Abstract
Dried blood spots (DBS) can provide accurate and valuable estimates of exposure to environmental toxicants, and the use of information derived from archived newborn DBS information has enormous potential to open up new research on the impacts of early chemical exposure on disease. Broad application of DBS for the purpose of quantitative exposure estimation requires robust and validated methods. This study investigates the suitability of DBS analyses for population studies of exposure to three chemical groups: polychlorinated biphenyls (PCBs), brominated flame retardants (BFRs), and chlorinated pesticides. It examines background (matrix) contamination, recovery and extraction variability, sensitivity, and storage stability. DBS samples prepared using 50 μL of adult blood were analyzed by GC/MS, and method performance was confirmed by using certified materials and paired DBS-blood samples from six volunteers. Several of the target compounds and their degradation products have not been previously measured in DBS. All target compounds were detected in DBS samples collected from the volunteers. Sample DBS cards showed background contamination of several compounds. When stored at room temperature, target compounds, excluding PBDEs, were stable for up to one month. When refrigerated or frozen, stability was acceptable for all compounds up to one year, and multiyear storage appears acceptable at colder (e.g., −80 °C) temperatures. Multicompartment models may be used to estimate or correct for storage losses. Considering concentrations of contaminants for adults and children reported in the literature, and experimental values of detection limits and background contamination, DBS samples are suitable for quantifying exposures to many PCBs, BFRs and persistent pesticides.
Keywords: Blood, bloodspot, DDT, exposure, organic compounds, PBDEs, PCBs, Biomonitoring, child exposure, flame retardants, pesticides, emerging contaminants
1. Introduction
All U.S. states have mandatory newborn screening programs for diseases (e.g., phenylketonuria) in their health preventative services that use the collection and analysis of biological specimens, most commonly newborn dried blood spots (DBSs). Fourteen states store biological specimens for at least 21 years, and eight programs report indefinite storage [1, 2]. States including Michigan and California have extensive archives that can be utilized for a wide variety of scientific purposes. For example, the Michigan Neonatal Biobank (MNB) has been collecting DBSs for every birth in Michigan since 1984, and its repository now includes millions of specimens. Such archives provide a unique and potentially powerful resource for retrospective assessment of environmental exposures during the prenatal period. The potential of DBS archives for epidemiological and other purposes has been recognized for several decades [3]. Potentially, a huge range of analytes could be examined using DBS, and the use of this information in cohort studies of well-phenotyped individuals has enormous potential to open up new research on the impacts of early chemical exposure on disease. The use of DBS samples also has several advantages as a biomarker sampling and storage technique for prospective investigations: the method is simple; less invasive than venipuncture; collection, transport and storage costs are low; and increased safety given the minimal volume of blood and reduced risk of infection [4, 5]. As a result, DBS may provide a cost-effective alternative to venipuncture.
DBS collection involves applying small volumes of blood, e.g., from a heel stick, to a sample collection card. Typically, 4 or 5 spots are made, dried at ambient temperature, and then sent to laboratory for analysis and then archived. Transport and storage conditions have changed over time. In Michigan, for example, DBS cards collected from 2009 to date are stored at −20°C, cards from 1998 through 2008 are refrigerated (controlled temperature and humidity), and older cards are stored at ambient temperature [6]. When received at the laboratory, a fixed area of the blood spot is extracted, typically using a 3.2 mm round punch. The size of the disk sampled provides the volumetric data for quantitation [7]. One limitation of the technique, however, is that the amount of blood in the punch depends on the spreading of blood, which is determined by the hematocrit concentration, which can vary for neonates over a relatively large range. [8] Multiple analytes can be determined from a single DBS using multiple punches. If a larger sample quantity is needed, e.g., due to detection limit issues, the entire DBS may be used or multiple punches may be used to form a composite sample [9].
Applications of DBS measurements for bioanalyses and pharmaceutical residues (and their derivatives) have been recently reviewed [4, 5, 7, 10–14]. The number of reports describing the methods and experience with DBS for exposure assessment purposes is comparatively sparse. Environmental applications have focused on several trace metals, e.g., lead and cadmium [15–17], and methods for the more prevalent metals, specifically lead, are considered to be reasonably well-validated [18]. However, DBS have been used for other analytes. For example, in mostly pilot studies, human and avian DBS samples were used to measure additional trace metals [15], cotinine [19], perchlorates [20], cysteinyl adducts of benzene oxide [21], bisphenol A [14, 22], and persistent organic compounds, e.g., pesticides [23–25], PCBs [24, 26], PBDEs [9, 24, 26], and fluorinated compounds like PFOS [14, 24, 27, 28].
