Abstract
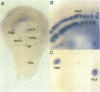
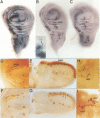
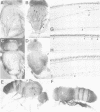
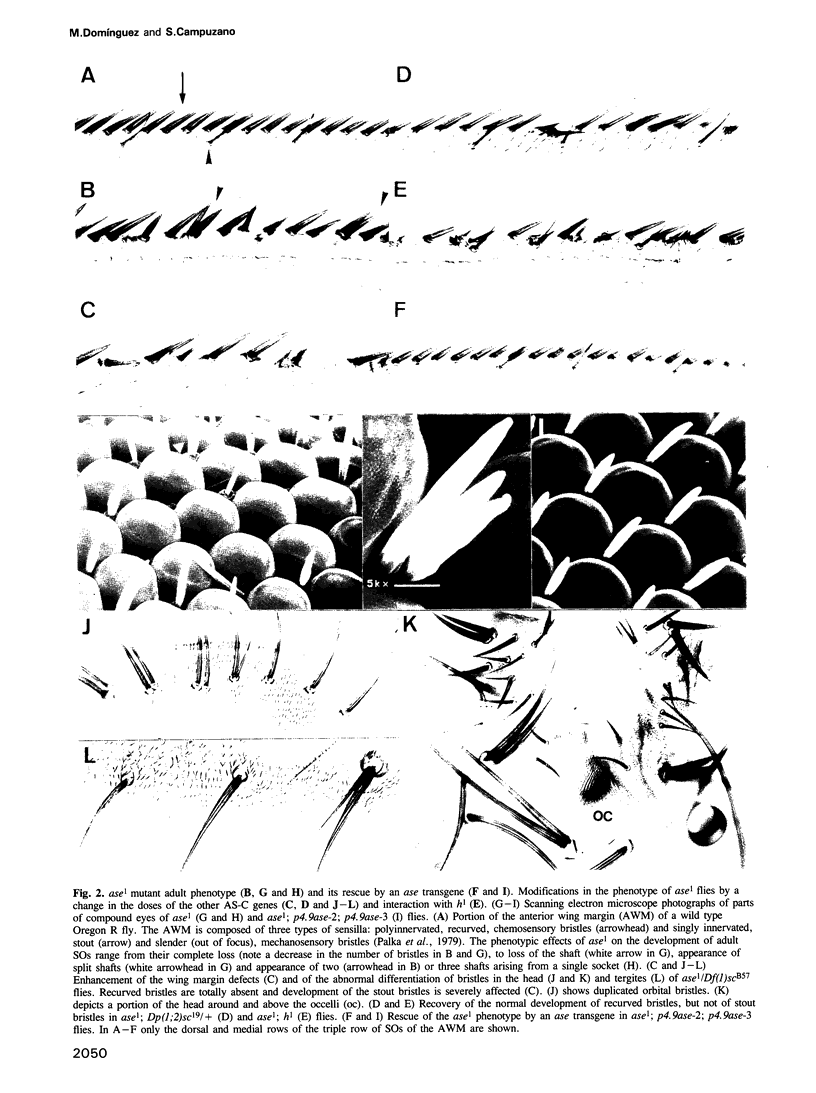
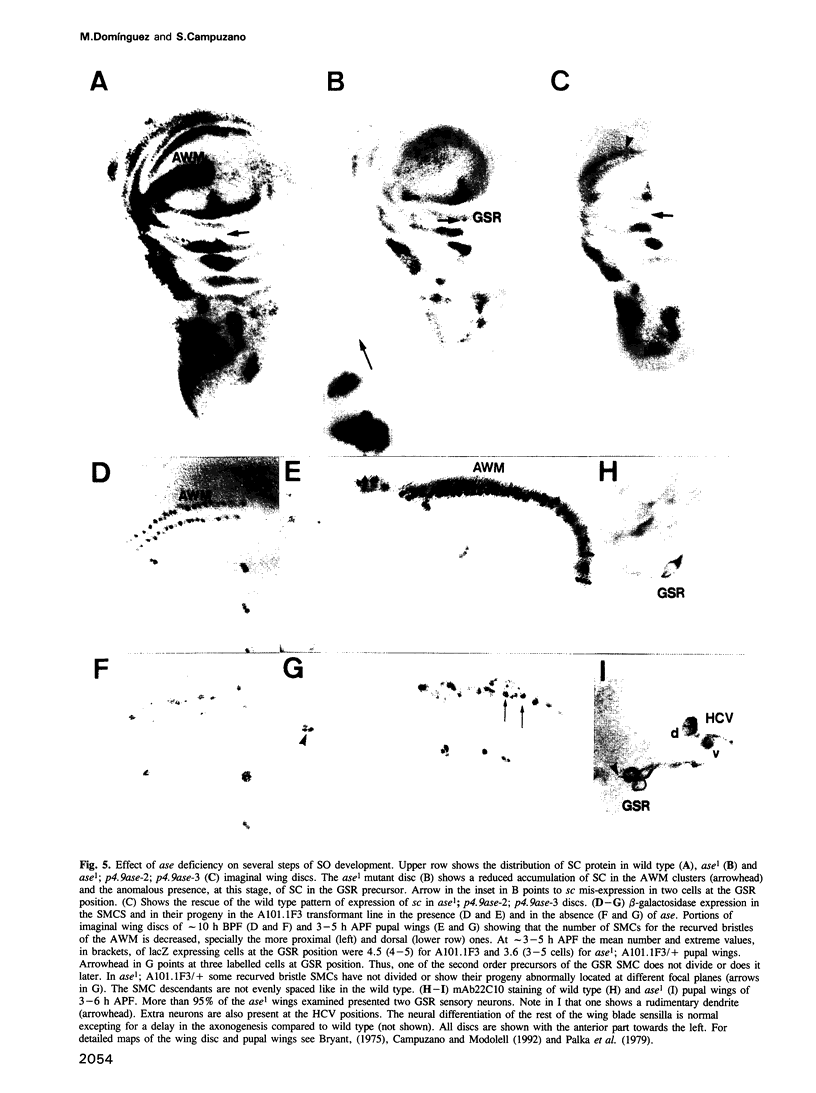
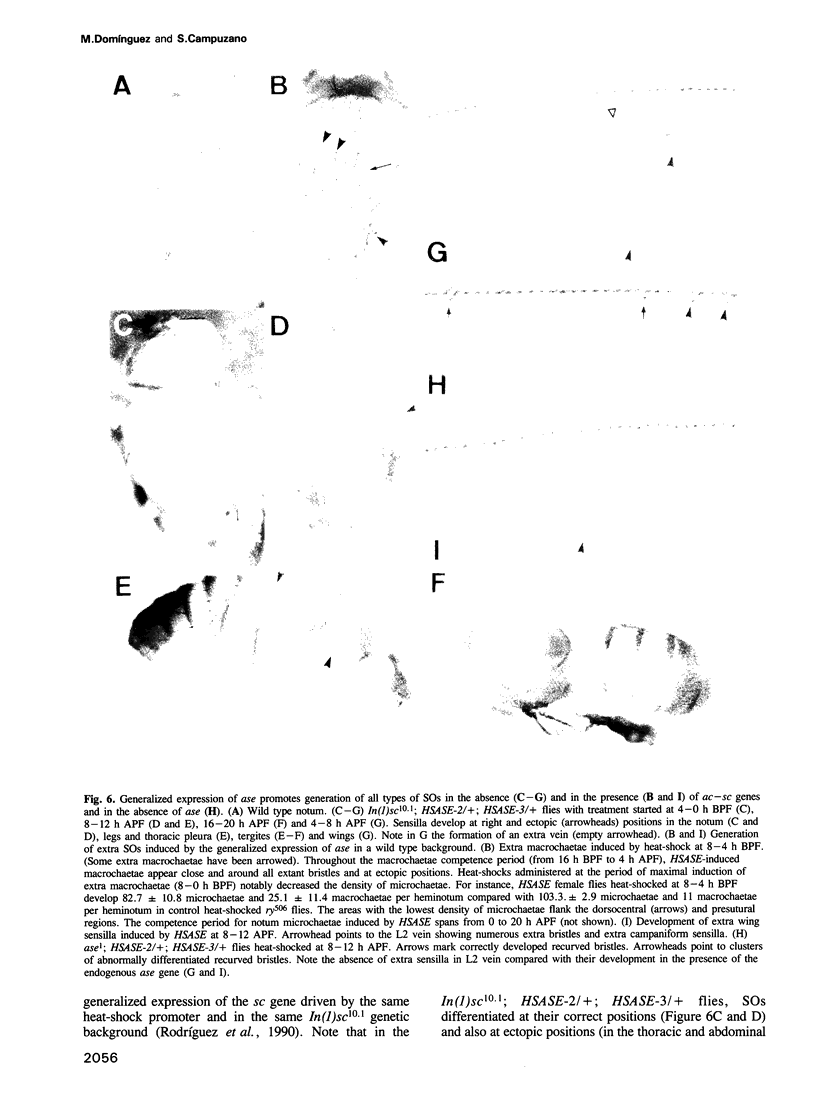
The asense (ase) gene of the achaete-scute complex (AS-C) is expressed in the precursors of all adult sensory organs (SOs), the sensory mother cells (SMCs) and in their immediate progeny. Its deletion causes the loss of some SOs and the abnormal differentiation of part of the remaining ones. These defects, which include malformations of the external part of the SOs, duplication of the innervating neuron etc, are enhanced by the haploid condition for the other AS-C genes and are corrected by an ase transgene. We conclude that ase participates, in combination with other members of the AS-C, in implementing the neural program of differentiation of the SMCs. ase also has a proneural function that participates in the singling out of the SMCs that give rise to the recurved bristles of the anterior wing margin. The proneural potential of ase is shown, in addition, by the generation of SOs induced by the generalized expression of an ase gene driven by a hsp70 promoter.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. C., Cabrera C. V. The achaete-scute gene complex of Drosophila melanogaster comprises four homologous genes. EMBO J. 1988 Aug;7(8):2585–2591. doi: 10.1002/j.1460-2075.1988.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells L., Modolell J., Ruiz-Gómez M. A unitary basis for different Hairy-wing mutations of Drosophila melanogaster. EMBO J. 1988 Dec 1;7(12):3899–3906. doi: 10.1002/j.1460-2075.1988.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang A. G., Hartenstein V., Posakony J. W. Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development. 1991 Jan;111(1):89–104. doi: 10.1242/dev.111.1.89. [DOI] [PubMed] [Google Scholar]
- Bang A. G., Posakony J. W. The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev. 1992 Sep;6(9):1752–1769. doi: 10.1101/gad.6.9.1752. [DOI] [PubMed] [Google Scholar]
- Blair S. S., Giangrande A., Skeath J. B., Palka J. The development of normal and ectopic sensilla in the wings of hairy and Hairy wing mutants of Drosophila. Mech Dev. 1992 Jul;38(1):3–16. doi: 10.1016/0925-4773(92)90033-g. [DOI] [PubMed] [Google Scholar]
