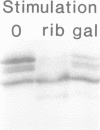
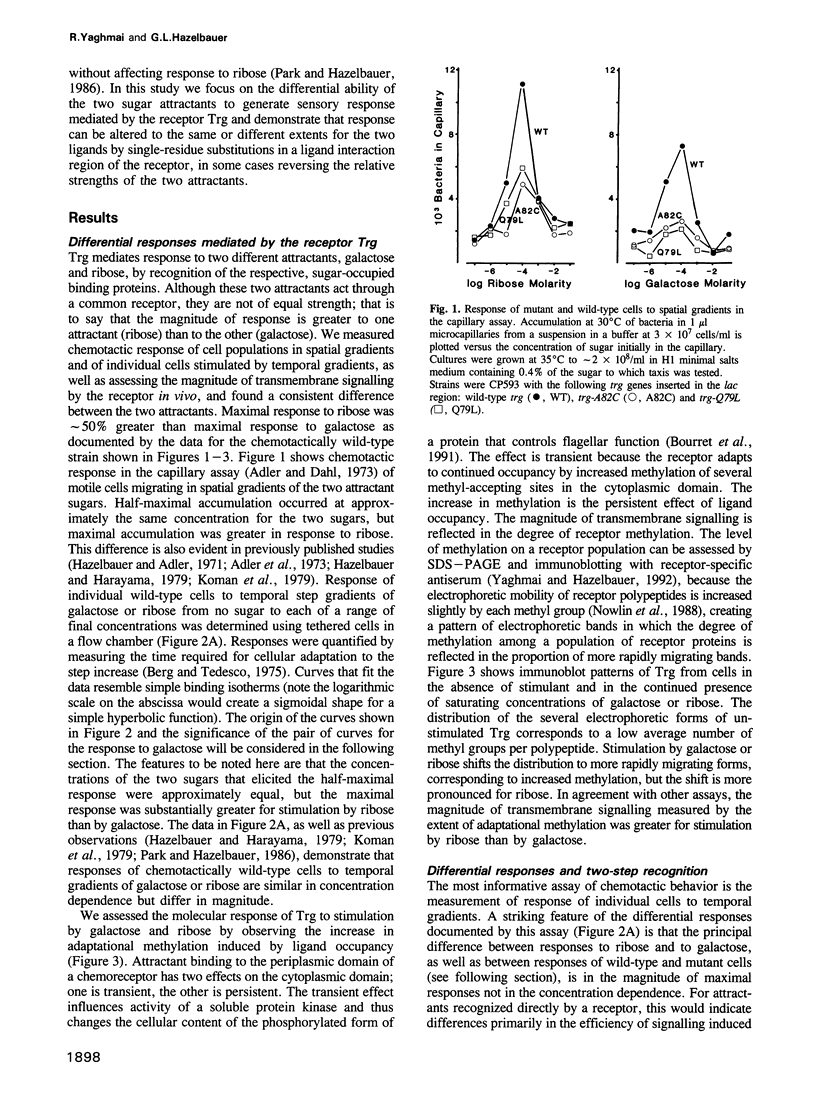
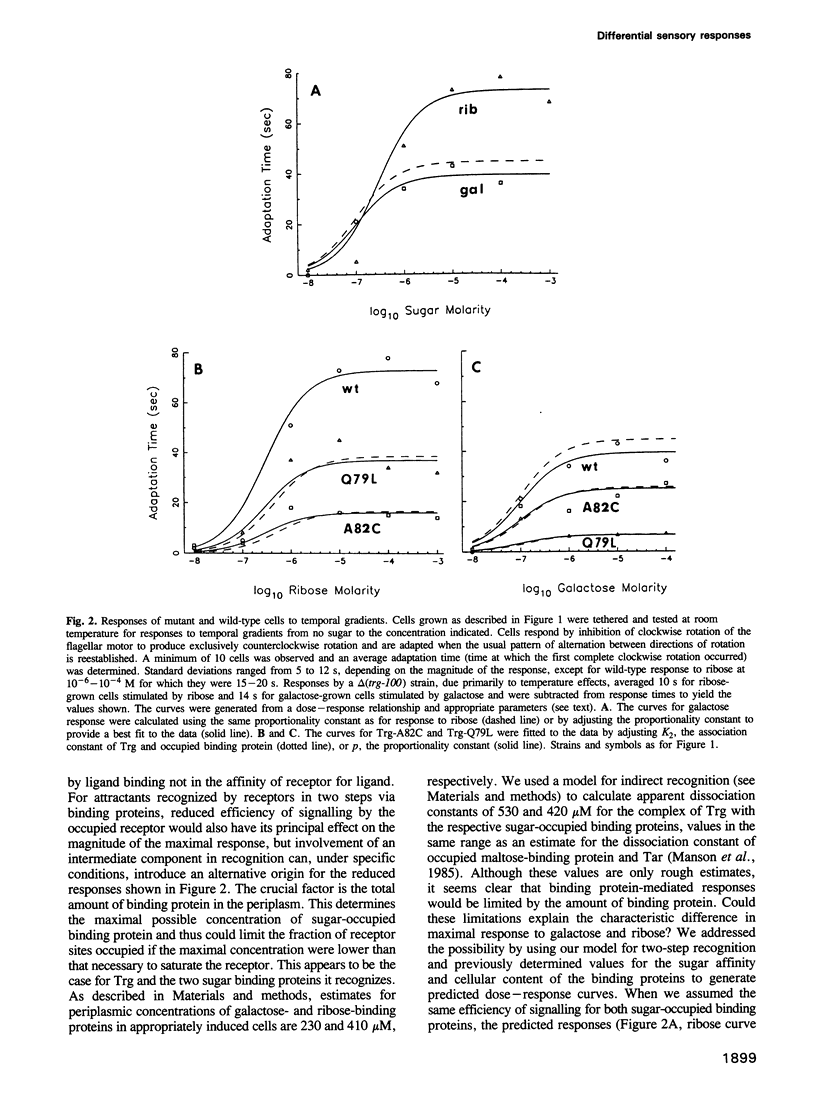
Abstract
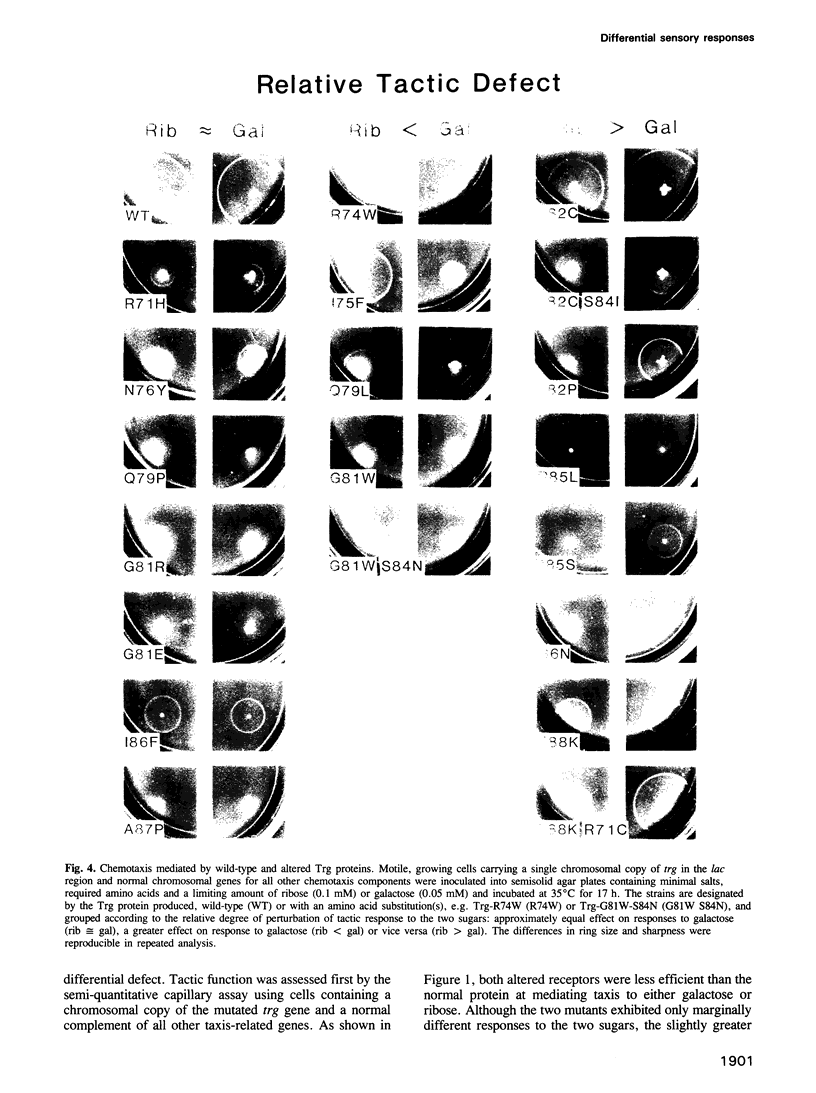
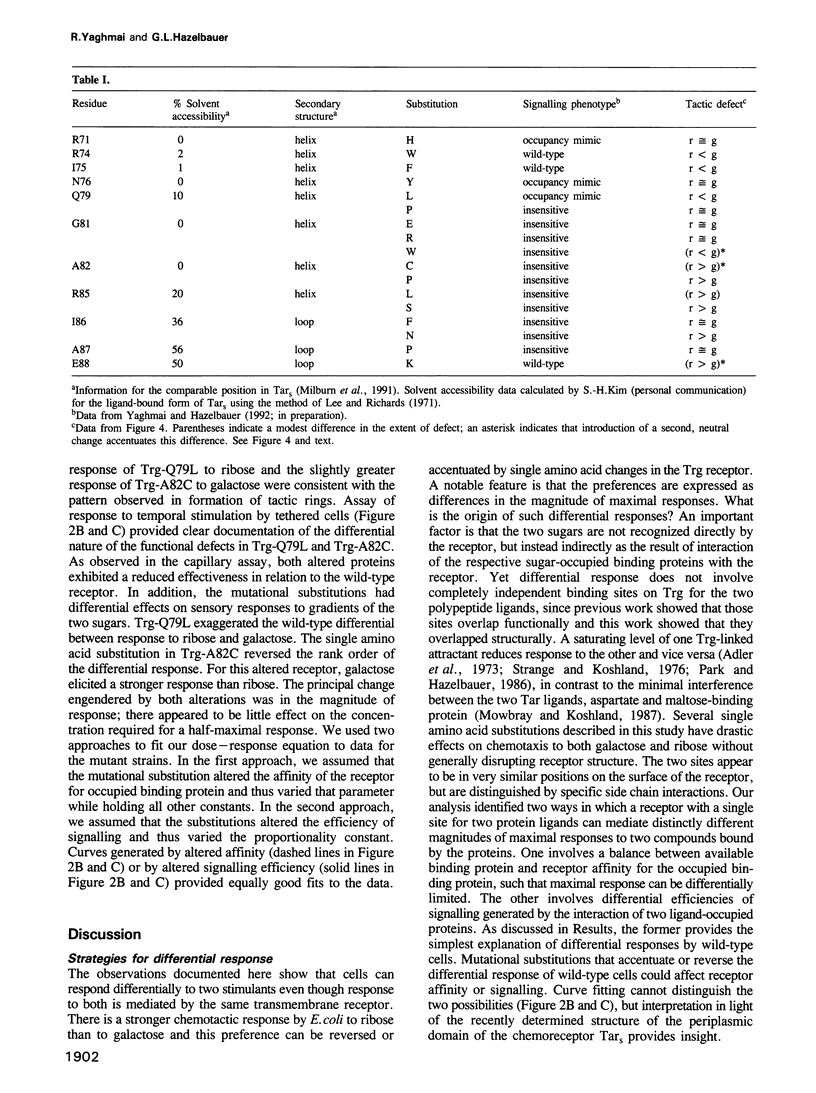
Trg mediates chemotaxis of Escherichia coli to galactose and ribose by recognition of respective, sugar-occupied binding proteins. Although both attractants act through one transmembrane receptor, maximal response is approximately 50% greater to ribose. This phenomenon was investigated by mutational analysis of a 20-residue segment of Trg implicated in ligand interaction and signalling. Among 17 defective receptors, responses to the two chemoattractants were reduced equivalently for seven and differentially for 10, in some cases reversing the preference order. Mutational substitutions with equivalent effects occurred throughout the segment, but those with a greater effect on galactose or ribose response were segregated to the amino-terminal two-thirds or the carboxy-terminal one-third, respectively, a segregation corresponding in large part to a functional division based on signalling phenotypes. A model for binding protein-mediated recognition revealed two strategies for differential responses. The wild-type preference for ribose probably reflects a balance of receptor affinities and a limiting supply of binding proteins. Mutants with reversed preference probably have differentially reduced receptor affinities and those with an