Abstract
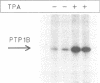
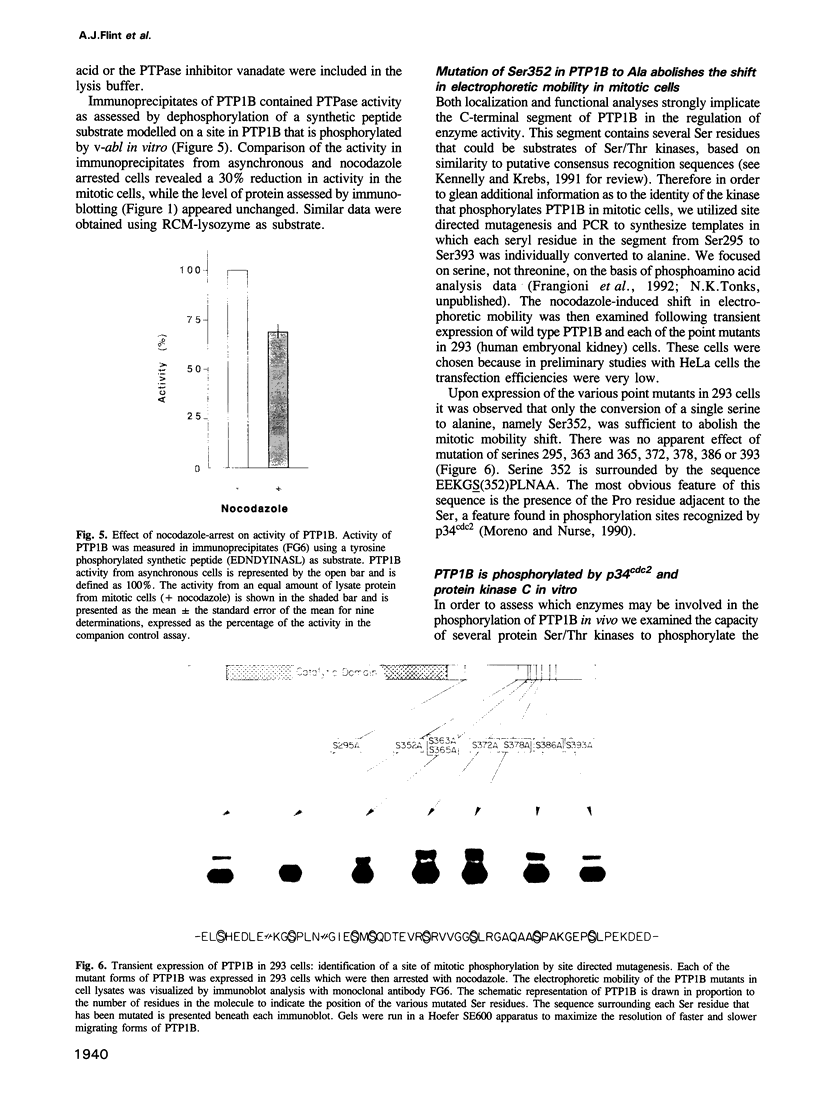
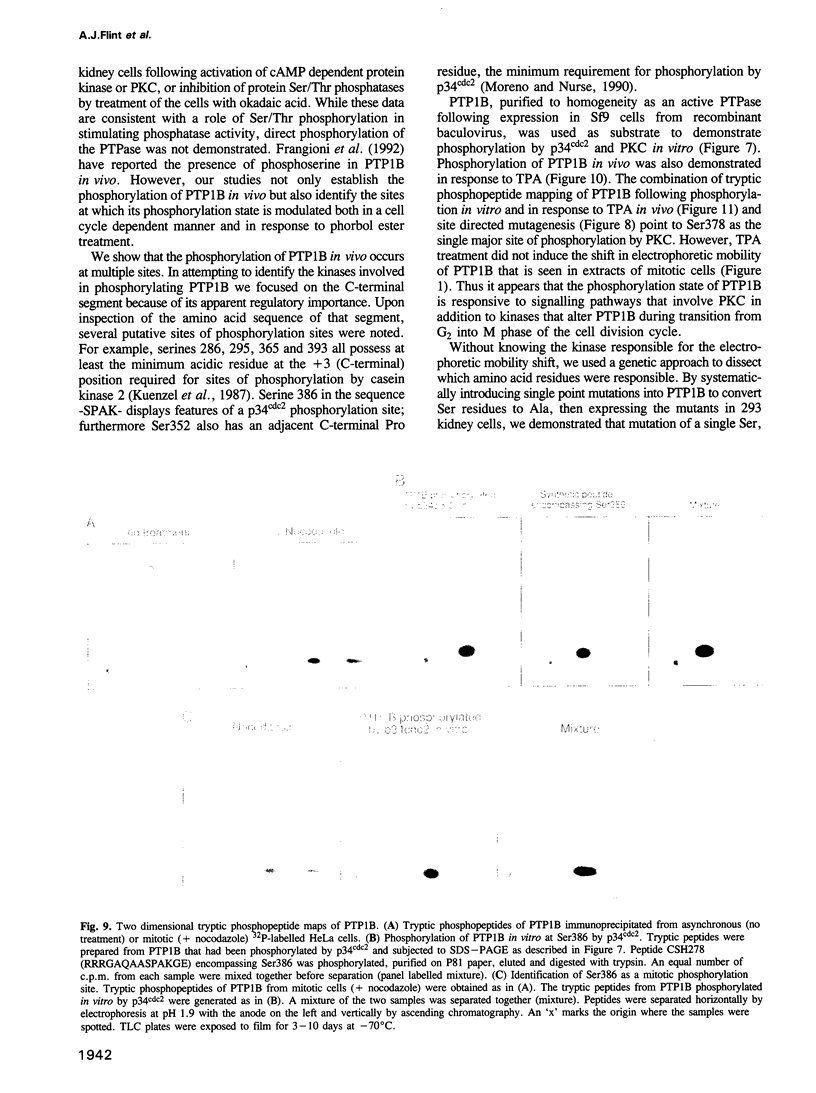
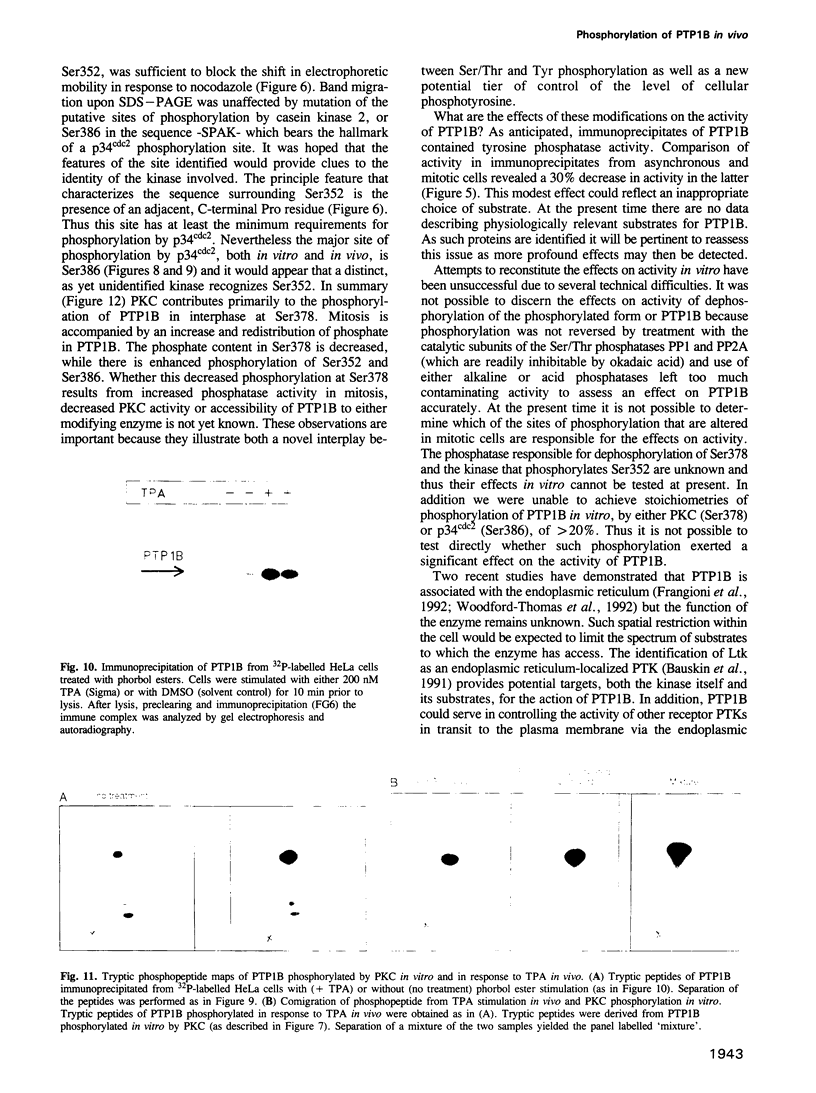
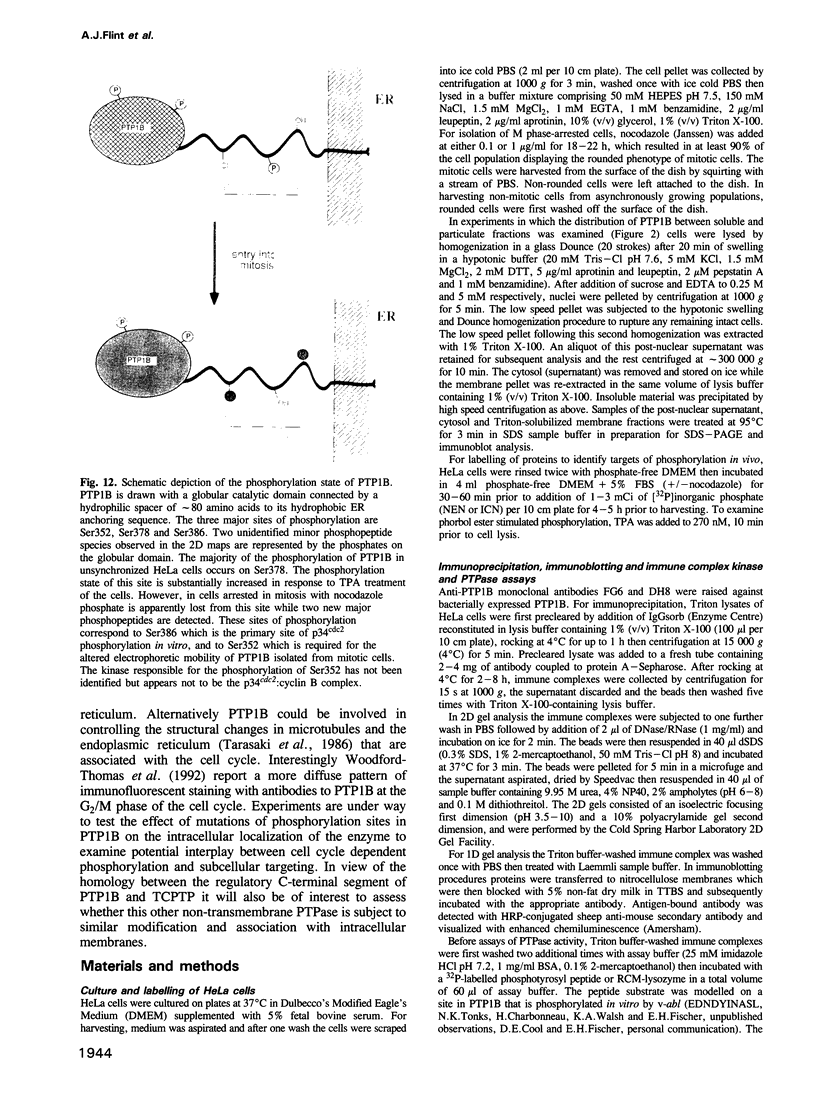
The non-transmembrane protein tyrosine phosphatase, PTP1B, comprises 435 amino acids, of which the C-terminal 114 residues have been implicated in controlling both localization and function of this enzyme. Inspection of the sequence of the C-terminal segment reveals a number of potential sites of phosphorylation. We show that PTP1B is phosphorylated on seryl residues in vivo. Increased phosphorylation of PTP1B is seen to accompany the transition from G2 to M phase of the cell cycle. Two major tryptic phosphopeptides appear in two-dimensional maps of PTP1B from mitotic cells. One of these comigrates with the peptide generated following phosphorylation of PTP1B in vitro at Ser386 by the mitotic protein Ser/Thr kinase p34cdc2:cyclin B. The site of phosphorylation that is responsible for the pronounced retardation in the electrophoretic mobility of PTP1B from mitotic cells has been identified by site directed mutagenesis as Ser352. The identify of the kinase responsible for this modification is presently unknown. We also show that stimulation of HeLa cells with the phorbol ester TPA enhances phosphorylation of PTP1B. Two dimensional phosphopeptide mapping reveals that the bulk of the phosphate is in a single tryptic peptide. The site, identified as Ser378, is also the site of phosphorylation by protein kinase C (PKC) in vitro. Thus the TPA-stimulated phosphorylation of PTP1B in vivo appears to result directly from phosphorylation by PKC. The effect of phosphorylation on the activity of PTP1B has been examined in immunoprecipitates from TPA-treated and nocodazole-arrested cells. TPA treatment does not appear to affect activity directly, whereas the activity of PTP1B from nocodazole-arrested cells is only 70% of that from asynchronous populations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autero M., Gahmberg C. G. Phorbol diesters increase the phosphorylation of the leukocyte common antigen CD45 in human T cells. Eur J Immunol. 