of highly active antiretroviral therapy on albuminuria in HIV-infected persons: results from a randomized trial. Nephrol Dial Transplant. 2005;20:2237–2242. doi: 10.1093/ndt/gfi053. [DOI] [PMC free article] [PubMed] [Google Scholar]