Broad application of DBS for quantitative exposure estimation purposes requires validation, reference materials, control samples and other quality assurance (QA) elements that meet data quality objectives [29, 30]. A number of issues in the collection, storage, processing, analysis and use of DBSs have been recognized [7, 10, 12, 13, 18], and most pertain to environmental toxicants. These include: loss of analytes by enzymatic action (or other mechanisms) during the collection, spotting and drying stages [31, 32]; differences between analyte profiles measured using DBS and plasma [33]; effects of storage times and storage conditions on sample integrity [34, 35]; the consistency of blood spot punches and chromatographic considerations on sample cards [36]; variability, recovery, sorption and other issues involved in extracting analytes from sample cards [11, 26, 37]; variability in blood volumes, irregular geometry of the DBS itself, and punch size [8, 38, 39]; background (matrix) contamination on the filter paper [15, 20]; within- and between-lot variability of the sample card properties and contamination [40]; chromatographic, carryover and other separation issues in analysis and quantitation [41]; limited sensitivity of methods, which can be particularly acute given the small volume of sample available [5, 10]; and a lack of standard reference materials (SRMs) to help ensure that measurements meet quality assurance criteria. (For example, SRMs are available from NIST for (persistent) organic contaminants in non-fortified human serum [SRM-1957] and metals in caprine (goat) blood [SRM-955c]). While progress is being made on many of these fronts, few investigators have reported basic performance criteria, including precision, accuracy, linearity, sensitivity, selectivity, and storage stability [7].
This study addresses the suitability of DBS measurements for population studies. It evaluates background (or matrix) contamination, recovery and extraction variability, sensitivity, and storage stability of persistent organic pollutants (POPs) on DBS. Three groups of POPs are tested: brominated flame-retardants (BFRs), chlorinated pesticides, and polychlorinated biphenyls (PCBs). Each group includes toxicants found in human blood. While dependent on many factors, e.g., age and diet, concentrations of the selected contaminants can be relatively high. The performance of DBS measurements and ability to meet QA goals are evaluated using both real and laboratory-prepared DBSs, reference materials, and tests over a one-year period.
1 Methods
1.1 Compound selection
Target compounds were selected considering prevalence and toxicity. We examined recent literature, including the latest National Health and Nutrition Examination Survey (NHANES) reports [42] and studies specific to newborns and children. While NHANES examines adults, it has the advantages of employing extensive and well-documented QA procedures and a large and nationally representative sample.
Four to 11 compounds in three groups of chemicals were selected. These included PCBs (congeners 28, 138, 153, 180, 105, 118, 194, 206, 209, 170, 187); persistent pesticides (trans-nonachlor, dichlorodiphenyldichloroethylene (p,p'-DDE), β-hexachlorcyclohexane (β HCH), hexachlorobenzene (HCB), and brominated flame retardants (BFRs) including 2,2',4,4',5,5'-hexabromobiphenyl (PBB-153), 2,2'6,6'-tetrabromo-4,4'-isopropylidene diphenol (TPBB-a), and PBDEs (congeners 28, 47, 99, 100, 153, 154). Table 1 shows the names and properties of these compounds.
Table 1.
Identification of target compounds, chemical properties from EpiSuite (http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm), instrumental detection limits (DL), and comparison to NIST standard reference material (SRM) to reconstituted serum and DBS measurements.