1987 Oct;17(10):1503–1506. doi: 10.1002/eji.1830171018. [DOI] [PubMed] [Google Scholar]
- Bauskin A. R., Alkalay I., Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991 Aug 23;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Pinault F. M. Activation of membrane protein-tyrosine phosphatase involving cAMP- and Ca2+/phospholipid-dependent protein kinases. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6696–6700. doi: 10.1073/pnas.88.15.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Shimer S., Johnson K. A., Hill D. E., Bruskin A. M. Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res. 1992 Jan 15;52(2):478–482. [PubMed] [Google Scholar]
- Brown-Shimer S., Johnson K. A., Lawrence J. B., Johnson C., Bruskin A., Green N. R., Hill D. E. Molecular cloning and chromosome mapping of the human gene encoding protein phosphotyrosyl phosphatase 1B. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5148–5152. doi: 10.1073/pnas.87.13.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Chernoff J., Schievella A. R., Jost C. A., Erikson R. L., Neel B. G. Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2735–2739. doi: 10.1073/pnas.87.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Fischer E. H., Krebs E. G. Expression of a human T-cell protein-tyrosine-phosphatase in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7280–7284. doi: 10.1073/pnas.87.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Walsh K. A., Fischer E. H., Krebs E. G. cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5257–5261. doi: 10.1073/pnas.86.