Abstract
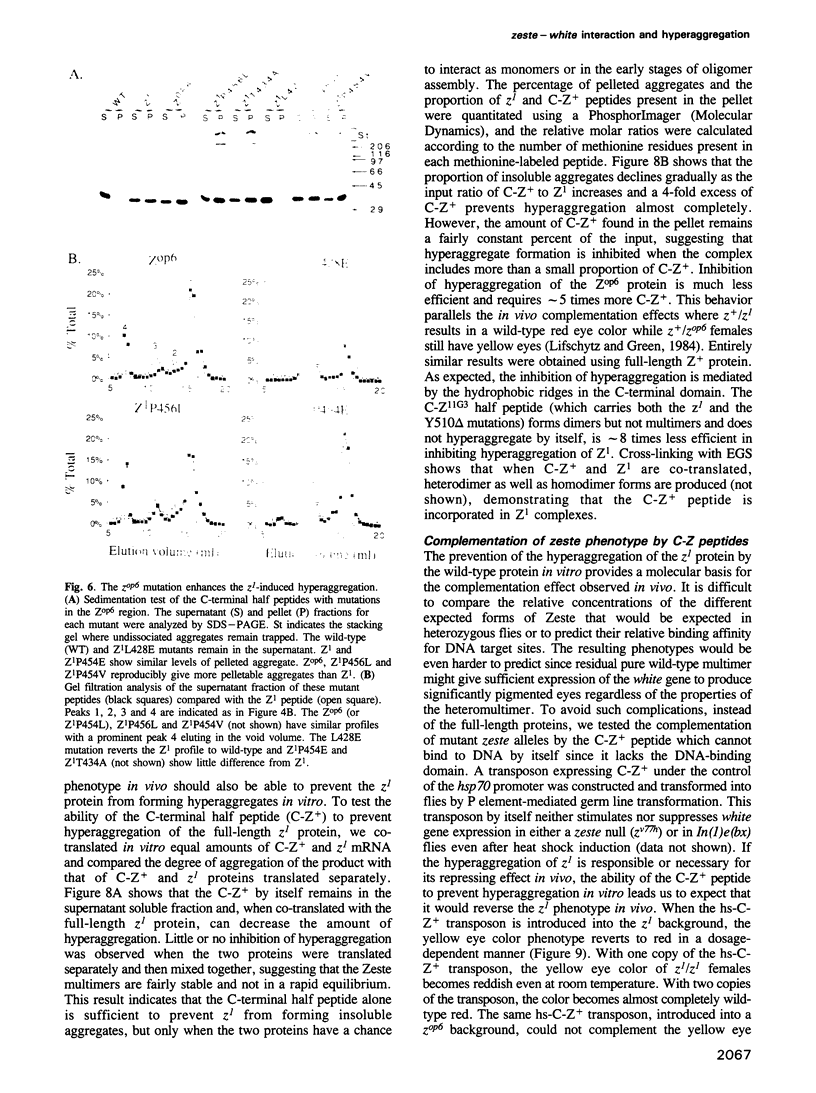
The zeste gene is involved in two chromosome pairing-dependent phenomena: transvection and the suppression of white gene expression. Both require the ability of zeste protein to multimerize, dependent on three interlaced hydrophobic heptad repeats in the C-terminal domain. The first step is dimerization through a leucine zipper. Two other heptad repeats are then required to form higher multimers. The zeta(1) mutation, which causes the pairing-dependent suppression of white, creates a new hydrophobic nucleus that allows the formation of a new and larger aggregate. The zeta(op6) mutation, which suppresses even unpaired copies of white, makes even larger aggregates. The phenotypic suppression of white by a series of mutants is strictly correlated with hyperaggregation and the larger the hyperaggregates, the weaker the requirement for the pairing of white. Hyperaggregation of the Z1 protein in vitro is suppressed by co-translation with the C-terminal peptide of wild-type protein, lacking the DNA-binding domain. This C-Z+ peptide also complements the zeta(1) allele in vivo and restores normal color, demonstrating that zeste product also exists in a multimeric complex in the cell. Complementation in vivo is strictly correlated with the prevention of hyperaggregation of the zeste mutant products in vitro, supporting the interpretation that excessive association of zeta(1) and zeta(op6) proteins is responsible for their repression of white gene expression.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella P. M., Smith P. K., Royer G. P. A new cleavable reagent for cross-linking and reversible immobilization of proteins. Biochem Biophys Res Commun. 1979 Apr 13;87(3):734–742. doi: 10.1016/0006-291x(79)92020-5. [DOI] [PubMed] [Google Scholar]
- Benson M., Pirrotta V. The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J. 1988 Dec 1;7(12):3907–3915. doi: 10.1002/j.1460-2075.1988.tb03277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S., Pirrotta V. Self-association of the Drosophila zeste protein is responsible for transvection effects. EMBO J. 1990 Sep;9(9):2959–2967. doi: 10.1002/j.1460-2075.1990.tb07488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Bickel S., Benson M., Pirrotta V., Tjian R. Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell. 1988 Jun 3;53(5):713–722. doi: 10.1016/0092-8674(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Zachar Z. Evidence that two mutations, wDZL and z1, affecting synapsis-dependent genetic behavior of white are transcriptional regulatory mutations. Cell. 1985 Apr;40(4):819–825. doi: 10.1016/0092-8674(85)90341-1. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Chan C. S., Pirrotta V. Conserved DNA binding and self-association domains of the Drosophila zeste protein. Mol Cell Biol. 1992 Feb;12(2):598–608. doi: 10.1128/mcb.12.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Pirrotta V. Multimerization of the Drosophila zeste protein is required for efficient DNA binding. EMBO J. 1993 May;12(5):2075–2083. doi: 10.1002/j.1460-2075.1993.tb05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A., Milner J. Evidence for allosteric variants of wild-type p53, a tumour suppressor protein. Br J Cancer. 1990 Apr;61(4):548–552. doi: 10.1038/bjc.1990.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gelbart W. M. Synapsis-dependent allelic complementation at the decapentaplegic gene complex in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2636–2640. doi: 10.1073/pnas.79.8.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart W. M., Wu C. T. Interactions of zeste mutations with loci exhibiting transvection effects in Drosophila melanogaster. Genetics. 1982 Oct;102(2):179–189. doi: 10.1093/genetics/102.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G. Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J. 1990 Jul;9(7):2247–2256. doi: 10.1002/j.1460-2075.1990.tb07395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Colvin R. A., Mellin A. F. The Drosophila zeste locus is nonessential. Genetics. 1989 Sep;123(1):145–155. doi: 10.1093/genetics/123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., O'Shea E. K., Kim P. S., Sauer R. T. Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science. 1990 Dec 7;250(4986):1400–1403. doi: 10.1126/science.2147779. [DOI] [PubMed] [Google Scholar]