| Group | Chemical | Chemical name | CAS No. | Formula | Properties from Episuite |
Detection Limit (ng/L) |
Certified SRM |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mole. Wt. |
Log Kow (oct-water) |
Vapor Pres (mm Hg) |
NIST (1) (ng/kg) |
Serum (2) (ng/kg) |
DBS (3) (ng/kg) |
||||||
| PCBs | PCB-28 | 2,4,4'-trichlorobiphenyl | 7012-37-5 | C12H7C13 | 257.6 | 5.62 | 4.00E-04 | 30 | 8.6 | 10.4 | <DL |
| PCB-138 | 2,2',3,4,4',5'-hexachlorobiphenyl | 35065-28-2 | C12H4C16 | 360.9 | 7.44 | 5.80E-07 | 10 | 36.9 | 42.2 | 38.3 | |
| PCB-153 | 2,2',4,4',5,5'-hexachlorobiphenyl | 35065-27-1 | C12H4C16 | 360.9 | 7.75 | 3.43E-06 | 10 | 58.2 | 67.1 | 65.3 | |
| PCB-180 | 2,2',3,4,4',5,5'-heptachlorobiphenyl | 35065-29-3 | C12H3C17 | 395.3 | 8.27 | 9.77E-07 | 17 | 54.5 | 61.3 | 62.5 | |
| PCB-105 | 2,3,3',4,4'-pentachlorobiphenyl | 32598-14-4 | C12H5C15 | 326.4 | 6.79 | 6.53E-06 | 5 | 4.1 | 5.2 | 5.7 | |
| PCB-118 | 2,3',4,4',5-pentahlorobiphenyl | 31508-00-6 | C12H5C15 | 326.4 | 7.12 | 8.97E-06 | 30 | 18.9 | 17.3 | <DL | |
| PCB-194 | 2,2',3,3',4,4',5,5'-octachlorobiphenyl | 35694-08-7 | C12H2C18 | 429.8 | 8.68 | 2.87E-08 | 12 | 11.9 | 14.3 | 15.3 | |
| PCB-206 | 2,2',3,3',4,4',5,5',6-nonachlorobiphenyl | 40186-72-9 | C12H1C19 | 464.2 | 9.14 | 7.60E-09 | 25 | 7.0 | 8.3 | <DL | |
| PCB-209 | decachlorobiphenyl | 2051-24-3 | C12C110 | 498.7 | 8.27 | 1.06E-07 | 50 | 4.3 | <DL | ||
| PCB-170 | 2,2',3,3',4,4',5-heptachlorobiphenyl | 35065-30-6 | C12H3C17 | 395.3 | 8.27 | 6.28E-07 | 30 | 16.2 | 17.4 | <DL | |
| PCB-187 | 2,2',3,4',5,5',6-heptachlorobiphenyl | 52663-68-0 | C12H3C17 | 395.3 | 8.27 | 1.30E-07 | 30 | 15.5 | 16.3 | <DL | |
| BFRs-PBDEs | BDE-28 | 2,4,4'-tribromodiphenyl ether | 41318-75-6 | C12H7Br3O | 406.9 | 5.88 | 6.70E-07 | 30 | 20.0 | 17.5 | <DL |
| BDE-47 | 2,2',4,4'-tetrabromodiphenyl ether | 5436-43-1 | C12H6Br4O | 485.8 | 6.77 | 7.00E-08 | 30 | 268.0 | 277.4 | 278.3 | |
| BDE-99 | 2,2',4,4'5-pentabromodiphenyl ether | 60348-60-9 | C12H5Br5O | 564.7 | 6.84 | 3.10E-08 | 30 | 76.0 | 81.3 | 78.2 | |
| BDE-100 | 2,2'4,4',6-pentabromodiphenyl ether | 189084-64-8 | C12H5Br5O | 564.7 | 7.66 | 2.44E-08 | 30 | 49.7 | 58.2 | 53.2 | |
| BDE-153 | 2,2',4,4',5,5'-hexabromodiphenylether | 68631-49-2 | C12H4Br6O | 643.6 | 8.55 | 2.87E-09 | 15 | 61.0 | 67.2 | 64.7 | |
| BDE-154 | 2,2',4,4',5,6'-hexabromodiphenylether | 207122-15-4 | C12H4Br6O | 643.6 | 8.55 | 2.87E-09 | 15 | 7.0 | 6.1 | <DL | |
| BFRs | PBB-153 | 2,2',4,4',5,5'-hexabromobiphenyl | 59080-40-9 | C12H4Br6 | 627.6 | 9.10 | 3.32E-07 | 15 | 15.8 | 16.5 | 15.4 |
| TBBP-a | 2,2',6,6'-tetrabromo-4,4'-isopropylidene diphenol | 79-94-7 | C15H12Br4O2 | 543.9 | 7.20 | 3.46E-11 | 12 | 120.0 | 113.8 | 122.3 | |
| Pesticides | trans-Nonachlor trans-nonachlor | 039765-80-5 | C10H5C19 | 444.2 | 6.35 | 2.16E-06 | 50 | 58.3 | 55.6 | 53.9 | |
| p,p'-DDE | Dichlorodiphenyldichloroethylene | 72-55-9 | C14H8C14 | 318.0 | 6.51 | 6.00E-06 | 90 | 921.0 | 893.2 | 926.1 | |
| P-HCH | (β) hexachlorcyclohexane | 319-85-7 | C6H6C16 | 290.8 | 4.14 | 3.52E-05 | 60 | 31.3 | 36.6 | <DL | |
| HCB | hexachlorobenzene | 118-74-1 | C6C16 | 284.8 | 5.73 | 1.80E-05 | 55 | 29.7 | 38.5 | <DL | |
(1) Concentration reported in standard reference material (SRM).[43]
(2) Concentration measured in 1 ml samples of reconstituted SRM.
