Abstract
Mycoplasma iowae is a well-established avian pathogen that can infect and damage many sites throughout the body. One potential mediator of cellular damage by mycoplasmas is the production of H2O2 via a glycerol catabolic pathway whose genes are widespread amongst many mycoplasma species. Previous sequencing of M. iowae serovar I strain 695 revealed the presence of not only genes for H2O2 production through glycerol catabolism but also the first documented mycoplasma gene for catalase, which degrades H2O2. To test the activity of M. iowae catalase in degrading H2O2, we studied catalase activity and H2O2 accumulation by both M. iowae serovar K strain DK-CPA, whose genome we sequenced, and strains of the H2O2-producing species Mycoplasma gallisepticum engineered to produce M. iowae catalase by transformation with the M. iowae putative catalase gene, katE. H2O2-mediated virulence by M. iowae serovar K and catalase-producing M. gallisepticum transformants were also analyzed using a Caenorhabditis elegans toxicity assay, which has never previously been used in conjunction with mycoplasmas. We found that M. iowae katE encodes an active catalase that, when expressed in M. gallisepticum, reduces both the amount of H2O2 produced and the amount of damage to C. elegans in the presence of glycerol. Therefore, the correlation between the presence of glycerol catabolism genes and the use of H2O2 as a virulence factor by mycoplasmas might not be absolute.
Introduction
Mycoplasma iowae is an economically important avian mycoplasma primarily associated with turkeys but also occasionally found in chickens [1]. Naturally occurring infections in turkeys commonly result in late embryo mortality, leading to a 2–5% decrease in hatchability, and leg abnormalities in offspring [2]–[4]. Experimental infections give rise to airsacculitis, stunting, poor feathering, and/or leg and joint problems [3], [4]. M. iowae can be recovered from the respiratory tract, gastrointestinal tract, spleen, and kidneys of orally infected day-old poults, suggesting invasiveness [5]. Atypically among cultured mycoplasmas, M. iowae exhibits a predilection for the intestinal tract. Yolk sac inoculation of eight-day old turkey embryos results in intestinal binding and colonization [6]. Oral inoculation of day-old poults also results in intestinal colonization with no signs of disease, with mycoplasmas isolated more frequently from the intestinal walls than its contents, for at least 21 d after inoculation [5].
Phylogenetically, M. iowae is in the Mycoplasma muris cluster within the pneumoniae group, with Mycoplasma penetrans as its best-characterized relative [7]. M. iowae has a terminal organelle with an internal cytoskeleton; this organelle attaches to host cells and is the leading cell pole during gliding motility [6], [8]. M. iowae is one of two known avian mycoplasmas, along with Mycoplasma lipofaciens, that can obtain energy from catabolism of both glucose and arginine [9], [10]. Despite considerable understanding of disease pathology resulting from M. iowae infection, as well as some knowledge about its cellular biology and biochemistry, the virulence factors of M. iowae are unidentified.
A draft genome sequence for M. iowae serovar I strain 695 [11] suggests some potential primary virulence factors, including two copies of genes for proteins related to an ADP-ribosylating and vacuolating toxin characterized in Mycoplasma pneumoniae [12] as well as genes for production of H2O2 through glycerol catabolism, a pathway that is widespread among mycoplasmas. In this pathway glycerol is imported into the cell via either the glycerol facilitator GlpF or an ABC transporter, phosphorylated by GlpK, and finally oxidized to dihydroxyacetone phosphate by GlpO (also known as GlpD in some species), using O2 and resulting in production of H2O2 [13], [14]. The H2O2 generated by GlpO can cause cellular damage either directly or through altering host gene expression [15]. H2O2 has been described as a virulence factor for several pathogenic mycoplasmas, including Mycoplasma mycoides subsp. mycoides, Mycoplasma pneumoniae, and Mycoplasma pulmonis. Onset time of pneumonia caused by M. pulmonis infection is shortened in mice lacking catalase activity, implicating H2O2 [16]. A glpD mutant of M. pneumoniae produces less H2O2 and causes less cytotoxicity than wild-type cells [14]. An M. mycoides strain that lacks components of the glycerol ABC transporter produces less H2O2 and causes disease with delayed onset and decreased severity in experimentally infected cattle as compared with a strain that has all the transport [17]–[19]. Several enzymes have been implicated in reversing H2O2-mediated damage to mycoplasma cells [20], [21], but direct degradation of H2O2 has not been described for these organisms.
It is unclear how well the existence of the glycerol catabolic pathway correlates with actual use of H2O2 as a virulence factor by mycoplasmas. The M. iowae serovar I genome sequence indicates the presence of glpF, glpO, and glpK [11], which could be taken to support a role for H2O2 in virulence of this organism. Thus far uniquely among mycoplasmas, it also contains a gene encoding a putative catalase, an enzyme that degrades H2O2, potentially interfering with H2O2-mediated virulence. In this report, we present the genome sequence of M. iowae serovar K strain DK-CPA, which also contains both the glycerol catabolism genes and the catalase gene, which we designate katE. The activity of the katE gene product as a catalase was tested by assaying for catalase activity in both M. iowae serovar K and katE transformants of Mycoplasma gallisepticum, a prolific H2O2 producer. All strains were also analyzed for the impact of M. iowae catalase on H2O2 production and resulting virulence via Caenorhabditis elegans toxicity assays modified for use with mycoplasmas. Our results, which constitute the first account of an active catalase in mycoplasmas, allow us to address the question of H2O2 as an M. iowae virulence factor.
Materials and Methods
Bacterial strains and growth conditions
Strains used in this study include M. iowae serovar K strain DK-CPA, Mycoplasma genitalium strain G37, M. gallisepticum strain Rlow, and M. gallisepticum transformants 55A–C and 56 A and C. All strains were grown at 37°C in 175-cm2 tissue culture flasks containing 50 mL SP-4 media [22] to mid-log phase (orange color). Transformants 55A-C and 56 A and C were grown in the presence of 4 µg mL−1 tetracycline. Escherichia coli strain DH5α was used for cloning and was grown in Luria-Bertani broth in the presence of 100 µg mL−1 ampicillin or 4 µg mL−1 tetracycline.
