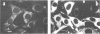Abstract
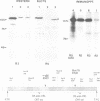
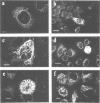
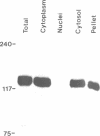
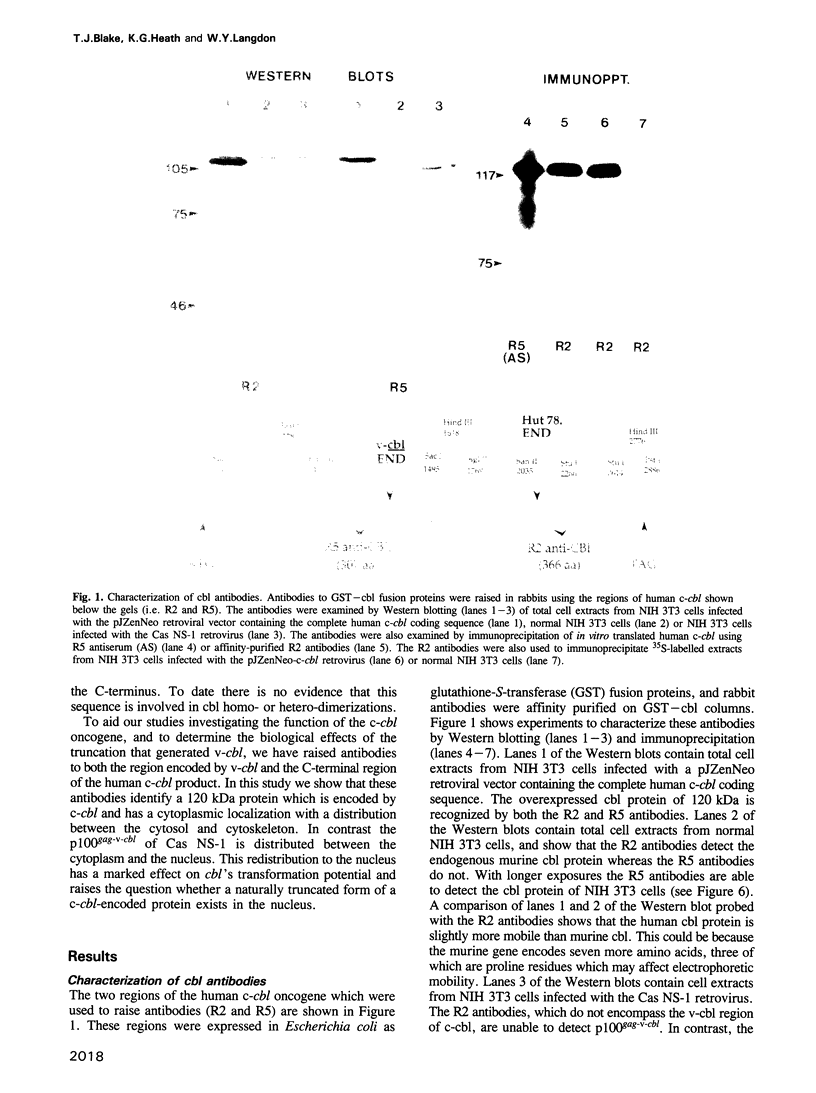
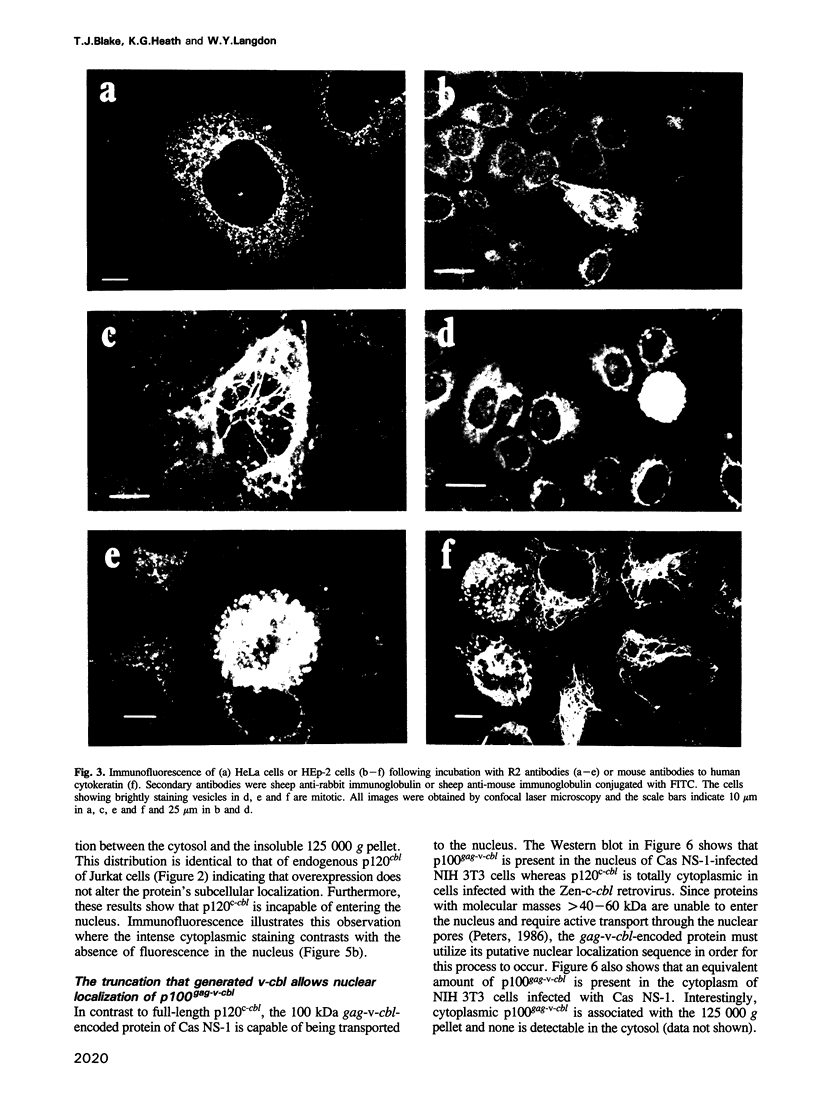
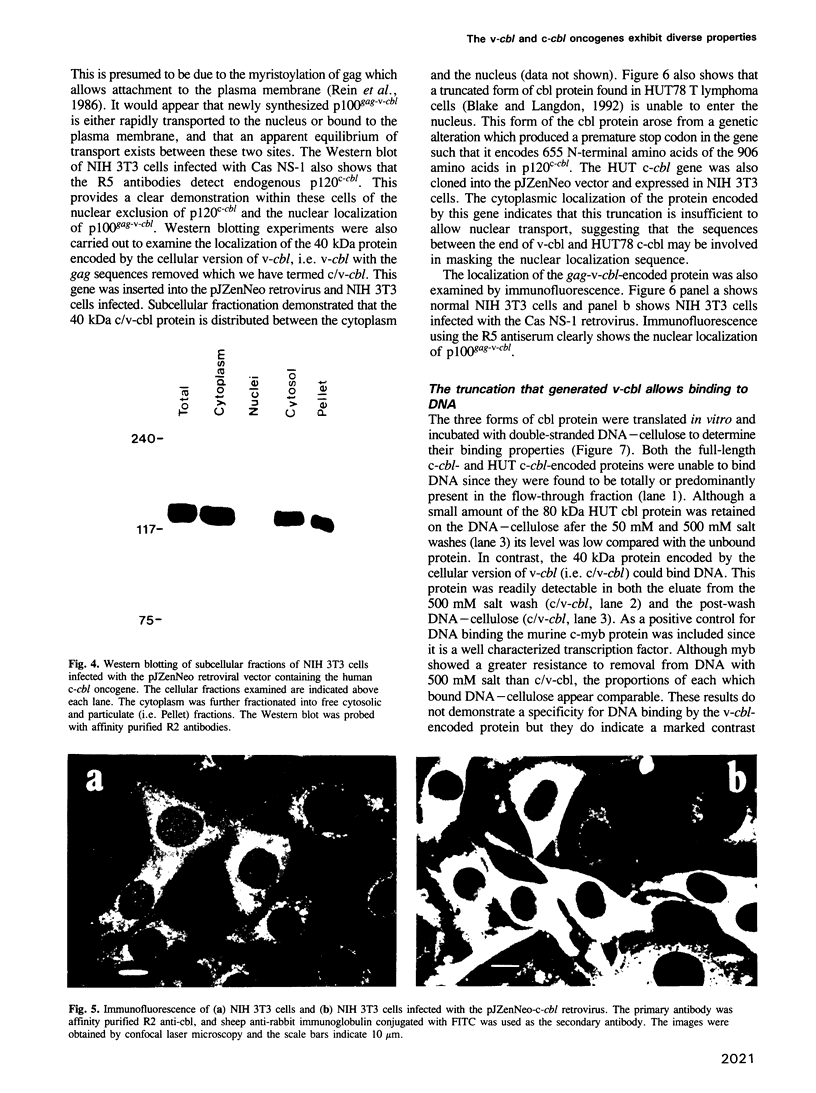
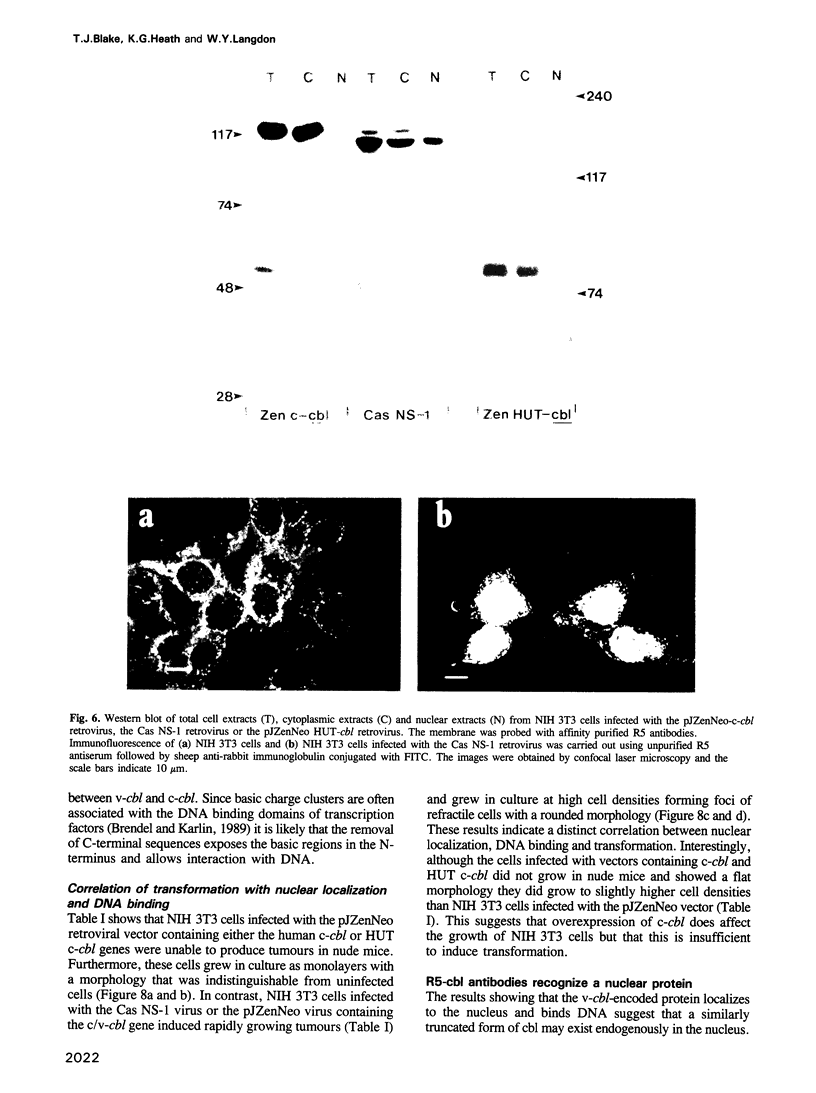
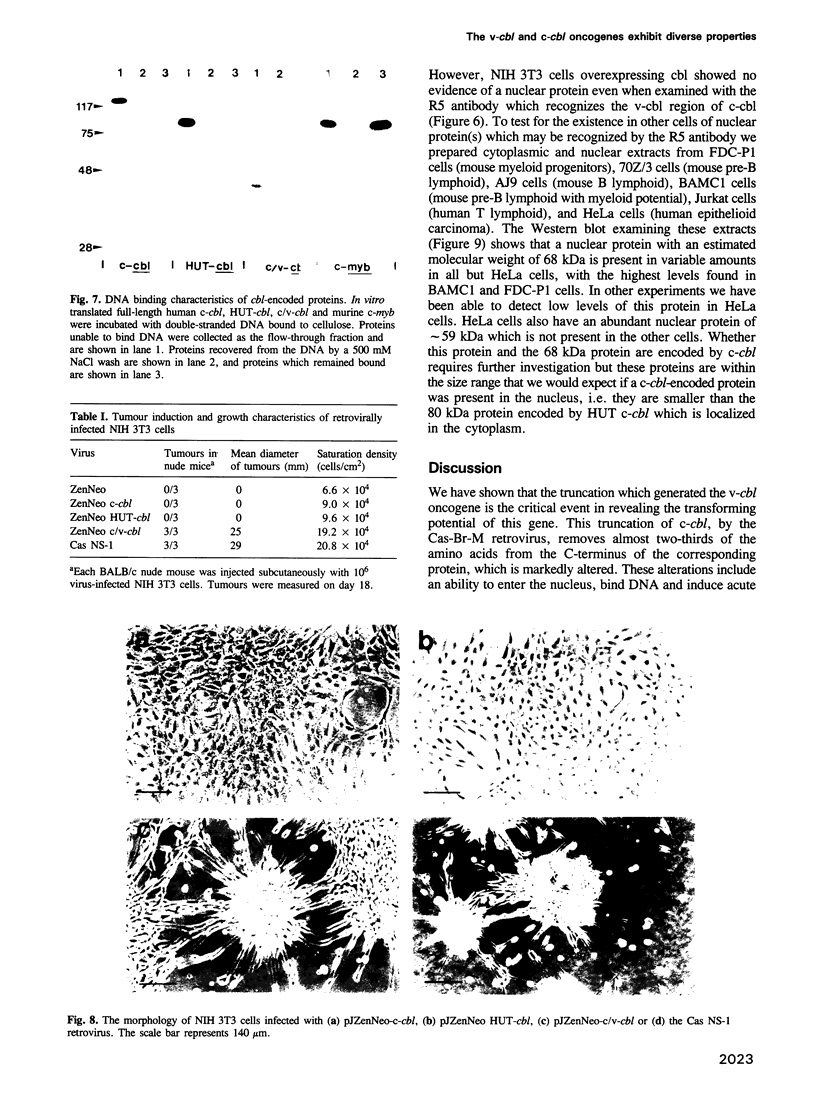
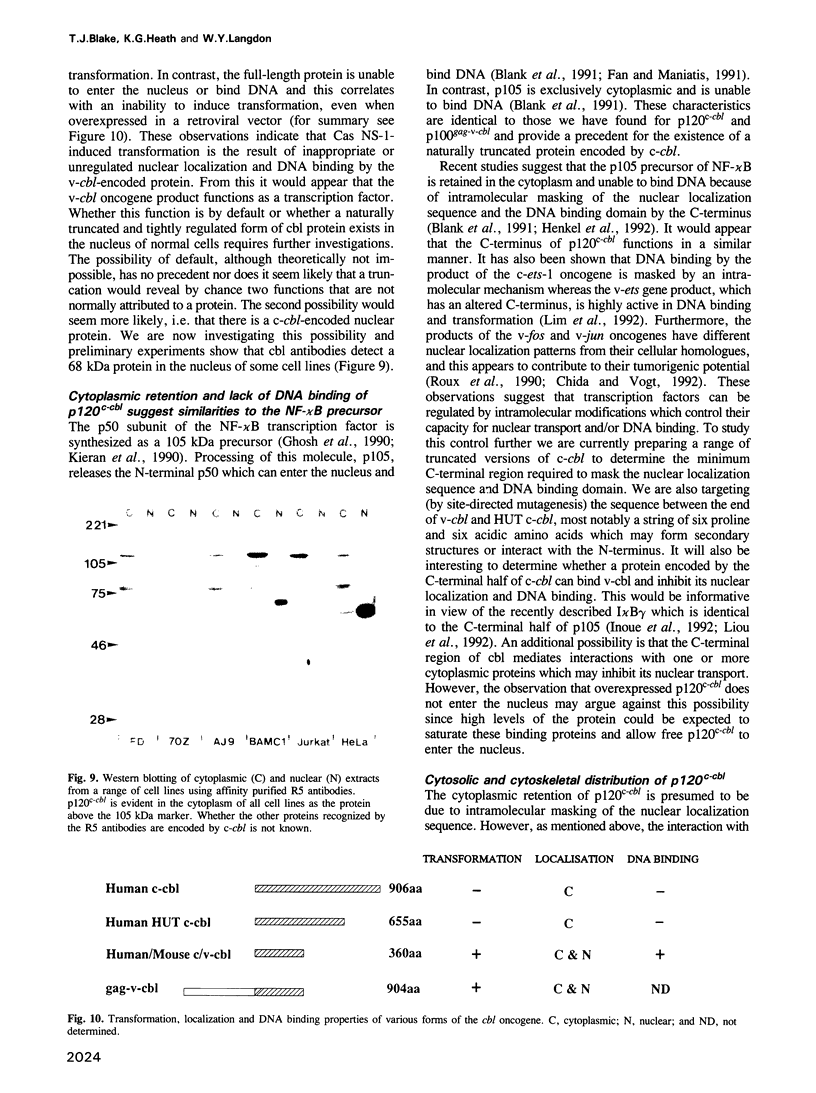
The v-cbl oncogene is the transforming gene of the murine Cas NS-1 retrovirus which induces pre-B cell lymphomas and myeloid leukaemias. Sequencing of c-cbl has revealed that v-cbl was generated by a large truncation that removed 60% of the C-terminus of the corresponding protein. In this study we prepared antibodies to cbl and found that c-cbl encodes a 120 kDa protein which is localized in the cytoplasm with a cytosolic and cytoskeletal distribution. Immunofluorescence studies show a striking pattern of brightly staining vesicles in mitotic cells similar to that observed with cytokeratin antibodies. In contrast to p120c-cbl, which is exclusively cytoplasmic, the p100gag-v-cbl encoded by Cas NS-1 is