(3) Concentration measured in 50 μl blood spot samples using reconstituted SRM.
1.2 Background contamination
Background (or matrix) contamination on sample cards used to collect DBS was evaluated using two types of new cards used by the MNB and many other states (CF10, CF12, W-903, Whatman, Sigma Aldrich, St. Louis, MO; and A-226, Ahlstrom, Alpharetta, GA). Circular punches (15 mm dia) were taken from empty areas (without blood) of the card. To increase sensitivity, representativeness and efficiency, composite samples were used, each consisting of two punches from a single card. Ten cards of each type were tested, each from a single lot.
1.3 Volunteer tests
Measurements of POPs in DBS and whole blood were compared among samples collected from six adult volunteers (aged 20 to 64 years) from different countries. Blood was collected in 7 mL tubes (Pink Top, Becton, Dickinson and Co., Research Triangle Park, NC). DBSs were prepared by placing a 50 μL drop of whole blood using a 100 μL syringe on unused cards (Whatman-903), which were dried overnight in a desiccator. Triplicate DBS and duplicate whole blood samples for each individual were analyzed using procedures described below. All protocols were approved by our institutional review board.
1.4 Preparation of storage stability and QC samples
DBSs for quality control (QC) and sample storage tests were prepared by aliquoting fresh blood (composite from volunteers) treated with EDTA. The sample was mixed for 10 min, allowed to equilibrate overnight, and 50 μL aliquots were applied to unused sample cards (Whatman-903) as described above. This blood volume was selected as typical of the quantity found in NBS. [7] Cards were dried overnight and stored at several temperatures.
A total of 125 DBSs and 125 blanks were prepared using 25 sample cards for each subsample type. (Additional cards were prepared and stored for possible future studies, e.g., further aging tests and interlaboratory comparisons.) Prepared DBS cards were individually wrapped in cleaned aluminum foil (rinsed with acetone and dichloromethane (DCM), and then baked overnight at 260 °C), and placed in groups of five in sealed polyethylene bags.
1.5 Sample stability tests
A series of tests was designed to simulate DBS transport and storage at four temperatures: 20 °C (room temperature), 4 °C (refrigerated), −20 °C (frozen), and −80 °C (ultralow freeze). The first three conditions correspond to those used in the MNB. Tests were run at nine time points (0, 1, 3, 10, 30, 100, 190, 251 and 365 days measured after sample preparation). Three replicates were used for each time point and temperature, along with QC samples, blanks and calibration checks.
1.6 Sample preparation and analysis
Sample analyses followed procedures described as NIST method 1. [43] In brief, DBS punches (15 mm dia) containing the DBS were placed in a 15 mL glass tube (Fisher Scientific, Pittsburgh, PA), and treated with hydrochloric acid (1 ml, 6 M) and then 1:1 ethanol/isopropanol mixture (6 mL). The sample was extracted twice using 1:1 hexane/methyl-t-butyl ether (6 mL, MTBE). After rotating for 2 min, the organic layer was transferred to a new tube with 1% potassium chloride (4 mL) and re-extracted with 1:1 hexane/MTBE (3 mL). The organic phases were combined, and the solvent volume was reduced to 1 mL by evaporation under a gentle N2 flow. Next, the concentrated sample was eluted through a sulfuric acid/silica gel column (6.6 g silica, 3.3 g sulfuric acid at the top; 0.1 g silica, 1 g sulfuric acid at the bottom). Prior to use, the silica gel was baked overnight at 280 °C and rinsed with 1:1 hexane/DCM mix (9 ml). Analytes were eluted from the column with 1:1 hexane/DCM mix (8 ml), and then evaporated under N2 to a volume of 0.5 mL. The final extract was prepared with the addition of 0.5 mL n-nonane and evaporated under N2 to a volume of 250 μL.
