Abstract
Background
Infliximab, a chimeric, monoclonal, anti-TNF antibody has been shown to be safe and efficacious for refractory sarcoidosis, we investigated whether adalimumab, a fully human, anti-TNF monoclonal antibody, is similarly safe and efficacious in refractory pulmonary sarcoidosis.
Methods
An open-label, single-center study was conducted in 11 patients with refractory pulmonary sarcoidosis. Patients received adalimumab 40 mg weekly for 45 weeks, with a final follow-up at Week 52. The primary endpoint was the percent change in predicted forced vital capacity (FVC) at 24 weeks. Secondary efficacy parameters included the 6-minute walk test (6MWT), Borg dyspnea score, and Physician’s (PGA) and Patient’s (PaGA) Global Assessments. A successful outcome of the study was defined as reduction in immunosuppressive therapy (prednisone to 10 mg or less), improvement in FVC of 5% or greater, improvement in 6-minute walk test distance (6MWD) of 50 meter or greater at the end of weeks 24 and 52.
Results
Eleven patients received adalimumab and had 24-week follow-ups. Only ten patients had a Week 52 evaluation. FVC stabilized in seven patients, and four patients showed improvement in FVC. Five patients had improved 6MWD, and nine had lower Borg dyspnea scores. PGA and PaGA improved at weeks 24 and 52 for all patients (P<0.008 for all comparisons). Among 11 patients who underwent adalimumab treatment, 9 (82%) and 8 (80%) had a successful outcome at the end of 24 and 52 weeks respectively. No severe adverse incidents were reported.
Conclusions
In this small, open-label study, adalimumab improved refractory pulmonary sarcoidosis and was well tolerated (ClinicalTrials.gov identifier NCT00311246).
Keywords: pulmonary sarcoidosis, anti-TNF-α antibody, adalimumab
Introduction
Sarcoidosis is a systemic disease that involves any organ mainly the lung.(1) The disease course is chronic and progressive in 10–30% of patients, and at least 10–20% of patients have extrapulmonary involvement (2). Sarcoidosis is fatal in 1–5% of patients, usually as a result of progressive lung disease, central nervous system complication, or myocardial involvement (2, 3).
Corticosteroids are considered the standard of care for sarcoidosis. There are currently no therapies approved for the treatment of sarcoidosis in the United States (U.S.). Anti-malarials, immunosuppressive agents, and non-steroidal anti-inflammatory drugs (NSAIDs), have been used as corticosteroid sparing-agents in patients whose disease requires chronic corticosteroid administration (4). However, the response to treatment is difficult to predict, and relapse may occur following discontinuation of therapy. Furthermore, the use of corticosteroids and corticosteroid-sparing agents in sarcoidosis do not represent disease-specific targeted approaches. Patients may experience considerable toxicities while on these medications (5, 6). Therefore, alternative therapies that improve patient outcomes and quality of life in progressive sarcoidosis are urgently needed.
Studies that have characterized the pathogenesis of sarcoidosis have found that tumor necrosis factor (TNF) plays a major role in the inflammatory processes underlying this disease (7, 8). Accordingly, TNF is involved in the development of the non-caseating granulomas that are the hallmark of sarcoidosis (9). Published reports confirm that TNF inhibition improves symptoms in patients with sarcoidosis (10-13). In a multicenter, randomized, double-blind, placebo-controlled study, the anti-TNF agent infliximab (Remicade®, Centocor Ortho Biotech Inc.; Horsham, PA) significantly improved the forced vital capacity (FVC) in patients with chronic sarcoidosis with pulmonary involvement after 24 weeks of therapy (13). Case reports from patients successfully treated with infliximab (10) and adalimumab have also been described (11, 14-16).
Adalimumab, a fully human monoclonal anti-TNF antibody, binds to and neutralizes TNF, there-by inhibiting its action after release from pulmonary macrophages and other cells (17). It is approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, Crohn’s disease and Ulcerative Colitis in the U.S., Europe, and elsewhere. Recently, adalimumab significantly improved disease activity, as measured by FDG-PET, in 9 out of 10 patients with refractory sarcoidosis. In these patients, pulmonary function tests and blood lymphocyte concentrations remained stable (18). Adalimumab has also been shown to improve intraocular inflammation, resolve choroidal involvement, and clear vitreous fluid in patients with sarcoidosis and refractory chronic non-infectious uveitis (19). Sarcoid-like granulomas were also improved with adalimumab in one patient with rheumatoid arthritis who developed both cutaneous and pulmonary sarcoid-like manifestations while receiving etanercept (20). Furthermore, adalimumab, but not etanercept, achieved responses in a patient with refractory sarcoidosis in one study (21). Given that various case reports and small studies have reported encouraging results with adalimumab in sarcoidosis, the present study was designed to assess safety and efficacy of adalimumab in a prospective, non-randomized, open-label study in patients with refractory pulmonary sarcoidosis. Interim results derived from this study have been previously presented (22, 23).