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992 Feb 7;68(3):545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Gu M. X., York J. D., Warshawsky I., Majerus P. W. Identification, cloning, and expression of a cytosolic megakaryocyte protein-tyrosine-phosphatase with sequence homology to cytoskeletal protein 4.1. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5867–5871. doi: 10.1073/pnas.88.13.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Warshawsky I., Majerus P. W. Cloning and expression of a cytosolic megakaryocyte protein-tyrosine-phosphatase with sequence homology to retinaldehyde-binding protein and yeast SEC14p. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2980–2984. doi: 10.1073/pnas.89.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Lombroso P. J., Murdoch G., Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. J., Bowne D. B., Flores E., Thomas M. L. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992 May;12(5):2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Trowbridge I. S. Negative regulation of CD45 protein tyrosine phosphatase activity by ionomycin in T cells. Science. 1991 Sep 20;253(5026):1423–1425. doi: 10.1126/science.1654595. [DOI] [PubMed] [Google Scholar]
- Ota I. M., Varshavsky A. A gene encoding a putative tyrosine phosphatase suppresses lethality of an N-end rule-dependent mutant. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2355–2359. doi: 10.1073/pnas.89.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottilie S., Chernoff J., Hannig G., Hoffman C. S., Erikson R. L. A fission-yeast gene encoding a protein with features of protein-tyrosine-phosphatases. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3455–3459. doi: 10.1073/pnas.88.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzky J., Neel B. G., Rosenberg R. D. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Bastien L., Posner B. I., Chrétien P. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature. 