Genome sequencing and analysis
Genomic DNA from M. iowae serovar K was sequenced at the Ohio State University Plant-Microbe Genomics Facility using the GS FLX system (454 Life Sciences). It was prepared with the GS FLX Titanium Rapid Library Preparation Kit (Roche) and sequenced using GS FLX Titanium Sequencing Kit XLR70. Shotgun sequencing data were assembled with the GS De Novo Assembler version 2.5.3 (Roche). Annotation was performed as previously described [23]. The draft genome project has been deposited at DDBJ/EMBL/GenBank under accession number AWQU00000000. The draft genomes of serovars K and I were compared using wgVISTA [24].
Catalase sequence analysis
The predicted amino acid sequence of M. iowae serovar K catalase was subjected to PSI-BLAST [25]. Matches reported as having e values of 0 were aligned by CLUSTALX 2.1 [26] using default parameters and the phylogram was inspected using NJplot [27]. Sequences that clustered separately from the group that included M. iowae catalase were removed and the remaining sequences were again subjected to alignment and tree generation. Secondary structure was predicted using SOPMA [28].
Detection of catalase activity
Cells from mid-log phase cultures of M. iowae, M. gallisepticum, and M. gallisepticum transformants were collected by centrifugation at 20,000×g for 20 min and washed three times with cold phosphate-buffered saline (PBS). Cells were smeared onto a clean microscope slide and one drop of 3% H2O2 was added. Catalase activity was indicated by the generation of bubbles.
To analyze H2O2 degradation, M. iowae and M. genitalium were grown in 24-well plate wells containing 1 mL of SP-4 media supplemented with 3% glycerol. Upon reaching mid-log phase, 1 mL of SP-4 containing 3% glycerol or mid-log phase M. iowae culture was added to M. genitalium culture and the amount of H2O2 remaining was measured over time using colorimetric test strips (EM Quant, range 0.5–25 mg L−1). Statistical analysis of results was calculated using repeated measures ANOVA and unpaired Student's T-test. Results represent three biological replicates with one technical replicate each.
Construction of plasmids and transformation with M. iowae katE
To amplify M. iowae katE with a SalI cloning site with or without the addition of a C-terminal 6xHis tag, primers upstream (5′-ATCGGTCGACAAATGCTGCAACAGCTGCAC-3′) and downstream (5′-ATCGGTCGACTAAACACAAAATTTGATTTAATC-3′ or 5′-ATCGGTCGACTTAATGATGGTGATGGTGGTGACCATATGCGTTTAATGGCAAGGT-3′, respectively) were synthesized (Fisher Scientific) and used for PCR with LongAmp Polymerase (NEB). Following addition of A overhangs using Taq polymerase (NEB), PCR products were ligated into linearized plasmid pCR2.1 (Invitrogen) and transformed into chemically competent E. coli cells. Sequencing at the Miami University Center for Bioinformatics and Functional Genomics confirmed 100% sequence identity to M. iowae katE. The resulting plasmids were named pOO54 and pOO53, respectively. These plasmids were digested with SalI and the resulting 1.8-kb DNA fragments were ligated together with pTF20 [29] that had been linearized using SalI. The resulting plasmids were named pOO56 and pOO55, respectively.
To produce M. gallisepticum katE transformants, electrocompetent M. gallisepticum cells were transformed with pOO55 and pOO56 as previously described [30]. Transformants were plated on SP-4 plates containing 4 µg mL−1 tetracycline for selection. Transformants were subjected to three rounds of filter cloning and more than one transformant from each series (named 55A–C and 56 A and C) was analyzed to ensure that results observed were not due to polar effects from the chromosomal transposon insertion site.
H2O2 assay
Methods were adapted from Hames et al. [14]. Fifty-mL cultures of mycoplasmas were grown to mid-log phase. Cells were collected by centrifugation at 20,000×g for 20 min and washed three times with cold buffer containing 67.6 mM HEPES, 140 mM NaCl, and 7 mM MgCl2. Following resuspension to an OD550 of 1.0, 1-mL aliquots were placed in microcentrifuge tubes and incubated at 37°C for 1 h. 100 µM glycerol was added to tubes and samples were incubated at 37°C for an additional 2 h. H2O2 levels were measured using colorimetric test strips. All analyses were performed in quadruplicate. Median and median absolute deviations values were calculated. Statistical significance of results was calculated using one-way ANOVA and Tukey' post hoc test. Results represent three biological replicates with at least two technical replicates each.
Preparation of cell extracts
Fifty-mL cultures of mycoplasma cells were collected by centrifugation at 20,000×g for 20 min and washed three times with cold PBS. The resulting cell pellet was resuspended in 1 mL cold PBS containing 1% sodium dodecyl sulfate and incubated at 37°C for 30 min. Protein concentration in cell lysates was determined using BCA assays (Pierce).
Western blot analysis
Five µg of cell lysates was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel. Following electrophoresis, proteins were transferred to a nitrocellulose membrane (GE) overnight at 100 mAmp. Membrane was probed with anti-6XHis primary antibodies (Immunology Consultants Laboratory, Inc.) at a dilution of 1∶1,000 followed by anti-rabbit immunoglobulin G-alkaline phosphatase secondary antibodies (Promega) at a dilution of 1∶7,500. Bands were visualized with 5-bromo-4-chloro-3-indolyl phosphate and nitro-blue tetrazolium.
PCR analysis
Primers were designed to encompass M. gallisepticum glpF, glpO, and glpK to confirm absence of transposon insertion at this locus. Primers (5′-TCAAGTTCTGCTAGTAGCGG-3′ and 5′-AATGTATCAGATCACGCACC-3′) were synthesized (Fisher Scientific) and used with Taq polymerase for PCR with chromosomal DNA. PCR products were subjected to agarose gel electrophoresis and transformants were compared to M. gallisepticum Rlow.
Caenorhabditis elegans growth conditions
All assays were performed with C. elegans N2 (Bristol). Nematodes were cultivated using standard practices [31]. Briefly, worms were cultivated on Nematode Growth Media (NGM) plates seeded with E. coli strain OP50 as a food source at room temperature on the bench top.
C. elegans killing assays
Plates containing many large, gravid nematodes were bleached to obtain sterile eggs using standard procedures [31]. Eggs were hatched overnight in 10 mL of M9 buffer to obtain L1 larvae. Following incubation with 1 mM glycerol for 5–6 h, L1 larvae were washed with and resuspended in M9 buffer, and approximately 20–40 larvae were aliquotted into 24-well plate wells. The number of live worms per well (indicated by the presence of movement) was counted prior to adding any additional sample. Plates were incubated at room temperature on the bench top for 24 h and live worms were counted to measure survival. Worms were considered dead if they showed no movement in response to shaking of the plate.