Materials and Methods
Study Design and Patients
This study was a prospective, single-center, non-randomized, open-label clinical trial. The University of Chicago’s Institutional review boards approved the study, and it was registered on clinicaltrials.gov (ClinicalTrials.gov identifier: NCT00311246). All patients provided written, informed consent to participate in this clinical trial.
Patients enrolled in this study had been diagnosed with refractory pulmonary sarcoidosis defined as symptomatic pulmonary sarcoidosis despite optimal treatment with oral corticosteroids and/or corticosteroid-sparing therapies for at least one year. We included patients who had histologically-proven sarcoidosis, evidence of parenchymal disease on chest radiograph, Stage II/III disease, a forced vital capacity (FVC) >40% and <80% of the predicted value, and Borg dyspnea score (24) of at least grade 1. Changes in Borg Dyspnea scores are reflective of alterations in patients’ dyspnea symptoms (25). Exclusion criteria included any serious infection within 2 months of screening or opportunistic infections within 6 months of screening, class III or IV of New York Heart Association congestive heart failure classification, active systemic lupus erythematosus, malignancy within the past five years, lymphoproliferative disease, and history of latent tuberculosis infection.
Treatment Protocol
Patients must have been on at least 7.5 mg per day of prednisone (or corticosteroid equivalent) or one or more immunosuppressants for at least three months prior to screening. Doses of these medications had to be stable for at least 4 weeks before entering the study. During the study, doses of concomitant medications for sarcoidosis were to remain stable unless medically indicated.
Subcutaneous adalimumab at a dose of 40 mg was administered weekly to each study participant for 45 weeks. AbbVie Inc (North Chicago, IL), provided the study agent. Patients were seen at least monthly. Last follow-up visit was conducted at Week 52.
Outcome Assessment and Follow-up
The primary endpoint was defined as the percent change in predicted forced vital capacity (FVC) at 24 weeks. FVC was measured by a standardized, calibrated spirometer in the pulmonary function laboratory at the University of Chicago. Each patient acted as his or her own control, with FVC measurements obtained at baseline, week 24, and week 52. Secondary outcomes measures included Borg dyspnea score (before and after 6MWT), Physician’s Global Assessment of disease activity (PGA) and Patient’s Global Assessment of disease activity (Pa-GA) (26). Changes in Borg Dyspnea scores are reflective of alterations in patients’ dyspnea symptoms (25). The PGA and PaGA scores range from 0 (no pulmonary manifestations of sarcoidosis) to 100 (worsening of pulmonary symptoms) on a visual analog scale. Chest radiographs were taken at baseline and 24 weeks and were scored by a radiologist and a pulmonologist independently who were blinded to the time-point of the radiograph. Images were evaluated for extent (score 0–4) and profusion (score 0–4) for each of the four types of shadows commonly seen in sarcoidosis: reticulonodular (R), mass (M), confluent (C), and fibrosis (F) (27).
Additional outcomes were evaluated in post-hoc analyses based on previously published recommendations (28). These included reduction in immunosuppressive therapy (prednisone to 10 mg or less), improvement in FVC of 5% or greater, improvement in 6-minute walk test distance (6MWD) of 50 meter or greater at the end of weeks 24 and 52. The study defined treatment success if a patient had one or more of these outcomes. Failure was defined as meeting none of these outcomes.
Statistical Analysis
The data are presented as the mean ± SD or the median (25th to 75th percentile) for continuous variables and percentages for categorical variables. The baseline characteristics of patients determined to be treatment success vs. failure were compared using the two-sample t test for continuous variables and the Fisher exact test for categorical variables. The Wilcoxon’s signed rank test was used to compare Physician’s global assessment scores because data distribution failed normality testing by D’Agostino & Pearson omnibus normality test. The Patient’s Global Assessment scores of the patients were compared using a Paired t-test (the data were normally distributed). In all cases, p values of ≤ 0.05 were considered to be statistically significant.
Results
Patient Epidemiologic Characteristics
Eleven African American (AA) patients were enrolled into the study and treated with adalimumab. Ten completed the Week 52 visit. One patient (patient #9) withdrew from the study because the patient moved out of the study area before week 52. Patient number five refused PFT at week 52. The patient characteristics are listed in Table 1.
Table 1.