- Bodmer R., Barbel S., Sheperd S., Jack J. W., Jan L. Y., Jan Y. N. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987 Oct 23;51(2):293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Bodmer R., Carretto R., Jan Y. N. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989 Jul;3(1):21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Boulianne G. L., de la Concha A., Campos-Ortega J. A., Jan L. Y., Jan Y. N. The Drosophila neurogenic gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. EMBO J. 1991 Oct;10(10):2975–2983. doi: 10.1002/j.1460-2075.1991.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant P. J. Pattern formation in the imaginal wing disc of Drosophila melanogaster: fate map, regeneration and duplication. J Exp Zool. 1975 Jul;193(1):49–77. doi: 10.1002/jez.1401930106. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development. 1990 Sep;110(1):733–742. [PubMed] [Google Scholar]
- Campos-Ortega J. A., Jan Y. N. Genetic and molecular bases of neurogenesis in Drosophila melanogaster. Annu Rev Neurosci. 1991;14:399–420. doi: 10.1146/annurev.ne.14.030191.002151. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Balcells L., Villares R., Carramolino L., García-Alonso L., Modolell J. Excess function hairy-wing mutations caused by gypsy and copia insertions within structural genes of the achaete-scute locus of Drosophila. Cell. 1986 Jan 31;44(2):303–312. doi: 10.1016/0092-8674(86)90764-6. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992 Jun;8(6):202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Caudy M., Vässin H., Brand M., Tuma R., Jan L. Y., Jan Y. N. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988 Dec 23;55(6):1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Cubas P., Modolell J. The extramacrochaetae gene provides information for sensory organ patterning. EMBO J. 1992 Sep;11(9):3385–3393. doi: 10.1002/j.1460-2075.1992.tb05417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas P., de Celis J. F., Campuzano S., Modolell J. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 1991 Jun;5(6):996–1008. doi: 10.1101/gad.5.6.996. [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Jamet E., Burri M., Bopp D., Basler K., Hafen E., Dumont N., Spielmann P., Ghysen A., Noll M. The paired box gene pox neuro: a determinant of poly-innervated sense organs in Drosophila. Cell. 1992 Apr 3;69(1):159–172. doi: 10.1016/0092-8674(92)90127-x. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Spann D. R., Posakony J. W. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990 Apr 6;61(1):27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- Fujita S. C., Zipursky S. L., Benzer S., Ferrús A., Shotwell S. L. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7929–7933. doi: 10.1073/pnas.79.24.