To determine the susceptibility of worms to H2O2 using our assay conditions, L1 larvae were incubated with 1 mL of buffer containing 67.6 mM HEPES, 140 mM NaCl, and 7 mM MgCl2 and various concentrations of H2O2. Survival was measured at 24 h as described above. Statistical significance of results was calculated using one-way ANOVA. Results represent three biological replicates with at least two technical replicates each.
Mycoplasma samples were processed as described for hydrogen peroxide assays. Following initial 1-h incubation at 37°C, 1-mL samples were added to 24-well plate wells containing counted nematodes and glycerol. Worm survival was measured after 24 h incubation at room temperature. Conditions were optimized for maximum H2O2 production at room temperature by varying the OD550 of M. gallisepticum Rlow and concentrations of glycerol. The concentration of H2O2 under these optimized conditions was established at 2-h intervals for each strain using the colorimetric test strips. Final nematode killing assays were performed with mycoplasmas at an OD550 of 0.75 in the presence or absence of 1 mM glycerol. Statistical significance of results was calculated using one-way ANOVA with Tukey' post hoc test and unpaired Student's T-test. Results represent three biological replicates with at least two technical replicates each.
Results
Analysis of M. iowae serovar K for catalase activity
We obtained a draft genome sequence of M. iowae serovar K, isolated from a turkey embryo [32]. The genomes of serovars K and I, which was isolated from a turkey air sac [32], are well conserved, with >99% nucleotide sequence conservation in most genes, and more substantive differences in genes encoding predicted lipoproteins and some hypothetical proteins. Differences in gene content are almost completely restricted to restriction systems, transposase fragments, and lipoprotein paralogs, plus the deletion in serovar K of two putative glycoconjugate synthesis genes.
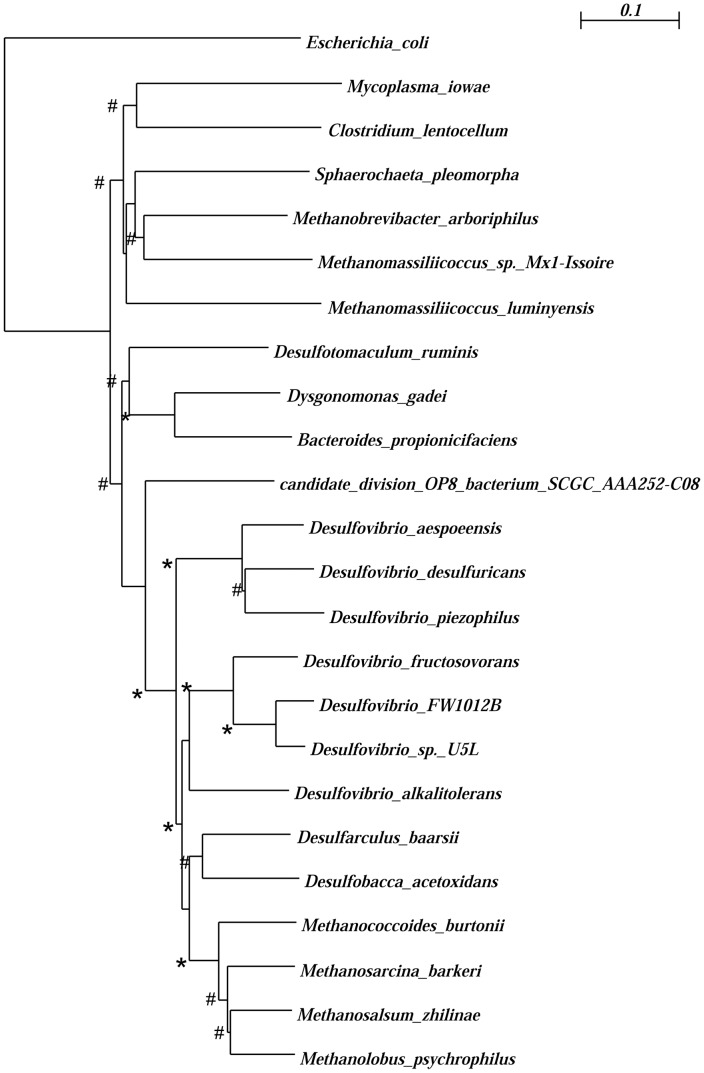
glpF, glpK, and glpO were present, constituting a complete pathway for assimilation of free glycerol and H2O2 production. However, glpU and glpQ, which import and break down host glycerophospholipids to glycerol-3-phosphate, the substrate for GlpO [33], [34], were absent. These findings suggest that M. iowae cannot use host cell phospholipids as a source of glycerol, leaving free glycerol, perhaps derived from host cells and/or other microflora, as the only source of substrate for GlpO. Although the genome had a typical complement of mycoplasmal genes associated with antioxidant functions, including genes encoding lipid hydroperoxide peroxidase, glutathione peroxidase, thioredoxins and thioredoxin reductases, flavodoxin and flavodoxin reductase, peroxiredoxin, and an OsmC-like protein possibly functioning in organic hydroperoxide reduction, two other genes stood out. One was a gene for superoxide dismutase, which among mycoplasmas has been found only in Mycoplasma haemofelis and Mycoplasma haemocanis [35], [36]. The other was a gene for catalase, which we call katE. It is present in both serovar K and the previously published serovar I [11], and the nucleotide sequence is nearly identical both in the coding region and the 434-bp non-coding region upstream. The predicted amino acid sequence for M. iowae serovar K catalase had 61% sequence identity to a homolog in the archaeon Methanobrevibacter arboriphilus, whose heme-dependent catalase activity has been characterized [37]. The predicted catalase protein contains all conserved amino acids of clade 3 heme-binding catalases as indicated on the Conserved Domain Database (CDD) [38], a group which it matches with an e-value of less than 10-100 . Its closest relatives are all found in anaerobic prokaryotes (Fig. 1), among which Clostridium lentocellum and Desulfotomaculum ruminis are the only members of the closely related Firmicutes lineage. The lack of a close relationship with catalases from most Firmicutes is more consistent with M. iowae having acquired katE by horizontal gene transfer from an anaerobe than having retained it during evolution from a common ancestor with Gram-positive bacteria. Sequence alignment reveals conservation of predicted secondary structural elements and active site residues (Fig. S1).