localized in both the cytoplasm and the nucleus. This redistribution to the nucleus correlates with the ability of cbl to induce acute transformation. Furthermore the truncated protein encoded by v-cbl can bind DNA, unlike the full-length protein. These results suggest that the C-terminus of cbl is involved in the retention of p120c-cbl in the cytoplasm and the inhibition of DNA binding. The findings also suggest that a truncated protein encoded by c-cbl exists in the nucleus of normal cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Blake T. J., Langdon W. Y. A rearrangement of the c-cbl proto-oncogene in HUT78 T-lymphoma cells results in a truncated protein. Oncogene. 1992 Apr;7(4):757–762. [PubMed] [Google Scholar]
- Blake T. J., Shapiro M., Morse H. C., 3rd, Langdon W. Y. The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene. 1991 Apr;6(4):653–657. [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. Cytoplasmic retention, DNA binding and processing of the NF-kappa B p50 precursor are controlled by a small region in its C-terminus. EMBO J. 1991 Dec;10(13):4159–4167. doi: 10.1002/j.1460-2075.1991.tb04994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Karlin S. Association of charge clusters with functional domains of cellular transcription factors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5698–5702. doi: 10.1073/pnas.86.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida K., Vogt P. K. Nuclear translocation of viral Jun but not of cellular Jun is cell cycle dependent. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4290–4294. doi: 10.1073/pnas.89.10.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter F. E., Lillington D., Hampton G., Riddle P., Nasipuri S., Gibbons B., Young B. D. Gene mapping by microdissection and enzymatic amplification: heterogeneity in leukaemia associated breakpoints on chromosome 11. Genes Chromosomes Cancer. 1991 Jan;3(1):8–15. doi: 10.1002/gcc.2870030103. [DOI] [PubMed] [Google Scholar]
- Das S., Cotter F. E., Gibbons B., Dhut S., Young B. D. CD3G is within 200 kb of the leukemic t(4;11) translocation breakpoint. Genes Chromosomes Cancer. 1991 Jan;3(1):44–47. doi: 10.1002/gcc.2870030108. [DOI] [PubMed] [Google Scholar]