Characteristics of the study cohort.
| Characteristics | Mean (±SD) |
|---|---|
| Age (years) | 45.3(12.7) |
| Race | |
| African American | 11(100%) |
| Gender | |
| Female, n (%) | 10(91%) |
| Vital signs | |
| Respiration rate | 20(8) |
| Heart rate (beats per minute) | 92(18) |
| Blood pressure | 109/72 |
| Weight (kilograms) | 81.5(34) |
| 6 MW measures at the screening | |
| Distances (meter) | 303.2(158.8) |
| Before 6MW | |
| Borg Dyspnea score at baseline | 1.45(1.4) |
| Borg fatigue score | 4.4(3.7) |
| Heart rate (beats per minute) | 94 (14) |
| Oxygen saturation (%) | 97(1.4) |
| After 6MW | |
| Borg dyspnea score | 4.2(3.8) |
| Borg fatigue score | 2.15(2.2) |
| Heart rate | 118(21) |
| Oxygen saturation (%) | 95(4.2) |
| FVC (L) at the screening | 61(12) |
| Physicians Global Assessment, (0-100) mean | 81(12) |
| Patients Global Assessment, (0-100) mean | 54(5) |
Legend for table 1: 6MW: 6 minute walk, L: liter
Change in the Patient’s Medications
Concomitant therapy for each patient is detailed in Table 2. The protocol did not include any planned withdrawal of corticosteroids or other therapy. Five patients were on prednisone initially (range 7.5 to 40 mg daily). Among them, two patients initially on 20 mg/day (patients #10 and #11) were able to reduce their dose to 5 mg daily by week 24 and to 3 mg by week 52. One patient initially on 40 mg/day (patient #2) remained on that dose throughout the study because of asthma. The other two steroid-treated patients (patients #4 and #6) were maintained on the same low dose throughout the study (10 and 7.5 mg/day, respectively). All patients were taking immunosuppressants at the beginning of the study. One patient (patient #11) was off all therapy by week 52.
Table 2.
Medication history of the study cohort during this clinical trial.
| Patient | Patient Characteristics | Medication Status | |||
|---|---|---|---|---|---|
| Age | Race | Baseline | Week 24 | Week 52 | |
| 1 | 60 | AA | Mycophenolate 500 mg/day | Mycophenolate 500 mg/day | Mycophenolate 500 mg/day |
| 2 | 51 | AA | Prednisone 40 mg/day Mycophenolate 500 mg/day |
Prednisone 40 mg/day Mycophenolate 500 mg/day |
Prednisone 40 mg/day Mycophenolate 500 mg/day |
| 3 | 30 | AA | Methotrexate 10 mg/week | Methotrexate 10 mg/week | Methotrexate 10 mg/week |
| 4 | 52 | AA | Prednisone 10 mg/day Cyclosporine 125 mg/daily |
Prednisone 10 mg/day Cyclosporine 125 mg/daily |
Prednisone 10 mg/day Cyclosporine 125 mg/daily |
| 5 | 42 | AA | Leflunomide 20 mg every other day |
Leflunomide 20 mg every other day |
Leflunomide 20 mg every other day |
| 6 | 68 | AA | Prednisone 7.5 mg/day Mycophenolate 500 mg/day |
Prednisone 7.5 mg/day Mycophenolate 500 mg/day |
Prednisone 7.5 mg/day Mycophenolate 500 mg/day |
| 7 | 33 | AA | Methotrexate 10 mg/week | Methotrexate 10 mg/week | Methotrexate 10 mg/week |
| 8 | 33 | AA | Methotrexate 7.5 mg/week | Methotrexate 7.5 mg/week | Methotrexate 7.5 mg/week |
| 9 | 48 | AA | Methotrexate 17.5 mg/week | Methotrexate 12.5 mg/week | - |
| 10 | 36 | AA | Prednisone 20 mg/day Mycophenolate 500 mg/day |
Prednisone 5 mg/day Mycophenolate 500 mg/day |
Prednisone 3 mg/day Mycophenolate 500 mg/day |
| 11 | 39 | AA | Azathioprine 100 mg/day Prednisone 20 mg/day |
Azathioprine 100 mg/day Prednisone 5 mg/day |
No medications |
Legend for table 2: AA: African-American
Change in the Percentage of FVC
Pulmonary physiology, including FVC % predicted, 6MWD, and Borg dyspnea score before and after the 6MWT are shown in Tables 3 and 4.
Table 3.
Improvement of Sarcoidosis Disease Measures With Adalimumab Therapy
| Patient | FVC,% | 6-MW Distance (meter) | Borg Dyspnea Score, Before/After 6MWT | ||||
|---|---|---|---|---|---|---|---|
| Number | Screening | Week 24/52 | Baseline | Week 24/52 | Baseline | Week 24 | Week 52 |
| 1 | 66 | 65/75 | 185 | 309/329 | 3/3 | 0/3 | 1/2 |
| 2 | 67 | 64/59 | 215 | 123/82 | 2/10 | 3/7 | 4/10 |
| 3 | 51 | 57/56 | 391 | 411/411 | 0.5/0.5 | 0/0 | 0/0 |
| 4 | 44 | 43/40 | 188 | 297/247 | 0.5/10 | 2/7 | 0/5 |
| 5 | 61 | 60/missing | 310 | 276/219 | 3/7 | 3/3 | 5/7 |
| 6 | 54 | 58/56 | 247 | 535/335 | 0/1 | 0.5/2 | 1/2 |
| 7 | 78 | 91/87 | 411 | 535/547 | 3/7 | 0.5/2 | 0/1 |
| 8 | 59 | 64/52 | 433 | 320/329 | 1/2 | 0.5/0.5 | 2/2 |
| 9 | 79 | 84/missing | 597 | 553/missing | 0.5/1 | 0/0 | missing |
| 10 | 46 | 49/53 | 329 | 442/329 | 0/0 | 0.5/0.5 | 0.5/0.5 |
| 11 | 73 | 72/73 | 501 | 411/501 | 0.5/0.5 | 0/0 | 0.5/0 |
Table 4.