Figure 1. Phylogenetic tree of M. iowae serovar K catalase and close relatives, with Escherichia coli katE as an outgroup.
All amino acid sequences included have an e value reported as 0 by PSI-BLAST with respect to M. iowae catalase. Scale bar, 0.05 substitutions per site. Bootstrap values out of 1000 trials are reported at nodes.
The presence of katE in M. iowae constitutes the first published account of a catalase gene in any mycoplasma species to date. Catalase activity was assessed in whole cells of M. iowae serovar K and M. gallisepticum strain Rlow, which has no catalase gene. Upon the addition of 3% H2O2, M. iowae produced robust bubbling which was absent from M. gallisepticum (Table 1), consistent with the presence of catalase activity in only M. iowae. Furthermore, M. iowae was capable of breaking down H2O2 produced by M. genitalium, a species previously shown to be a robust H2O2-producer [39]. Cells of both species were grown to mid-log phase in media containing 3% glycerol, which stimulates H2O2 production [19], [39]. Upon reaching mid-log phase, the cultures were combined and H2O2 concentration was monitored over time (Fig. 2). When an equal volume of M. iowae culture was added to the M. genitalium culture, the H2O2 concentration fell below detectable levels within 2 min. This decrease in the amount of H2O2 was not observed when warm SP-4 broth was added to M. genitalium. A repeated measures ANOVA revealed a significant difference between these two groups (F(1,8) = 56.783, p<0.05), and significant differences were observed from 45 sec onward during the assay as determined by unpaired Student's T-test (p<0.05). These data are consistent with catalase activity by M. iowae.
Table 1. Catalase activity and H2O2 production by M. iowae serovar K, M. gallisepticum Rlow, and M. gallisepticum 55 and 56 series transformants containing M. iowae katE.
| Strain | Catalase activity | H2O2 production (mg L−1) | |
| 100 µM glucose | 100 µM glycerol | ||
| M. iowae serovar K | + | 0±0* | 0±0* |
| M. gallisepticum Rlow | − | 1.6±0.1 | 3.8±0.3 |
| 55A | + | 0±0* | 0±0* |
| 55B | + | 0±0* | 0±0* |
| 55C | + | 0±0* | 0.1±0.1* |
| 56A | + | 0±0* | 0±0* |
| 56C | + | 0.3±0.3* | 0.6±0.4* |
*P<0.05 compared to M. gallisepticum Rlow H2O2 production with respective carbohydrate.
Figure 2. Degradation of H2O2 produced by an exogenous source of by M. iowae.
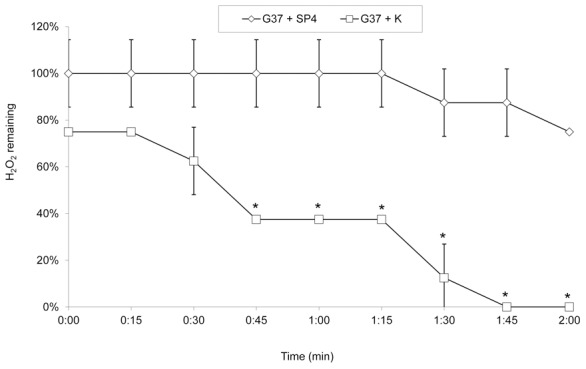
H2O2 present in a mid-log phase culture of M. genitalium G37 was measured following the addition of an equal amount of SP-4 media (◊) or mid-log phase M. iowae serovar K culture (□). Error bars, SD. *, p<0.05 compared to control as determined by Student's T-test.
Expression of M. iowae katE in a hydrogen peroxide-producing mycoplasma
To test the role of katE in the catalase activity of M. iowae, M. gallisepticum was transformed with the gene either without or with a C-terminal 6X-His tag to create transformant series 55 and 56, respectively [30]. This species was chosen as the recipient due to its ability to produce H2O2 and lack of other known toxins [40], [41]. We included the coding region of katE plus 239 nucleotides upstream of the start codon, anticipating that this region included a promoter, although no promoter sequences could be unambiguously identified by sequence inspection (not shown). Immunoblotting with anti-6X-His antibodies confirmed production by transformants of a protein migrating at approximately 60 kDa, the predicted size of M. iowae catalase, that was absent from untransformed M. gallisepticum (Fig. 3a). Addition of 3% H2O2 to all transformants resulted in bubbling, indicative of the presence of catalase activity (Table 1). Transformants were also analyzed for H2O2 production in the presence of 100 µM glucose or glycerol as previously described [14]. M. iowae produced no detectable H2O2 under any test conditions, including incubation with up to 3 mM glycerol (Table 1 and not shown). Although the parent strain produced robust H2O2 in the presence of 100 µM glycerol, all katE transformants displayed decreased H2O2 production, approaching or at undetectable levels. Individual transformants produced varying amounts of H2O2, but always less than the parent strain. There was a statistically significant difference between strains as determined by a one-way ANOVA (F(7,21) = 73.102, p<0.0001 for 100 µM glucose and F(7,22) = 96.076, p<0.0001 for 100 µM glycerol). A Tukey post hoc test revealed significant differences between M. gallisepticum and all catalase-containing strains, but no statistical differences between M. iowae and catalase-containing transformants following treatment with both carbohydrates, suggesting that katE is responsible for the catalase activity present in M. iowae. Because it was possible that introduction of the transposon carrying katE interfered with glycerol metabolism and therefore H2O2 production, PCR was performed on the glpFOK putative operon, which constitute the only genes known to be involved in glycerol metabolism in this organism. Wild-type M. gallisepticum and all transformants displayed a product of ∼5.7 kb (Fig. 3b). These results are consistent with reduction in H2O2 accumulation in transformants being due solely to catalase activity provided by M. iowae katE.
Figure 3. Production of catalase and integrity of glycerol metabolism operon in M. gallisepticum transformants.