Analytes were separated and quantified using a gas chromatograph and mass spectrometer (GC/MS; 5890/5963, Agilent Industries, Palo Alto, CA, USA) equipped with a DB-5 column (30 m length, 0.25 mm id, 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA). Each run used a 2 μL injection, 10 μL injector syringe, injector temperature of 280 °C, helium as the GC carrier gas (flow rate of 0.7 ml/min, pressure of 5.43 psi, and average velocity of 31 cm/s), and required from 34.5 (PBDEs) to 69.5 min (pesticides).
GC temperature programs and MS settings were optimized for each compound group. Five temperature programs were used. For PCBs, the initial oven temperature setting was 80 °C, which was held for 1 min, then ramped at 20 °C/min to 150 °C, then ramped at 2 °C/min to 250 °C, held for 4 min, ramped at 30 °C/min to 300 °C, and finally held for 6 min (total run time of 66.17 min). For pesticides other than toxaphene, the initial temperature was 80 °C, held for 1 min, ramped at 20 °C/min to 150 °C, ramped at 2 °C/min to 250 °C, held for 4 min, ramped at 10 °C/min to 300 °C, and then held for 6 min (total of 69.5 min). For toxaphene, the initial temperature was 80 °C, held for 1 min, increased at 10 °C/min to 200 °C, increased at 1.5 °C/min to 230 °C, increased at 10 °C/min to 300 °C, and held for 6 min (total of 46 min). For tetra- through octa-homologues of PBDEs, temperatures started at 80 °C, held for 2 min, and then ramped at 10 °C/min to 300 °C (total of 34 min). For nona- through deca-homologues of PBDEs, the temperature started at 80 °C, held for 2 min, increased at 50 °C/min to 300 °C, and then was held for 55 min (total of 61.4 min). The MS detector was operated in negative chemical ionization selective ion monitoring (NCI SIM) mode using methane as the reagent gas. Ions and retention times are listed in Supplemental Table 1.
Analytes designated as total were determined as the sum of individually quantified constituents calibrated against authentic standards, e.g., Σ11PCB is the sum of the 11 congeners noted above, and Σ6PBDE is the sum of 6 congeners. In this application, measurements below the detection limit in sums were set to zero; this made no practical difference since concentrations of most samples considerably exceeded detection limits, and only data for the most abundant compounds are presented in this paper.
1.7 Calibration and quality assurance (QA)
Calibration standards were prepared using certified calibration standards (CDC persistent pesticides (PPC), PCB and PBDE calibration solutions, Cambridge Isotope Laboratories) at concentrations of 0.01, 0.1, 0.5, 1, 10 and 50 ng/mL. This range was selected to span the range expected in the adult samples.
Table 1 lists instrumental detection limits (IDLs) for the target compounds, defined as the amount of analyte for which the signal-to-noise ratio of the peak is equal to three, based on analyses of low concentration samples. The IDLs assume analyses using an extraction volume of 250 μL. They are appropriate for the sample integrity and other analyses in this paper. Because they may not include all sources of error, they are defined as IDLs, and not as method detection limits.
A minimum of three replicates were analyzed for each sample type and time point. Internal standards (IS), quantified in each run, consisted of 200 μL of labeled PCB, pesticide and PBDE mixtures (EO-5275, EC-4060, ES-5478 CIL, Tewksbury, MA), and 15 μL of the labeled internal standard, which were injected into each GC vial just before the GC/MS run. Labeled PCB standards were used as internal standards for the PBDEs. Each set of analyses used quality control (QC) samples processed in parallel with samples, and QC analyses included: drift checks (using repeated analysis of a standard injected every fifth sample), blanks, linearity, spike recovery, and SRM checks. Results were accepted only when duplicate values varied by less than 10%, linearity defined using a R2 exceeded 0.999; and measurements of SRMs were within 20% of certified values (see below). All spike recoveries were in the range of 78 to 104%. Blank contamination in standards and blood collection materials was negligible (<detection limit), although (background) contamination was found on unused cards, as discussed later.
The accuracy of both DBS and blood measurements were determined using a standard reference material (SRM 1957, Organic Contaminants in Non-Fortified Human Serum, NIST, Gaithersburg MD USA), which was reconstituted by adding 10.7 mL of HPLC-grade water, sonicating for 15 min, and equilibrating overnight under refrigeration. Then, reconstituted serum was analyzed as described in Section 1.6. The accuracy of DBS measurements was evaluated by preparing paper cards with 50 μl of reconstituted serum (applied in stages so that the DBS did not exceed a diameter of 15 mm). Cards were then dried, extracted and analyzed. With several exceptions, these measurements ranged from 81 to 119% of certified values for blood, and from 98 to 115% for DBS. Measurements were slightly elevated (127 to 139% of certified values) for PCB-105 and PCB-194 in the DBS samples, and for HCB in blood (127%), which may have resulted from imperfect peak separation. Measurement variability was 15%, below the 20% limit. Overall, the blood and serum measurements closely matched certified values, demonstrating excellent performance of extraction and analysis steps. (Section 2.6 of this paper, Strengths and Limitations, discusses several additional design and methodological issues that might affect QA and study results.)