Improvement of Sarcoidosis Disease With Adalimumab Therapy
| Pt | Week 24 | Week 52 | ||||||
|---|---|---|---|---|---|---|---|---|
| # | Δ Medicine | Δ FVC% | Δ 6MWD | Improve | Medicine | Δ FVC | Δ 6MWD | Improve |
| 1 | NS | NS | S | Y | NS | S | S | Y |
| 2 | NS | NS | NS | N | NS | NS | NS | N |
| 3 | NS | S | NS | Y | NS | S | NS | Y |
| 4 | NS | NS | S | Y | NS | NS | S | Y |
| 5 | NS | NS | NS | N | NS | UK | NS | UK |
| 6 | NS | NS | S | Y | NS | NS | S | Y |
| 7 | NS | S | S | Y | NS | S | S | Y |
| 8 | NS | S | NS | Y | NS | S | NS | Y |
| 9 | S | S | NS | Y | UK | UK | UK | UK |
| 10 | S | NS | S | Y | S | S | NS | Y |
| 11 | S | NS | NS | Y | S | NS | NS | Y |
Legend for table 4: Pt: Patient, Δ Medicine: Changes in immunosuppressive therapy (prednisone to 10 mg or less), Δ FVC%: Changes in FVC of 5% or greater, Δ 6MWD: Changes in 6MWD of 50 meter or greater. UK: Unknown, S: Significant, NS: Non-significant, Y: Yes, N: No
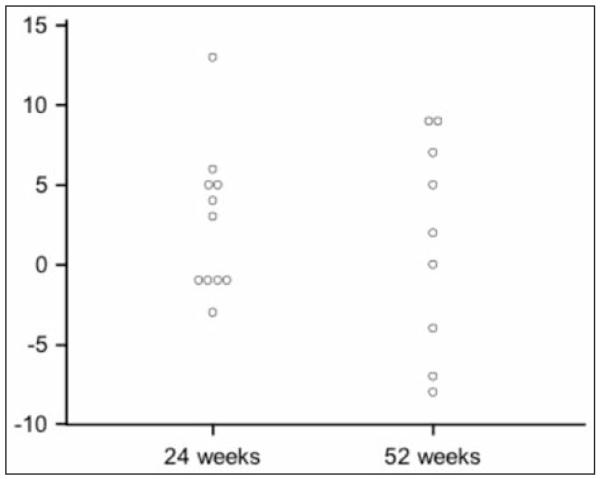
Figure 1 shows the absolute change in the FVC from the baseline at Week 24 (median, 3%; range, −3 to 13%) and Week 52 (median, 2%; range, −8 to 9%). At Week 24, 4 of 11 patients had a 5% or greater increase in FVC % (two with 5-10% improvement, two with >10% improvement). At week 52, 4 of 10 patients had a 5% or greater absolute increase in FVC %.
Fig. 1.
Change in FVC % predicted from baseline
Change in the 6MWD
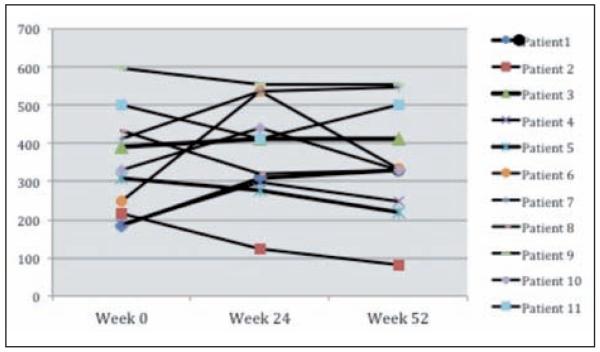
Figure 2 demonstrates the changes in 6MWD from baseline. At week 24, five of 11 patients had an increase in 6MWD with each exhibiting an increase of more than 100 meters (m). At week 52, 4 of 10 evaluated patients continued to exhibit a greater than 50m increase in 6MWD. One patient initially had a greater than 100m improvement in 6MWD at week 24 but not at week 52. This was due to the development of new musculoskeletal problems during the course of the study (pelvic fracture and clinically significant worsening of degenerative disease of the spine).