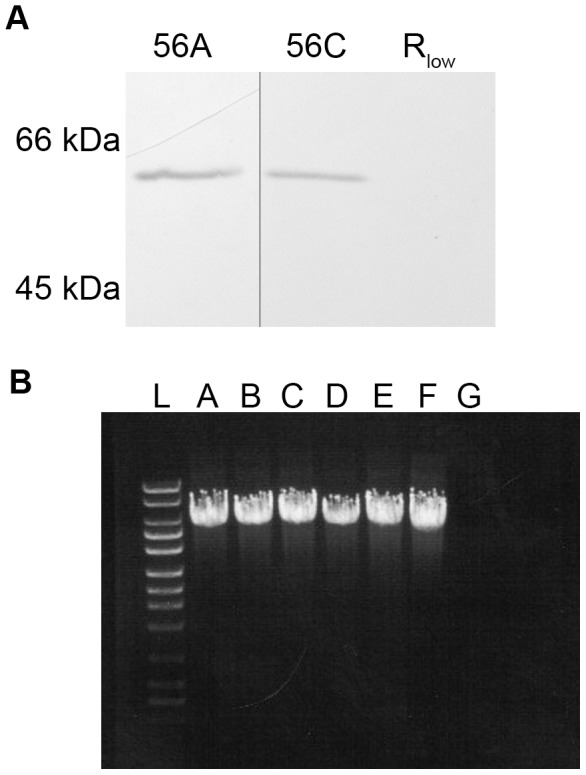
(a) Immunoblot of M. gallisepticum transformants 56A and 56C and M. gallisepticum Rlow with anti-6X-His antibodies. (b) PCR products using primers for the putative glpFOK operon of M. gallisepticum. L, ladder with bands at 10, 8, 6, 5, 4, 3, 2.5, 2, 1.5, 1, 0.7, and 0.5 kb; A, M. gallisepticum Rlow; B–D, M. gallisepticum transformants 55A–C; E–F, M. gallisepticum transformants 56A and C; G, M. iowae serovar K.
H2O2-mediated toxicity in a C. elegans model
Multiple studies have demonstrated the susceptibility of C. elegans to H2O2 [42], [43], but a C. elegans model for toxicity of H2O2 from mycoplasmas has never been reported. When C. elegans L1 larvae were incubated with increasing concentrations of H2O2 at room temperature, a significant decrease in survival at 24 h was evident as indicated by a one-way ANOVA (F(3,26) = 5.911, p = 0.003) (Fig. 4). To maximize H2O2 output by M. gallisepticum after 24 h, thereby allowing for the most easily discernible results in subsequent assays, various concentrations of glycerol and M. gallisepticum cells were analyzed at room temperature to mimic conditions necessary for C. elegans toxicity assays (Fig. 5). H2O2 production by wild-type M. gallisepticum reached a distinct maximum level when the glycerol concentration was increased to 1 mM (Fig. 5a). There was a statistically significant difference between different glycerol concentrations at the same time point as determined by a one-way ANOVA (F(7,6) = 124.842, p<0.0001 at 24 hr and F(7,6) = 20.591, p<0.0001 at 48 hr). A Tukey post hoc test revealed a significant difference associated with treatment with 100 µM glycerol but not with higher concentrations. No statistically significant differences were observed for any glycerol concentrations with an increase in incubation time from 24 to 48 hrs. OD550 values ranging from 0.5 to 1.0 yielded the same level of H2O2 (Fig. 5b); therefore, we chose to use cells at an OD550 of 0.75 in the C. elegans assays. When transformant 56A, representing the katE transformants, was analyzed for H2O2 production with cells at an OD550 of 0.75 in the presence of 1 mM glycerol at room temperature, H2O2 was detectable, but present at more than an order of magnitude lower than wild-type M. gallisepticum, with H2O2 production peaking early and becoming undetectable after 10 h (Fig. 5b). Thus, the amount of H2O2 produced by both wild-type M. gallisepticum and transformant 56A under the conditions used for the C. elegans studies is markedly greater than those originally obtained with 100 µM glycerol over 2 h of incubation at 37°C.
Figure 4. Survival of C. elegans upon exposure to increasing concentrations of H2O2.
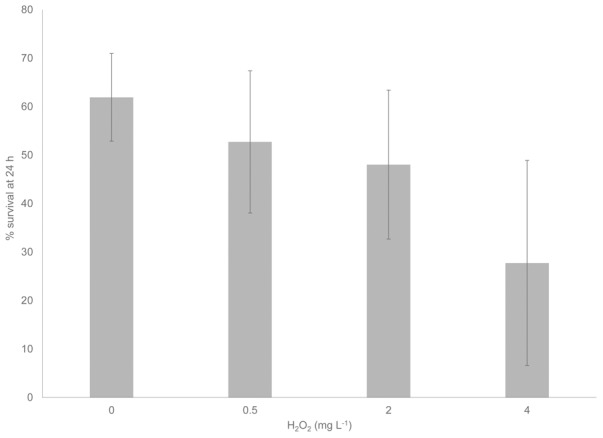
Error bars, SD.
Figure 5. H2O2 accumulation by different amounts of M. gallisepticum with varying glycerol concentrations.
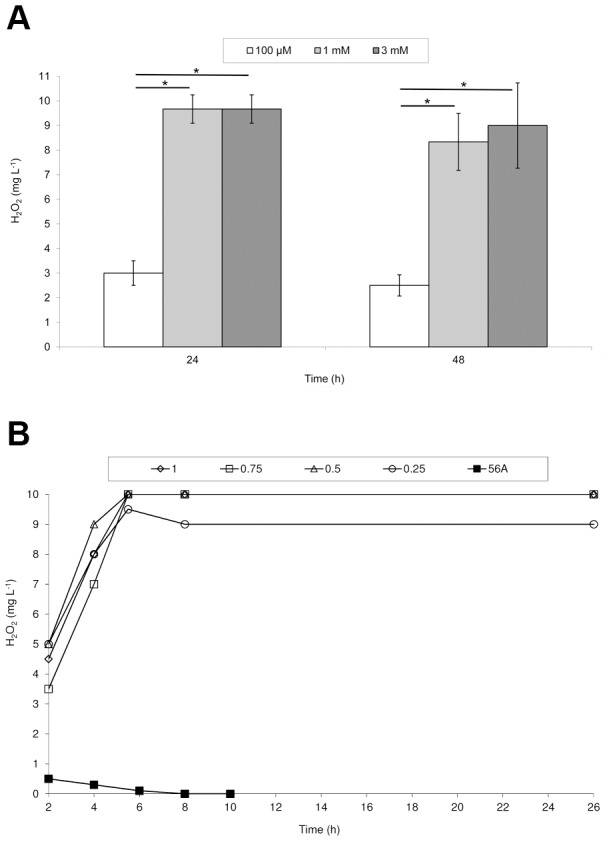
(a) M. gallisepticum Rlow at an OD550 of 1.0 with 100 µM (white bars), 1 mM (light grey bars), or 3 mM (dark grey bars) glycerol. Experiments were performed in triplicate. *, p<0.05 as determined by one-way ANOVA and Tukey's post hoc test. (b) M. gallisepticum Rlow at an OD550 of 1 (◊), 0.75 (□), 0.5 (Δ), or 0.25 (x) with 1 mM glycerol and M. gallisepticum catalase-producing transformant 56A at an OD550 of 0.75 with 1 mM glycerol. Results are from one representative experiment.