accentuated ribose preference probably have altered signalling abilities. Two-step recognition of ligand allows functional separation of detection and response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Tedesco P. M. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Park C., Nowlin D. M. Adaptational "crosstalk" and the crucial role of methylation in chemotactic migration by Escherichia coli. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1448–1452. doi: 10.1073/pnas.86.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L. The bacterial chemosensory system. Can J Microbiol. 1988 Apr;34(4):466–474. doi: 10.1139/m88-080. [DOI] [PubMed] [Google Scholar]
- Kossmann M., Wolff C., Manson M. D. Maltose chemoreceptor of Escherichia coli: interaction of maltose-binding protein and the tar signal transducer. J Bacteriol. 1988 Oct;170(10):4516–4521. doi: 10.1128/jb.170.10.4516-4521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lee L., Imae Y. Role of threonine residue 154 in ligand recognition of the tar chemoreceptor in Escherichia coli. J Bacteriol. 1990 Jan;172(1):377–382. doi: 10.1128/jb.172.1.377-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Mizuno T., Imae Y. Thermosensing properties of Escherichia coli tsr mutants defective in serine chemoreception. J Bacteriol. 1988 Oct;170(10):4769–4774. doi: 10.1128/jb.170.10.4769-4774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch B. A., Koshland D. E., Jr Disulfide cross-linking studies of the transmembrane regions of the aspartate sensory receptor of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10402–10406. doi: 10.1073/pnas.88.23.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Boos W., Bassford P. J., Jr, Rasmussen B. A. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J Biol Chem. 1985 Aug 15;260(17):9727–9733. [PubMed] [Google Scholar]
- Milburn M. V., Privé G. G., Milligan D. L., Scott W. G., Yeh J., Jancarik J., Koshland D. E., Jr, Kim S. H. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991 Nov 29;254(5036):1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- Milligan D. L., Koshland D. E., Jr Site-directed cross-linking. Establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J Biol Chem. 1988 May 5;263(13):6268–6275. [PubMed] [Google Scholar]
- Mowbray S. L., Foster D. L., Koshland D. E., Jr Proteolytic fragments identified with domains of the aspartate chemoreceptor. J Biol Chem. 1985 Sep 25;260(21):11711–11718. [PubMed] [Google Scholar]
- Mowbray S. L., Koshland D. E., Jr Additive and independent responses in a single receptor: aspartate and maltose stimuli on the tar protein. Cell. 1987 Jul 17;50(2):171–180. doi: 10.1016/0092-8674(87)90213-3. [DOI] [PubMed] [Google Scholar]
- Mowbray S. L., Koshland D. E., Jr Mutations in the aspartate receptor of Escherichia coli which affect aspartate binding. J Biol Chem. 1990 Sep 15;265(26):15638–15643. [PubMed] [Google Scholar]
