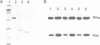Abstract
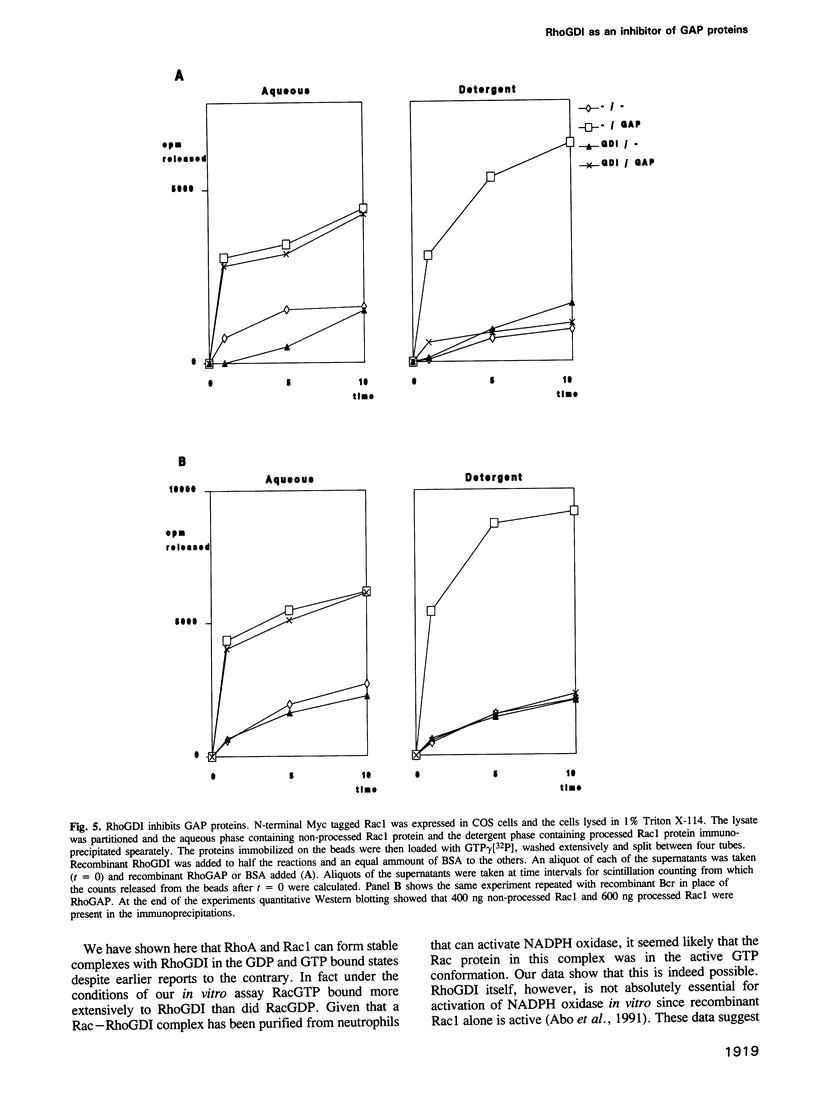
RhoGDI inhibits guanine nucleotide dissociation from post-translationally processed Rho and Rac proteins but its biochemical role in vivo is unknown. We show here that N-terminal effector site mutations in the Rac protein do not compromise its interaction with RhoGDI and that, whilst geranylgeranylation and -AAX proteolysis of the C-terminal CAAX motif of Rac1 and RhoA are required for efficient interaction with RhoGDI, methylesterification of the C-terminal cysteine residue is not required. In vitro, RhoGDI can form stable complexes with Rho and Rac proteins in both the GTP and GDP bound states. Furthermore the Rac-GTP--RhoGDI complex is resistent to the action of recombinant RhoGAP and recombinant BCR. Thus GDI, by complexing with Rac-GTP and preventing GAP stimulated GTP hydrolysis, may allow transit of the activated form of the Rac protein between physically separated activator and effector proteins in the cell.
Full text
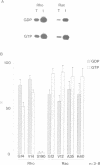
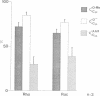
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Adamson P., Marshall C. J., Hall A., Tilbrook P. A. Post-translational modifications of p21rho proteins. J Biol Chem. 1992 Oct 5;267(28):20033–20038. [PubMed] [Google Scholar]
- Adamson P., Paterson H. F., Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992 Nov;119(3):617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K., Braun U., Rösener S., Just I., Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989 Jan 16;158(1):209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Créchet J. B., Poullet P., Mistou M. Y., Parmeggiani A., Camonis J., Boy-Marcotte E., Damak F., Jacquet M. Enhancement of the GDP-GTP exchange of RAS proteins by the carboxyl-terminal domain of SCD25. Science. 1990 May 18;248(4957):866–868. doi: 10.1126/science.2188363. [DOI] [PubMed] [Google Scholar]
- Fujioka H., Kaibuchi K., Kishi K., Yamamoto T., Kawamura M., Sakoda T., Mizuno T., Takai Y. Transforming and c-fos promoter/enhancer-stimulating activities of a stimulatory GDP/GTP exchange protein for small GTP-binding proteins. J Biol Chem. 1992 Jan 15;267(2):926–930. [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Hori Y., Fujioka H., Araki S., Ueda T., Kikuchi A., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990 Sep;5(9):1321–1328. [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Ras-related GTPases and the cytoskeleton. Mol Biol Cell. 1992 May;3(5):475–479. doi: 10.1091/mbc.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Signal transduction through small GTPases--a tale of two GAPs. Cell. 1992 May 1;69(3):389–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Paterson H., Marshall C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991 Dec;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Maru Y., Leonard D., Witte O. N., Evans T., Cerione R. A. A GDP dissociation inhibitor that serves as a GTPase inhibitor for the Ras-like protein CDC42Hs. Science. 1992 Oct 30;258(5083):812–815. doi: 10.1126/science.1439791. [DOI] [PubMed] [Google Scholar]
- Hiraoka K., Kaibuchi K., Ando S., Musha T., Takaishi K., Mizuno T., Asada M., Ménard L., Tomhave E., Didsbury J. Both stimulatory and inhibitory GDP/GTP exchange proteins, smg GDS and rho GDI, are active on multiple small GTP-binding proteins. Biochem Biophys Res Commun. 1992 Jan 31;182(2):921–930. doi: 10.1016/0006-291x(92)91820-g. [DOI] [PubMed] [Google Scholar]
- Hiroyoshi M., Kaibuchi K., Kawamura S., Hata Y., Takai Y. Role of the C-terminal region of smg p21, a ras p21-like small GTP-binding protein, in membrane and smg p21 GDP/GTP exchange protein interactions. J Biol Chem. 1991 Feb 15;266(5):2962–2969. [PubMed] [Google Scholar]
- Hori Y., Kikuchi A., Isomura M., Katayama M., Miura Y., Fujioka H., Kaibuchi K., Takai Y. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene. 1991 Apr;6(4):515–522. [PubMed] [Google Scholar]