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A. Genetic Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1979 Mar;91(3):491–520. doi: 10.1093/genetics/91.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A., Santamaria P. Developmental Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1978 Mar;88(3):469–486. doi: 10.1093/genetics/88.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell J., Campuzano S. The helix-loop-helix domain: a common motif for bristles, muscles and sex. Bioessays. 1991 Oct;13(10):493–498. doi: 10.1002/bies.950131002. [DOI] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. Genesis of the Drosophila peripheral nervous system. Trends Genet. 1989 Aug;5(8):251–255. doi: 10.1016/0168-9525(89)90097-8. [DOI] [PubMed] [Google Scholar]
- González F., Romani S., Cubas P., Modolell J., Campuzano S. Molecular analysis of the asense gene, a member of the achaete-scute complex of Drosophila melanogaster, and its novel role in optic lobe development. EMBO J. 1989 Dec 1;8(12):3553–3562. doi: 10.1002/j.1460-2075.1989.tb08527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. A dual function of the Notch gene in Drosophila sensillum development. Dev Biol. 1990 Nov;142(1):13–30. doi: 10.1016/0012-1606(90)90147-b. [DOI] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989 Oct;107(2):389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Higashijima S., Michiue T., Emori Y., Saigo K. Subtype determination of Drosophila embryonic external sensory organs by redundant homeo box genes BarH1 and BarH2. Genes Dev. 1992 Jun;6(6):1005–1018. doi: 10.1101/gad.6.6.1005. [DOI] [PubMed] [Google Scholar]
- Huang F., Dambly-Chaudière C., Ghysen A. The emergence of sense organs in the wing disc of Drosophila. Development. 1991 Apr;111(4):1087–1095. doi: 10.1242/dev.111.4.1087. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Genes required for specifying cell fates in Drosophila embryonic sensory nervous system. Trends Neurosci. 1990 Dec;13(12):493–498. doi: 10.1016/0166-2236(90)90083-m. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Neuronal specification. Curr Opin Genet Dev. 1992 Aug;2(4):608–613. doi: 10.1016/s0959-437x(05)80180-7. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Campos-Ortega J. A. Genes in subdivision 1B of the Drosophila melanogaster X-chromosome and their influence on neural development. J Neurogenet. 1987 Jun;4(4):179–200. [PubMed] [Google Scholar]
- Lee L. W., Gerhart J. C. Dependence of transdetermination frequency on the developmental stage of cultured imaginal discs of Drosophila melanogaster. Dev Biol. 1973 Nov;35(1):62–82. doi: 10.1016/0012-1606(73)90007-9. [DOI] [PubMed] [Google Scholar]