Fig. 2.
Change in 6MWD from baseline
Changes in Borg Dyspnea and PGA/PaGA scores
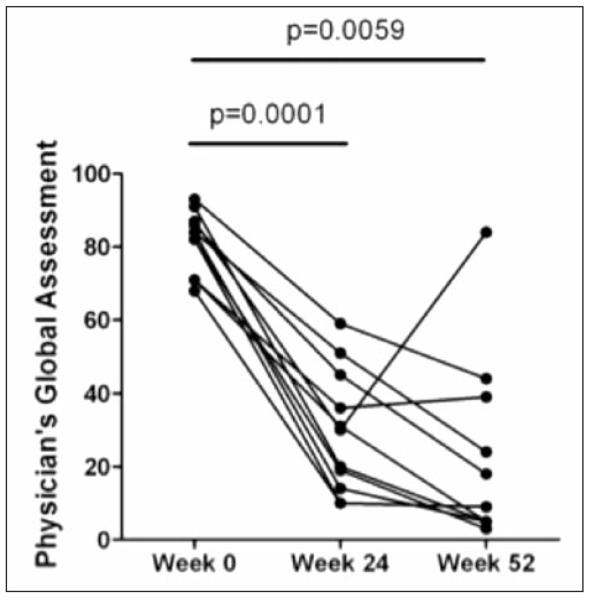
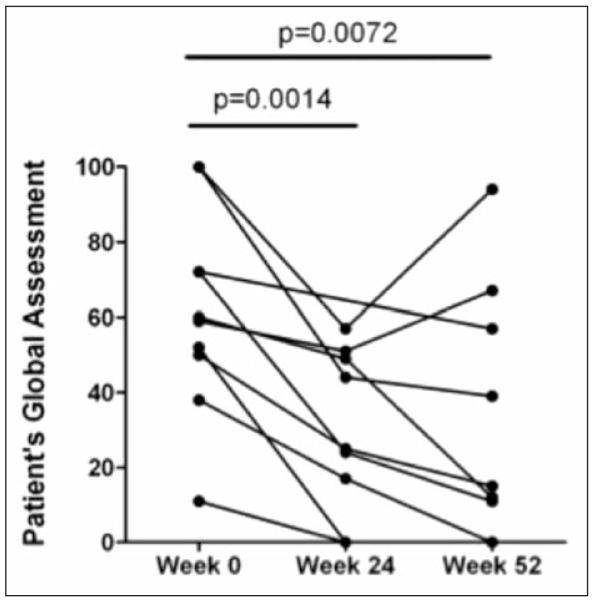
Nine of 11 patients had lower Borg dyspnea scores following the 24-week 6MWT. However, Borg scores remained stable between weeks 24 and 52 as shown in Tables 3 and 4. PGA (Figure 3) and PaGA (Figure 4) were measured at weeks 24 and 52. Significant improvements were reported for each assessment at both time-points when compared to baseline (p <0.008 for all comparisons), with striking improvements noted by the majority of physicians and patients at week 24 (P= 0.0001 and P= 0.0014, respectively).
Fig. 3.
Physician’s global assessment scores are significantly reduced at Weeks 24 and 52.
Fig. 4.
Patient’s global assessment significantly improves at Weeks 24 and 52.
Chest Radiographs Scores
Six patients had chest radiographs classified as stage 3, and 5 patients had stage 2 of sarcoidosis. Reticulonodular opacities, as measured by the R-score, improved in 2 and 4 patients by the end of weeks 24 and 52 respectively. Additively, adalimumab treatment resulted in an approximate 1-point improvement; representing an approximate 25% decrease in the reticulonodular infiltrates extension or profusion in 36% of patients. No significant changes occurred in any other score assessed including fibrosis.
Successful treatment
Among 11 patients who underwent adalimumab treatment 9 (82%) and 8(80%) had a successful outcome at weeks 24 and 52 respectively. One (10%) patient failed on adalimumab treatment at the end of 24 and another one (10%) at 52 weeks.
Adverse events
The administration of adalimumab was well tolerated. No patients discontinued the trial due to drug-related adverse events. During the study period, one patient experienced an upper respiratory tract infection but was not hospitalized. No serious adverse events were reported.
Discussion
In patients with active sarcoidosis, alveolar macrophages produce a variety of proinflammatory cytokines, including TNF, interleukin (IL)-1, and IL-6 (29). TNF appears to be essential for the development of non-caseating granulomas, the hallmark of sarcoidosis (9).Targeting TNF with infliximab has been associated with improvements in lung function of patients with refractory pulmonary sarcoidosis, likely involving direct effects on sarcoidosis-associated non-caseating granulomas (30).