1991 Aug 22;352(6337):736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- Stover D. R., Charbonneau H., Tonks N. K., Walsh K. A. Protein-tyrosine-phosphatase CD45 is phosphorylated transiently on tyrosine upon activation of Jurkat T cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7704–7707. doi: 10.1073/pnas.88.17.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Chen L. B., Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986 Oct;103(4):1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
- Woodford-Thomas T. A., Rhodes J. D., Dixon J. E. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992 Apr;117(2):401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Co D., Sommercorn J., Tonks N. K. Cloning and expression of PTP-PEST. A novel, human, nontransmembrane protein tyrosine phosphatase. J Biol Chem. 1993 Mar 25;268(9):6622–6628. [PubMed] [Google Scholar]
- Yang Q., Tonks N. K. Isolation of a cDNA clone encoding a human protein-tyrosine phosphatase with homology to the cytoskeletal-associated proteins band 4.1, ezrin, and talin. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5949–5953. doi: 10.1073/pnas.88.14.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T. L., Cleveland J. L., Ihle J. N. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992 Feb;12(2):836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander N. F., Lorenzen J. A., Cool D. E., Tonks N. K., Daum G., Krebs E. G., Fischer E. H. Purification and characterization of a human recombinant T-cell protein-tyrosine-phosphatase from a baculovirus expression system. Biochemistry. 1991 Jul 16;30(28):6964–6970. doi: 10.1021/bi00242a022. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Huber L. A., Mâle P., Goud B., Mellman I. Reversible phosphorylation--dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992 Dec;11(12):4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]