To compare H2O2 toxicity in the presence and absence of catalase-containing mycoplasmas, pre-counted C. elegans L1 larvae were incubated with 1 mL of M. iowae, M. gallisepticum, and M. gallisepticum transformant 56A with catalase activity at an OD550 of 0.75 in the presence or absence of 1 mM glycerol at room temperature, and survival was measured at 24 h (Fig. 6). In the absence of glycerol, leaving H2O2 production absent or minimal (see Table 1), incubation with all strains resulted in 60–80% survival of C. elegans, which were statistically similar as determined by a one-way ANOVA (F(2,21) = 0.962, p = 0.398). Statistically significant differences were found between experiments with and without glycerol for M. gallisepticum, but not for M. iowae or transformant 56A as determined by Student's T-test. Wild-type M. gallisepticum allowed no survival of C. elegans at 24 h due to the high level of H2O2 production. Transformant 56A allowed increased survival of C. elegans as compared to wild-type M. gallisepticum, paralleling differences observed in H2O2 production under assay conditions (Fig. 5b). Catalase-producing M. iowae and transformant 56A were both statistically significantly different from untransformed M. gallisepticum in the presence of glycerol as determined by a one-way ANOVA (F(2,21) = 292.531, p<0.001) and Tukey's post hoc test.
Figure 6. Toxicity of mycoplasmas to C. elegans.
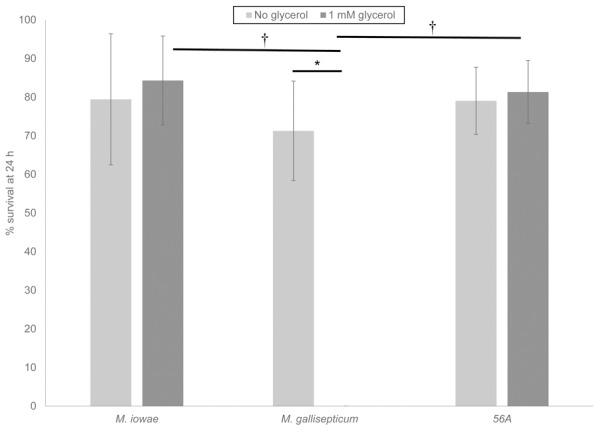
Toxicity was measured in the absence (light grey bars) and presence (dark grey bars) of 1 mM glycerol. Error bars, SD. *, p<0.05 as determined by unpaired Student's T-test. † p<0.05 as determine by one-way ANOVA and Tukey's post hoc test.
Discussion
Despite the presence in M. iowae of an entire set of genes sufficient for importing and using free glycerol as a metabolite, this organism produced no detectable H2O2 under any conditions tested. We speculated that the absence of H2O2 accumulation was due to the presence and activity of a catalase, likely intracellular, which provides M. iowae with the ability to break down exogenous H2O2 and might remove H2O2 generated through the glycerol catabolism pathway before it accumulates to detectable levels. Consistent with this hypothesis, M. gallisepticum displayed catalase activity upon transformation with M. iowae katE. Significantly, catalase activity resulted in reduced or even undetectable H2O2 production by M. gallisepticum transformants, and a C. elegans toxicity assay revealed a correlation between reduced H2O2 production and decreased toxicity. A possible explanation for the absence of H2O2 produced by M. iowae in the presence of glycerol is that the GlpFKO pathway experiences low flux and produces only a small amount of H2O2. A second possibility is that M. iowae has a defect in its GlpFKO pathway resulting in the inability to utilize glycerol as a metabolite and therefore an absence of H2O2 production. Finally, the catalase activity may simply be so great as to prevent any accumulation of H2O2 generated by glycerol catabolism. Although the presence of these genes suggests that there is a use for these proteins in normal cellular function, further testing is needed to determine their expression, activity, and regulation. There might, for example, be conditions under which either catalase activity is reduced or glycerol catabolism is increased. Preliminary data suggest that a close relative of M. iowae, M. penetrans, produces minimal H2O2 amounts from glycerol catabolism (J. T. Newman and M. F. B., unpublished data), raising the possibility that this pathway is not very productive in this clade of mycoplasmas. Development of genetic tools for M. iowae, which have not been described, would be valuable for directly addressing the question of H2O2 production.
Our finding of reduced or no H2O2 production in the presence of catalase suggests a fundamental incompatibility between the presence of catalase and the use of H2O2 as a virulence factor by mycoplasmas, and provides insight into the correlation between the presence of glycerol catabolism genes and the use of H2O2 for virulence. If H2O2 is a widespread virulence factor among mycoplasmas, then this incompatibility is likely to explain the general absence of catalase from other mycoplasmas. The findings of this report support the idea that, although glycerol catabolism genes are widespread amongst mycoplasmas, their presence does not necessarily signal the use of H2O2 for virulence. In addition to its function in utilizing glycerol as an energy source, recent work has illustrated the importance of this pathway in other biochemical functions, such as the production of lipids [44].