We have now conducted the first prospective, clinical trial to evaluate the use of adalimumab in patients with refractory pulmonary sarcoidosis and determined that 80% of patients receiving adalimumab showed some improvement during treatment in one or more pre-defined parameters of improvement. Adalimumab was not only effective in improving pulmonary function and 6MWD, but also in lowering Borg dyspnea scores. Although targeting TNF with adalimumab appeared to improve these clinical parameters for most patients, it failed in 20% of subjects. Adalimumab therapy led to improvement in the overall health status of the study subjects reflected by the improvement in patient’s and physician’s global assessments reported at weeks 24 and 52 compared to baseline.
Additional studies are needed to determine whether patients with refractory sarcoidosis would benefit more from adalimumab compared to patients with stable disease. We were able to decrease the dosage of prednisone in 3 (27%) patients. Providing patients the opportunity to potentially limit their need for long-term corticosteroids and immunomodulators may be of significant benefit, particularly with respect to short-term and long-term side effects of these medications.
There are conflicting reports on the association of malignancies, such as lymphomas, with the use of anti-TNF agents for the treatment of rheumatoid arthritis (31-33). No cases of malignancies or lymphomas were observed in this study of patients with refractory pulmonary sarcoidosis. However, there is a limited number of patients and duration of follow-up in this trial.
These agents have also been associated with an increase in serious infections, including tuberculosis primary infection and reactivation (34). It is important for clinicians to be aware of this potential complication of anti-TNF therapy since symptoms of sarcoidosis and tuberculosis occasionally overlap and mandate that tuberculosis always be ruled out before the diagnosis of sarcoidosis (2, 35). In addition to malignancies and tuberculosis infections, fungal infections have also been reported in patients with sarcoidosis after treatment with immunosuppressant therapies (36). In this trial, we had no cases of fungal infection, tuberculosis, or opportunistic infections. Specific guidelines for monitoring patients while on adalibmumab have been developed (37).
Milman et al showed treatment with adalimumab can reduce sarcoidosis disease activity, as assessed by FDG-PET with decreased FDG-PET uptake in nine of ten patients (P = 0.011) (18). The dose of adalimumab used in this study was 40 mg weekly. The usual dose of adlimumab is 40 mg every other week. However, It was reported in patients with Crohn’s disease that higher initial and maintenance doses of adalimumab were more effective (38). In a series of five patients with symptomatic, chronic pulmonary and extrapulmonary sarcoidosis, Kamphius et al used a higher loading dose of adlimumab and found a good response similar to that reported here (39). In a study using the standard dose for rheumatoid arthritis, the response rate was approximately fifty percent (40).
There is a single report of development of pulmonary sarcoidosis five months after commencing adalimumab for chronic plaque psoriasis (41) with signs and symptoms resolving within three months of cessation of adalimumab. Similar sarcoidosis-like reactions have been reported with other biologic agents directed against TNF (42). The etiology of these events is unclear.
The impact of ethnicity on adalimumab treatment outcome should be investigated. The ethnic differences in sarcoidosis have been shown previously (43). Pulmonary sarcoidosis tends to be more severe in AA patients (44). We showed that AA patients with sarcoidosis have higher serum TNF levels than non-AA patients (45). We propose a translational clinical trial approach to identify the potential molecular predictors of response to anti TNF therapy in different ethnicities.
Although, this study is limited because of open label design, small sample size and limited duration of follow up, but could show that adalimumab was associated with improvement in both objective and subjective outcome measures in patients with refractory pulmonary sarcoidosis. We found that adalimumab allowed decrease in dosages of corticosteroids and immunomodulators, or withdrawal of these medications in (27%) of patients. This suggests potential long-term benefit to patients on chronic corticosteroids for sarcoidosis. In the aspect of safety, the administration of adalimumab was well tolerated in all subjects. Further evaluation of anti–TNF therapy in patients with refractory sarcoidosis is warranted.
Acknowledgment
The authors thank Dr.Michael Ellman and Dr. Stephen Hanauer for their mentorship and support, James Curran for his support and Kelly McCoy for editorial help. We also thank the study participants for their unfailing commitment and enthusiasm; the staff of the pulmonary and rheumatology Clinics, and the Laboratory staffs in collaborating centers.
Abbreviations
- FVC
forced vital capacity
- 6MWT
6 minute walk test
- 6MWD
6 minute walk distances
- NSAID
non-steroidal anti-inflammatory drug
- TNF-α
tumor necrosis factor-alpha
Footnotes
Author Contributions
Conception, hypotheses delineation, and design of the study: N.J.S., Acquisition of the data or the analysis and interpretation of such information: N.J.S., M.M., I.N., W.Z., R.P.B., J.C, E.T.N., K.H., M.S., M.L.A., P.C., A.L.P., M.E., Writing the article or substantial involvement in its revision before submission: N.J.S., M.M, R.P.B., J.G.G.