- Martín-Bermudo M. D., Martínez C., Rodríguez A., Jiménez F. Distribution and function of the lethal of scute gene product during early neurogenesis in Drosophila. Development. 1991 Oct;113(2):445–454. doi: 10.1242/dev.113.2.445. [DOI] [PubMed] [Google Scholar]
- Martínez C., Modolell J. Cross-regulatory interactions between the proneural achaete and scute genes of Drosophila. Science. 1991 Mar 22;251(5000):1485–1487. doi: 10.1126/science.1900954. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Palka J., Lawrence P. A., Hart H. S. Neural projection patterns from homeotic tissue of Drosophila studied in bithorax mutants and mosaics. Dev Biol. 1979 Apr;69(2):549–575. doi: 10.1016/0012-1606(79)90311-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez I., Hernández R., Modolell J., Ruiz-Gómez M. Competence to develop sensory organs is temporally and spatially regulated in Drosophila epidermal primordia. EMBO J. 1990 Nov;9(11):3583–3592. doi: 10.1002/j.1460-2075.1990.tb07569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Macagno E. R., Modolell J. Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 1989 Jul;3(7):997–1007. doi: 10.1101/gad.3.7.997. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Ghysen A. The expression and role of a proneural gene, achaete, in the development of the larval nervous system of Drosophila. EMBO J. 1993 Mar;12(3):1121–1130. doi: 10.1002/j.1460-2075.1993.tb05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Modolell J. Deletion analysis of the achaete-scute locus of Drosophila melanogaster. Genes Dev. 1987 Dec;1(10):1238–1246. doi: 10.1101/gad.1.10.1238. [DOI] [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. 1987 Feb 26-Mar 4Nature. 325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Schweisguth F., Posakony J. W. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992 Jun 26;69(7):1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development. 1990 Jul;109(3):509–519. doi: 10.1242/dev.109.3.509. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991 Jun;5(6):984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development. 1992 Apr;114(4):939–946. doi: 10.1242/dev.114.4.939. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Marí-Beffa M., García-Bellido A. Cell-autonomous role of Notch, an epidermal growth factor homologue, in sensory organ differentiation in Drosophila. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):632–636. doi: 10.1073/pnas.88.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]