C. elegans has previously been used to assay toxicity of several bacterial species via production of toxic molecules and infectious processes. The susceptibility of C. elegans to H2O2 has been well established and H2O2-mediated toxicity assays on solid media and in liquid have been used to study virulence caused via this mechanism [42], [43], although this is the first report of a C. elegans assay addressing mycoplasma toxicity. There are several benefits of using this method as opposed to standard tissue culture models. First, C. elegans is a more complex model than tissue culture cells, possessing several of the same innate immune defense mechanisms as more complex organisms [45], although whether this feature is important in this particular study is not clear. Second, previous studies have established a time frame of 2–5 d until bacteria can adequately colonize the gut of C. elegans and therefore cause damage via an infectious process [43]. Any death observed in the first 48 h is therefore due to diffusible molecules like H2O2. Third, tissue culture models of virulence require infection for long periods of time, and they cannot distinguish damage caused by H2O2, which occurs rapidly, and damage caused by toxins, which occurs more slowly, without the use of mutants, which can be difficult at minimum to construct using currently available molecular techniques [14]. Although there are limitations associated with comparing results obtained from this method to those observed in the natural host, such as examining the impact of H2O2 produced from exogenous or host-derived carbohydrates, it serves as a good system for examination of strains deficient in H2O2 production, such as the catalase-positive strains examined in this study.
One potential concern about the use of C. elegans as a reporter of H2O2-mediated toxicity is the question of whether binding of mycoplasmas to C. elegans impacts toxicity during the time frame required for these assays. Whether M. iowae binds to C. elegans under our experimental conditions, or under any conditions, is not known. Although M. gallisepticum can bind red blood cells at room temperature within 5 min [46], other pathogens with cell-associated toxins also bind externally to C. elegans within 2 d yet still require 4 d before any decrease in worm survival is observed [47]. Therefore, even though mycoplasmas might or might not bind to C. elegans during the 24-h incubation required for the toxicity assay employed in this study, one can be certain that any decrease in survival during this time frame is due to the production of diffusible molecules such as H2O2 and not cell-associated toxins.
Supporting Information
Alignment of catalase proteins closely related to that of M. iowae . The uppermost track plots alignment entropy, with hotter colors at more variable positions than colder ones. Beneath the entropy plot the sequence coordinates are given for M. iowae catalase. The secondary structure for KatA from Enterococcus faecalis (PDB ID, 1SI8; [48]), the most closely related catalase for which structural information is available, is displayed beneath the alignment plot. Active site residues are indicated with asterisks. Red alpha-helices and yellow beta-strands predicted by SOPMA [26] are indicated at the bottom.
(PDF)
Acknowledgments
We are grateful to Naola Ferguson-Noel for providing bacterial strains, Danielle Hamill for providing C. elegans samples and training, Jaime Newman for preliminary data, Amy Wetzel for preparation of genomic DNA, and Jenny Panescu for genomic DNA sequencing at the Ohio State University Plant-Microbe Genome Facility. Other DNA sequencing and analysis was carried out at the Miami University Center for Bioinformatics and Functional Genomics.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All Mycoplasma iowae genome sequence files are available from the DDBJ/EMBL/GenBank database (accession number AWQU00000000).
Funding Statement
This work was supported by the National Institutes of Health (www.nih.gov) Public Service Grant R15 AI073994 to MFB, by funding from Hybrid Turkeys (www.hybridturkeys.com), Kitchener, Ontario, Canada to ZR, and by the Miami University Doctoral-Undergraduate Opportunities in Scholarship Program (miamioh.edu) to REP and AJP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Al-Ankari A-RS, Bradbury JM (1996) Mycoplasma iowae: a review. Avian Pathol 25: 205–229. [DOI] [PubMed] [Google Scholar]
- 2. Bradbury JM, Ideris A, Oo TT (1988) Mycoplasma iowae infection in young turkeys. Avian Pathol 17: 149–171. [DOI] [PubMed] [Google Scholar]
- 3. Trampel DW, Goll F Jr (1994) Outbreak of Mycoplasma iowae infection in commercial turkey poults. Avian Dis 38: 905–909. [PubMed] [Google Scholar]
- 4. Ley DH, Marusak RA, Vivas EJ, Barnes HJ, Fletcher OJ (2010) Mycoplasma iowae associated with chondrodystrophy in commercial turkeys. Avian Pathol 39: 87–93. [DOI] [PubMed] [Google Scholar]
- 5. Shah-Majid M, Rosendal S (1986) Oral challenge of turkey poults with Mycoplasma iowae . Avian Dis 31: 365–369. [PubMed] [Google Scholar]
- 6. Mirsalimi SM, Rosendal S, Julian RJ (1989) Colonization of the intestine of turkey embryos exposed to Mycoplasma iowae . Avian Dis 33: 310–315. [PubMed] [Google Scholar]
- 7.Johansson K-E, Pettersson B (2002) Taxonomy of Mollicutes. In: Razin S, Herrmann R, editors.Molecular Biology and Pathogenicity of Mycoplasmas.Boston: Kluwer Academic/Plenum Publishers. pp. 1–30. [Google Scholar]
- 8. Jurkovic DA, Newman JT, Balish MF (2012) Conserved terminal organelle morphology and function in Mycoplasma penetrans and Mycoplasma iowae . J Bacteriol 194: 2877–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barber TL, Fabricant J (1971) Identification of Mycoplasmatales: procedures for both characterization and purification. Appl Microbiol 21: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradbury JM (1983) Mycoplasma iowae – an avian mycoplasma with unusual properties. Yale J Biol Med 56: 912. [Google Scholar]
- 11. Wei S, Guo Z, Li T, Zhang T, Li X, et al. (2012) Genome sequence of Mycoplasma iowae strain 695, an unusual pathogen causing deaths in turkeys. J Bacteriol 194: 547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kannan TR, Baseman JB (2006) ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci USA 103: 6724–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pilo P, Vilei EM, Peterhans E, Bonvin-Klotz L, Stoffel MH, et al. (2005) A metabolic enzyme as a primary virulence factor for Mycoplasma mycoides subsp. mycoides small colony. J Bacteriol 187: 6824–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hames C, Halbedel S, Hoppert M, Frey J, Stülke J (2009) Glycerol metabolism is important for cytotoxicity of Mycoplasma pneumoniae . J Bacteriol 191: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baeuerle PA, Henkel T (1994) Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179. [DOI] [PubMed] [Google Scholar]