References
- 1.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, Terrin ML, Weinberger SE, Moller DR, McLennan G, Hunninghake G, DePalo L, Baughman RP, Iannuzzi MC, Judson MA, Knatterud GL, Thompson BW, Teirstein AS, Yeager H, Jr., Johns CJ, Rabin DL, Rybicki BA, Cherniack R. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 2.Joint Statement of the American Thoracic Society (ATS) European Respiratory Society (ERS) World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) ATS Board of Directors and by the ERS Executive Committee Statement on sarcoidosis. Am J Respir Crit Care Med. 1999 Feb;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. adopted by the. 1999. [DOI] [PubMed] [Google Scholar]
- 3.Rybicki BA, Major M, Popovich J, Jr., Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, Lower EE. New therapies for sarcoidosis. Clin Pulm Med. 2004;11:154–160. [Google Scholar]
- 5.Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med. 1995;155:846–851. [PubMed] [Google Scholar]
- 6.Martin WJ, 2nd, Iannuzzi MC, Gail DB, Peavy HH. Future directions in sarcoidosis research: summary of an NHLBI working group. Am J Respir Crit Care Med. 2004;170:567–571. doi: 10.1164/rccm.200308-1073WS. [DOI] [PubMed] [Google Scholar]
- 7.Bachwich PR, Lynch JP, 3rd, Larrick J, Spengler M, Kunkel SL. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol. 1986;125:421–425. [PMC free article] [PubMed] [Google Scholar]
- 8.Baughman RP, Strohofer SA, Buchsbaum J, Lower EE. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med. 1990;115:36–42. [PubMed] [Google Scholar]
- 9.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 10.Sweiss NJ, Welsch MJ, Curran JJ, Ellman MH. Tumor necrosis factor inhibition as a novel treatment for refractory sarcoidosis. Arthritis Rheum. 2005;53:788–791. doi: 10.1002/art.21468. [DOI] [PubMed] [Google Scholar]
- 11.Callejas-Rubio JL, Ortego-Centeno N, Lopez-Perez L, Benticuaga MN. Treatment of therapy-resistant sarcoidosis with adalimumab. Clin Rheumatol. 2006;25:596–597. doi: 10.1007/s10067-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 12.Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, Miller W, Jr., Sands BE, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, Albera C, Brutsche M, Davis G, Donohue JF, Muller-Quernheim J, Schlenker-Herceg R, Flavin S, Lo KH, Oemar B, Barnathan ES. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:201–208. [PubMed] [Google Scholar]
- 13.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, Albera C, Brutsche M, Davis G, Donohue JF, Muller-Quernheim J, Schlenker-Herceg R, Flavin S, Lo KH, Oemar B, Barnathan ES. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 14.Lahmer T, Knopf A, Lanzl I, Heemann U, Thuermel K, Kaiser CA, Cozzio A, Hofbauer GF, Kamarashev J, French LE, Navarini AA. Using TNF-alpha antagonist Adalimumab for treatment for multisystem sarcoidosis: a case study. Disfiguring annular sarcoidosis improved by adalimumab. Rheumatol Int. 2011;5:5. [Google Scholar]
- 15.Kaiser CA, Cozzio A, Hofbauer GF, Kamarashev J, French LE, Navarini AA. Disfiguring annular sarcoidosis improved by adalimumab. Case Rep Dermatol. 2011;3:103–106. doi: 10.1159/000328796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasni SA, Kunz D, Finzel K, Gruber BL. Osseous sarcoidosis treated with tumor necrosis factor-inhibitors: case report and review of the literature. Spine (Phila Pa 1976) 2010;35:E904–907. doi: 10.1097/brs.0b013e3181dc9a54. [DOI] [PubMed] [Google Scholar]
- 17.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Milman N, Graudal N, Loft A, Mortensen J, Larsen J, Baslund B. Effect of the TNF-alpha inhibitor adalimumab in patients with recalcitrant sarcoidosis: a prospective observational study using FDG-PET. Clin Respir J. 2011 doi: 10.1111/j.1752-699X.2011.00276.x. [DOI] [PubMed] [Google Scholar]
- 19.Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;27:27. doi: 10.1007/s00417-011-1844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns AM, Green PJ, Pasternak S. Etanercept-induced cutaneous and pulmonary sarcoid-like granulomas resolving with adalimumab. J Cutan Pathol. 2012;39:289–293. doi: 10.1111/j.1600-0560.2011.01795.x. [DOI] [PubMed] [Google Scholar]
- 21.Field S, Regan AO, Sheahan K, Collins P. Recalcitrant cutaneous sarcoidosis responding to adalimumab but not to etanercept. Clin Exp Dermatol. 2010;35:795–796. doi: 10.1111/j.1365-2230.2010.03829.x. [DOI] [PubMed] [Google Scholar]
- 22.Sweiss NJ, Noth I, Baughman RP, Ellman M, Curran J, Hogarth DK, Strek M, Alegre M, Clark M, Hires A, Caligiuri P, Pangan A. Adalimumab as a Novel Therapy for Refractory Sarcoidosis: Preliminary Results of a 52-Week Trial; American College of Rheumatology Annual Meeting; Boston, MA. 2007. [Google Scholar]