- Nowlin D. M., Bollinger J., Hazelbauer G. L. Site-directed mutations altering methyl-accepting residues of a sensory transducer protein. Proteins. 1988;3(2):102–112. doi: 10.1002/prot.340030205. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Hazelbauer G. L. Mutations specifically affecting ligand interaction of the Trg chemosensory transducer. J Bacteriol. 1986 Jul;167(1):101–109. doi: 10.1128/jb.167.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard B. L., Koshland D. E., Jr Prediction of the structure of a receptor-protein complex using a binary docking method. Nature. 1992 Aug 27;358(6389):774–776. doi: 10.1038/358774a0. [DOI] [PubMed] [Google Scholar]
- Strange P. G., Koshland D. E., Jr Receptor interactions in a signalling system: competition between ribose receptor and galactose receptor in the chemotaxis response. Proc Natl Acad Sci U S A. 1976 Mar;73(3):762–766. doi: 10.1073/pnas.73.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C., Parkinson J. S. Aspartate taxis mutants of the Escherichia coli tar chemoreceptor. J Bacteriol. 1988 Oct;170(10):4509–4515. doi: 10.1128/jb.170.10.4509-4515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghmai R., Hazelbauer G. L. Ligand occupancy mimicked by single residue substitutions in a receptor: transmembrane signaling induced by mutation. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7890–7894. doi: 10.1073/pnas.89.17.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]