- Isomura M., Kaibuchi K., Yamamoto T., Kawamura S., Katayama M., Takai Y. Partial purification and characterization of GDP dissociation stimulator (GDS) for the rho proteins from bovine brain cytosol. Biochem Biophys Res Commun. 1990 Jun 15;169(2):652–659. doi: 10.1016/0006-291x(90)90380-6. [DOI] [PubMed] [Google Scholar]
- Isomura M., Kikuchi A., Ohga N., Takai Y. Regulation of binding of rhoB p20 to membranes by its specific regulatory protein, GDP dissociation inhibitor. Oncogene. 1991 Jan;6(1):119–124. [PubMed] [Google Scholar]
- Kaibuchi K., Mizuno T., Fujioka H., Yamamoto T., Kishi K., Fukumoto Y., Hori Y., Takai Y. Molecular cloning of the cDNA for stimulatory GDP/GTP exchange protein for smg p21s (ras p21-like small GTP-binding proteins) and characterization of stimulatory GDP/GTP exchange protein. Mol Cell Biol. 1991 May;11(5):2873–2880. doi: 10.1128/mcb.11.5.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R., Lutz R. J., Cox A. D., Conroy L., Bourne J. R., Sinensky M., Balch W. E., Buss J. E., Der C. J. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella B. T., Maltese W. A. rab GTP-binding proteins with three different carboxyl-terminal cysteine motifs are modified in vivo by 20-carbon isoprenoids. J Biol Chem. 1992 Feb 25;267(6):3940–3945. [PubMed] [Google Scholar]
- Ma Y. T., Chaudhuri A., Rando R. R. Substrate specificity of the isoprenylated protein endoprotease. Biochemistry. 1992 Dec 1;31(47):11772–11777. doi: 10.1021/bi00162a014. [DOI] [PubMed] [Google Scholar]
- Ma Y. T., Rando R. R. A microsomal endoprotease that specifically cleaves isoprenylated peptides. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6275–6279. doi: 10.1073/pnas.89.14.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martegani E., Vanoni M., Zippel R., Coccetti P., Brambilla R., Ferrari C., Sturani E., Alberghina L. Cloning by functional complementation of a mouse cDNA encoding a homologue of CDC25, a Saccharomyces cerevisiae RAS activator. EMBO J. 1992 Jun;11(6):2151–2157. doi: 10.1002/j.1460-2075.1992.tb05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kaibuchi K., Ando S., Musha T., Hiraoka K., Takaishi K., Asada M., Nunoi H., Matsuda I., Takai Y. Regulation of the superoxide-generating NADPH oxidase by a small GTP-binding protein and its stimulatory and inhibitory GDP/GTP exchange proteins. J Biol Chem. 1992 May 25;267(15):10215–10218. [PubMed] [Google Scholar]
- Mizuno T., Kaibuchi K., Yamamoto T., Kawamura M., Sakoda T., Fujioka H., Matsuura Y., Takai Y. A stimulatory GDP/GTP exchange protein for smg p21 is active on the post-translationally processed form of c-Ki-ras p21 and rhoA p21. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6442–6446. doi: 10.1073/pnas.88.15.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. M., Giannakouros T., Hancock J. F., Fawell E. H., Armstrong J., Magee A. I. Post-translational processing of Schizosaccharomyces pombe YPT proteins. J Biol Chem. 1992 Jun 5;267(16):11329–11336. [PubMed] [Google Scholar]
- Peter M., Chavrier P., Nigg E. A., Zerial M. Isoprenylation of rab proteins on structurally distinct cysteine motifs. J Cell Sci. 1992 Aug;102(Pt 4):857–865. doi: 10.1242/jcs.102.4.857. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Hiroyoshi M., Isomura M., Araki S., Sasaki T., Takai Y. Inhibition of the action of the stimulatory GDP/GTP exchange protein for smg p21 by the geranylgeranylated synthetic peptides designed from its C-terminal region. J Biol Chem. 1991 Nov 5;266(31):20672–20677. [PubMed] [Google Scholar]
- Shou C., Farnsworth C. L., Neel B. G., Feig L. A. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992 Jul 23;358(6384):351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tan E. W., Pérez-Sala D., Cañada F. J., Rando R. R. Identifying the recognition unit for G protein methylation. J Biol Chem. 1991 Jun 15;266(17):10719–10722. [PubMed] [Google Scholar]
- Ueda T., Kikuchi A., Ohga N., Yamamoto J., Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Jun 5;265(16):9373–9380. [PubMed] [Google Scholar]