- 23.Sweiss NJ, Noth IN, Baughman RP, Curran J, Naureckas E, Hogarth DK, Strek M, Caligiuri P, Alegre M, Niewold T, Pangan A, Ellman M. 52-Week Trial Results of Adalimumab as Novel Therapy for Refractory, Progressive Pulmonary Sarcoidosis; American Thoracic Society 2010 International Conference; New Orleans, LA. 2010; Abstract A2370. [Google Scholar]
- 24.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 25.Borg G. Human Kinetics. Champaign, IL: 1998. [Google Scholar]
- 26.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42:2220–2230. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Muers MF, Middleton WG, Gibson GJ, Prescott RJ, Mitchell DN, Connolly CK, Harrison BD. A simple radiographic scoring method for monitoring pulmonary sarcoidosis: relations between radiographic scores, dyspnoea grade and respiratory function in the British Thoracic Society Study of Long-Term Corticosteroid Treatment. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:46–56. [PubMed] [Google Scholar]
- 28.Baughman RPCD, Grutters JC, et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:90–98. [PubMed] [Google Scholar]
- 29.Steffen M, Petersen J, Oldigs M, Karmeier A, Magnussen H, Thiele HG, Raedler A. Increased secretion of tumor necrosis factor-alpha, interleukin-1-beta, and interleukin-6 by alveolar macrophages from patients with sarcoidosis. J Allergy Clin Immunol. 1993;91:939–949. doi: 10.1016/0091-6749(93)90352-g. [DOI] [PubMed] [Google Scholar]
- 30.Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest. 2005;127:1064–1071. doi: 10.1378/chest.127.3.1064. [DOI] [PubMed] [Google Scholar]
- 31.Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119–130. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 32.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. Jama. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 33.Dixon WG, Watson KD, Lunt M, Mercer LK, Hyrich KL, Symmons DP. Influence of anti-tumor necrosis factor therapy on cancer incidence in patients with rheumatoid arthritis who have had a prior malignancy: results from the British Society for Rheumatology Biologics Register. Arthritis Care Res (Hoboken) 2010;62:755–763. doi: 10.1002/acr.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 35.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–1118. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 36.Baughman RP, Lower EE. Fungal infections as a complication of therapy for sarcoidosis. Qjm. 2005;98:451–456. doi: 10.1093/qjmed/hci073. [DOI] [PubMed] [Google Scholar]
- 37.Baughman RP, Meyer KC, Nathanson I, Angel L, Bhorade SM, Chan KM, Culver D, Harrod CG, Hayney MS, Highland KB, Limper AH, Patrick H, Strange C, Whelan T. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: american college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;142:e1S–e111S. doi: 10.1378/chest.12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, Li J, Pollack PF. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamphuis LS, Lam-Tse WK, Dik WA, van Daele PL, van Biezen P, Kwekkeboom DJ, Kuijpers RW, Hooijkaas H, van Laar JA, Bastiaans J, Baarsma GS, van Hagen PM. Efficacy of adalimumab in chronically active and symptomatic patients with sarcoidosis. Am J Respir Crit Care Med. 2011;184:1214–1216. doi: 10.1164/ajrccm.184.10.1214. [DOI] [PubMed] [Google Scholar]
- 40.RP B. Tumor necrosis factor inhibition in treating sarcoidosis: the American experience. Revista Portuguesa de Pneumonologia. 2007;13:S47–S50. [Google Scholar]
- 41.Marcella S, Welsh B, Foley P. Development of sarcoidosis during adalimumab therapy for chronic plaque psoriasis. Australas J Dermatol. 2011;52:e8–11. doi: 10.1111/j.1440-0960.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- 42.Daien CI, Monnier A, Claudepierre P, Constantin A, Eschard JP, Houvenagel E, Samimi M, Pavy S, Pertuiset E, Toussirot E, Combe B, Morel J. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford) 2009;48:883–886. doi: 10.1093/rheumatology/kep046. [DOI] [PubMed] [Google Scholar]
- 43.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183:573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunewald J. Genetics of sarcoidosis. Curr Opin Pulm Med. 2008;14:434–439. doi: 10.1097/MCP.0b013e3283043de7. [DOI] [PubMed] [Google Scholar]
- 45.Sweiss NJ, Zhang W, Franek BS, Kariuki SN, Moller DR, Patterson KC, Bennett P, Girijala LR, Nair V, Baughman RP, Garcia JG, Niewold TB. Linkage of type I interferon activity and TNF-alpha levels in serum with sarcoidosis manifestations and ancestry. PLoS One. 2011;6:e29126. doi: 10.1371/journal.pone.0029126. [DOI] [PMC free article] [PubMed] [Google Scholar]