- Der C. J., Cox A. D. Isoprenoid modification and plasma membrane association: critical factors for ras oncogenicity. Cancer Cells. 1991 Sep;3(9):331–340. [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991 Dec 5;354(6352):395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Henkel T., Zabel U., van Zee K., Müller J. M., Fanning E., Baeuerle P. A. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell. 1992 Mar 20;68(6):1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Kakizuka A., Verma I. M. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992 Mar 20;68(6):1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Hartley J. W., Klinken S. P., Ruscetti S. K., Morse H. C., 3rd v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F., Kraut N., Framptom J., Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992 Feb;11(2):643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H. C., Nolan G. P., Ghosh S., Fujita T., Baltimore D. The NF-kappa B p50 precursor, p105, contains an internal I kappa B-like inhibitor that preferentially inhibits p50. EMBO J. 1992 Aug;11(8):3003–3009. doi: 10.1002/j.1460-2075.1992.tb05370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Thorley-Lawson D. Posttranslational processing of the Epstein-Barr virus-encoded p63/LMP protein. J Virol. 1987 Jul;61(7):2100–2108. doi: 10.1128/jvi.61.7.2100-2108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim Biophys Acta. 1986 Dec 22;864(3-4):305–359. doi: 10.1016/0304-4157(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Raimondi S. C., Peiper S. C., Kitchingman G. R., Behm F. G., Williams D. L., Hancock M. L., Mirro J., Jr Childhood acute lymphoblastic leukemia with chromosomal breakpoints at 11q23. Blood. 1989 May 1;73(6):1627–1634. [PubMed] [Google Scholar]
- Regnier D. C., Kozak C. A., Kingsley D. M., Jenkins N. A., Copeland N. G., Langdon W. Y., Morse H. C., 3rd Identification of two murine loci homologous to the v-cbl oncogene. J Virol. 1989 Sep;63(9):3678–3682. doi: 10.1128/jvi.63.9.3678-3682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P., Blanchard J. M., Fernandez A., Lamb N., Jeanteur P., Piechaczyk M. Nuclear localization of c-Fos, but not v-Fos proteins, is controlled by extracellular signals. Cell. 1990 Oct 19;63(2):341–351. doi: 10.1016/0092-8674(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Diaz M. O., Espinosa R., 3rd, Patel Y. D., van Melle E., Ziemin S., Taillon-Miller P., Lichter P., Evans G. A., Kersey J. H. Mapping chromosome band 11q23 in human acute leukemia with biotinylated probes: identification of 11q23 translocation breakpoints with a yeast artificial chromosome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage P. D., Shapiro M., Langdon W. Y., Geurts van Kessel A. D., Seuanez H. N., Akao Y., Croce C., Morse H. C., 3rd, Kersey J. H. Relationship of the human protooncogene CBL2 on 11q23 to the t(4;11), t(11;22), and t(11;14) breakpoints. Cytogenet Cell Genet. 1991;56(2):112–115. doi: 10.1159/000133062. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., McGuire R. S. A physical linkage group in human chromosome band 11q23 covering a region implicated in leukocyte neoplasia. Genomics. 1990 Nov;8(3):447–453. doi: 10.1016/0888-7543(90)90030-x. [DOI] [PubMed] [Google Scholar]
- Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992 Sep 24;359(6393):336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]