- 16. Brennan PC, Feinstein RN (1969) Relationship of hydrogen peroxide production by Mycoplasma pulmonis to virulence for catalase-deficient mice. J Bacteriol 98: 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Houshaymi BM, Miles RJ, Nicholas RAJ (1997) Oxidation of glycerol differentiates African from European isolates of Mycoplasma mycoides subspecies mycoides SC (small colony). Vet Record 140: 182–183. [DOI] [PubMed] [Google Scholar]
- 18. Abdo E-M, Nicolet J, Miserez R, Gonçalves R, Regalla J, et al. (1998) Humoral and bronchial immune responses in cattle experimentally infected with Mycoplasma mycoides subsp. mycoides small colony type. Vet Microbiol 59: 109–122. [DOI] [PubMed] [Google Scholar]
- 19. Vilei EM, Frey J (2001) Genetic and biochemical characterization of glycerol uptake in Mycoplasma mycoides subsp. mycoides SC: its impact on H2O2 production and virulence. Clin Diagn Lab Immunol 8: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhandayuthapani S, Blaylock MW, Bebear CM, Rasmussen WG, Baseman JB (2001) Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium . J Bacteriol 183: 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang W, Baseman JB (2014) Functional characterization of osmotically inducible protein C (MG_427) from Mycoplasma genitalium . J Bacteriol 196: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tully JG, Rose DL, Whitcomb RF, Wenzel RP (1979) Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium. J Infect Dis 139: 478–482. [DOI] [PubMed] [Google Scholar]
- 23. Simmons WL, Daubenspeck JM, Osborne JD, Balish MF, Waites KB, et al. (2013) Type 1 and type 2 strains of Mycoplasma pneumoniae form different biofilms. Microbiol 159: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Couronne O, Poliakov A, Bray N, Ishkhanov T, Ryaboy D, et al. (2003) Strategies and tools for whole-genome alignments. Genome Res 13: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 27. Perrière G, Gouy M (1996) WWW-Query: An on-line retrieval system for biological sequence banks. Biochimie 78: 364–369. [DOI] [PubMed] [Google Scholar]
- 28. Geourjon C, Deleage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 13: 209–212. [DOI] [PubMed] [Google Scholar]
- 29. French CT, Lao P, Loraine AE, Matthews BT, Yu H, et al. (2008) Large-scale transposon mutagenesis of Mycoplasma pulmonis . Mol Microbiol 69: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hedreyda CT, Lee KK, Krause DC (1993) Transformation of Mycoplasma pneumoniae with Tn4001 by electroporation. Plasmid 30: 170–175. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JA, Fleming JT (1995) Basic culture methods. In: Epstein, HF, Shakes, DC, editors.Methods in Cell Biology, Volume 48, Caenorhabditis elegans: Modern Biological Analysis of an Organism.Boston: Academic Press. pp. 3–29. [Google Scholar]
- 32. Hong Y, Garciá M, Levisohn S, Lysnyansky I, Leiting V, et al. (2005) Evaluation of amplified fragment length polymorphism for differentiation of avian mycoplasma species. J Clin Microbiol 43: 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidl SR, Otto A, Lluch-Senar M, Piñol J, Busse J, et al. (2011) A trigger enzyme in Mycoplasma pneumoniae: impact of the glycerophosphodiesterase GlpQ on virulence and gene expression. PLoS Pathog 7: e1002263 doi:10.1371/journal.ppat.1002263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Groβhennig S, Schmidl SR, Busse J, Stülke J (2013) Implication of glycerol and phospholipid transporters in Mycoplasma pneumoniae growth and virulence. Infect Immun 81: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berent LM, Messick JB (2003) Physical map and genome sequencing survey of Mycoplasma haemofelis (Haemobartonella felis). Infect Immun 71: 3657–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. do Nasciemento N, Guimaraes AM, Santos AP, SanMiguel PJ, Messick JB (2012) Complete genome sequence of Mycoplasma haemocanis strain Illinois. J Bacteriol 194: 1605–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shima S, Sordel-Klippert M, Brioukhanov A, Netrusov A, Linder D, et al. (2001) Characterization of a heme-dependent catalase from Methanobrevibacter arboriphilus . Appl Environ Microbiol 67: 3041–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, et al. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41: D348–D352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez MA, Das K, Saikolappan S, Materon LA, Dhandayuthapani S (2013) A serine/threonine phosphatase encoded by MG_207 of Mycoplasma genitalium is crucial for its virulence. BMC Microbiol 13: 44 doi: 10.1186/1471-2180-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor RR, Mohan K, Miles RJ (1996) Diversity of energy-yielding substrates and metabolism in avian mycoplasmas. Vet Microbiol 51: 291–304. [DOI] [PubMed] [Google Scholar]
- 41. Papazisi L, Gorton TS, Kutish G, Markham PF, Browning GF, et al. (2003) The complete genome sequence of the avian pathogen Mycoplasma gallisepticum strain Rlow . Microbiol 149: 2307–2316. [DOI] [PubMed] [Google Scholar]
- 42. Jansen WTM, Bolm M, Balling R, Chhatwal GS, Schnabel R (2002) Hydrogen peroxide-mediated killing of Caenorhabditis elegans by Streptococcus pyogenes . Infect Immun 70: 5202–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moy TI, Mylonakis E, Calderwood SB, Ausubel FM (2004) Cytotoxicity of hydrogen peroxide produced by Enterococcus faecium . Infect Immun 72: 4512–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yus E, Maier T, Michalodimitrakis K, van Noort V, Yamada T, et al. (2009) Impact of genome reduction on bacterial metabolism and its regulation. Science 326: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 45. Ewbank JJ (2002) Tackling both sides of the host-pathogen equation with Caenorhabditis elegans . Microbes Infect 4: 247–256. [DOI] [PubMed] [Google Scholar]
- 46. Banai M, Kahane I, Razin S, Bredt W (1978) Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect Immun 21: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Couillault C, Ewbank JJ (2002) Diverse bacteria are pathogens of Caenorhabditis elegans . Infect Immun 70: 4705–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hakansson KO, Brugna M, Tasse L (2004) The three-dimensional structure of catalase from Enterococcus faecalis . Acta Crystallogr D Biol Crystallogr 60: 1374–1380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of catalase proteins closely related to that of M. iowae . The uppermost track plots alignment entropy, with hotter colors at more variable positions than colder ones. Beneath the entropy plot the sequence coordinates are given for M. iowae catalase. The secondary structure for KatA from Enterococcus faecalis (PDB ID, 1SI8; [48]), the most closely related catalase for which structural information is available, is displayed beneath the alignment plot. Active site residues are indicated with asterisks. Red alpha-helices and yellow beta-strands predicted by SOPMA [26] are indicated at the bottom.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All Mycoplasma iowae genome sequence files are available from the DDBJ/EMBL/GenBank database (accession number AWQU00000000).