- Jack J. W., Judd B. H. Allelic pairing and gene regulation: A model for the zeste-white interaction in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1368–1372. doi: 10.1073/pnas.76.3.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman T. C., Tasaka S. E., Suzuki D. T. The interaction of two complex loci, zeste and bithorax in Drosophila melanogaster. Genetics. 1973 Oct;75(2):299–321. doi: 10.1093/genetics/75.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Laney J. D., Biggin M. D. zeste, a nonessential gene, potently activates Ultrabithorax transcription in the Drosophila embryo. Genes Dev. 1992 Aug;6(8):1531–1541. doi: 10.1101/gad.6.8.1531. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Lifschytz E., Green M. M. The zeste-white interaction: induction and genetic analysis of a novel class of zeste alleles. EMBO J. 1984 May;3(5):999–1002. doi: 10.1002/j.1460-2075.1984.tb01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Crickmore A., Sherwood P. W., Goldberg M. L. DNA-binding properties of the Drosophila melanogaster zeste gene product. Mol Cell Biol. 1988 Feb;8(2):615–623. doi: 10.1128/mcb.8.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Gunaratne P. H., Sherwood P. W., Sneath B. J., Goldberg M. L. Nucleotide sequence and structural analysis of the zeste locus of Drosophila melanogaster. Mol Gen Genet. 1988 Jan;211(1):121–128. doi: 10.1007/BF00338402. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Klemm J. D., Kim P. S., Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991 Oct 25;254(5031):539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- Pan D. J., Huang J. D., Courey A. J. Functional analysis of the Drosophila twist promoter reveals a dorsal-binding ventral activator region. Genes Dev. 1991 Oct;5(10):1892–1901. doi: 10.1101/gad.5.10.1892. [DOI] [PubMed] [Google Scholar]
- Paro R. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 1990 Dec;6(12):416–421. doi: 10.1016/0168-9525(90)90303-n. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bickel S., Mariani C. Developmental expression of the Drosophila zeste gene and localization of zeste protein on polytene chromosomes. Genes Dev. 1988 Dec;2(12B):1839–1850. doi: 10.1101/gad.2.12b.1839. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Manet E., Hardon E., Bickel S. E., Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987 Mar;6(3):791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. Transvection and long-distance gene regulation. Bioessays. 1990 Sep;12(9):409–414. doi: 10.1002/bies.950120903. [DOI] [PubMed] [Google Scholar]
- Qian S., Varjavand B., Pirrotta V. Molecular analysis of the zeste-white interaction reveals a promoter-proximal element essential for distant enhancer-promoter communication. Genetics. 1992 May;131(1):79–90. doi: 10.1093/genetics/131.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman R. R., Pirrotta V., Geyer P. K. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 1993 Feb;12(2):435–442. doi: 10.1002/j.1460-2075.1993.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Goldberg M. L. The Drosophila zeste gene and transvection. Trends Genet. 1989 Jun;5(6):189–194. doi: 10.1016/0168-9525(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Jones R. S., Lasko P. F., Gelbart W. M. Homeosis and the interaction of zeste and white in Drosophila. Mol Gen Genet. 1989 Sep;218(3):559–564. doi: 10.1007/BF00332424. [DOI] [PubMed] [Google Scholar]