1.8 Data analysis
Analyte stability was assessed by comparing concentrations after various storage periods to concentrations obtained in the initial determination, measuring what has been termed “incurred” sample stability (as distinguished by comparison to the nominal values of QC samples, which is called “QC” sample stability) [5]. Using data from the storage tests, loss rates per year were estimated using linear regression models and multi-compartment exponential models, which represent fast and slow loss processes separately, and which can provide excellent explanatory ability [44–46]. Model parameters were estimated by minimizing sum of squared residuals.
2 Results and Discussion
2.1 Background contamination
Trace levels of several target POPs were found on unused cards (Table 2). PCB-170+190, 105 and 194 were found at blood concentrations equivalent to 5±2, 8±1 and 19±3 ng/L, respectively, and at higher levels (35±3, 17±2 and 24±3 ng/L) in the Ahlstrom cards (mean and standard deviation). BDE-47 was found in both cards at similar levels (35±3 and 33±3 ng/L for Whatman and Ahlstrom cards, respectively.) Background contamination was quite consistent across the card samples (typical coefficient of variation around 10%), all of which were drawn from the same lot. These contaminants were confirmed to be in the blanks, and not in materials used for extraction and sample processing. No additional contamination was found after storing samples for 1 year, with the exception of PCB-209 found in the final months (as discussed later in the sample storage tests). In the storage tests, the background contamination was consistent (coefficients of variation from 6 to 10%, n=96), indicating that the contamination source was the original card, and that storage did not add further contamination.
Table 2.
Comparison of concentrations measured in NHANES to estimated limit of quantitation (LOQ) to determine suitability of DBS measurements. BG is background contamination in cards.
| Compound | LOQ (ng/L) |
BG Contam. (ng/L) |
NHANES Concentration |
Ratio to LOQ |
Overall suitability for DBS measurements / BG Notes, if any |
||||
|---|---|---|---|---|---|---|---|---|---|
| 50th (ng/kg) |
90th (ng/kg) |
95th (ng/kg) |
50th (ratio) |
90th (ratio) |
95th (ratio) |
||||
| PCB-28 | 100 | 0 | 30 | 57 | 67 | 0.3 | 0.6 | 0.7 | Poor |
| PCB-138 | 33 | 0 | 95 | 359 | 477 | 2.9 | 10.8 | 14.3 | Excellent |
| PCB-153 | 33 | 0 | 135 | 477 | 624 | 4.1 | 14.3 | 18.7 | Excellent |
| PCB-180 | 57 | 35 | 114 | 409 | 534 | 2.0 | 7.2 | 9.4 | Excellent, BG Correction |
| PCB-105 | 17 | 17 | 7 | 27 | 43 | 0.4 | 1.6 | 2.5 | Good, BG Correction |
| PCB-118 | 100 | ND | 32 | 143 | 216 | 0.3 | 1.4 | 2.2 | Good |
| PCB-194 | 40 | 24 | 26 | 96 | 129 | 0.7 | 2.4 | 3.2 | Good, BG Correction |
| PCB-206 | 83 | ND | NA | NA | NA | NA | NA | NA | - |
| PCB-209 | 167 | ND | NA | NA | NA | NA | NA | NA | - |
| PCB-170 | 100 | ND | 41 | 144 | 188 | 0.4 | 1.4 | 1.9 | Good |
| PCB-187 | 100 | ND | 29 | 115 | 167 | 0.3 | 1.2 | 1.7 | Good |
| p,p'-DDE | 300 | ND | 1,260 | 7,070 | 12,100 | 4.2 | 23.6 | 40.3 | Excellent |
| β-HCH | 200 | ND | NA | 216 | 372 | NA | 1.1 | 1.9 | Good |
| HCB | 183 | ND | 90 | 157 | 186 | 0.5 | 0.9 | 1.0 | Fair |
| PBB-153 | 50 | ND | 14 | 84 | 178 | 0.3 | 1.7 | 3.6 | Good |
| TBBP-a | 40 | ND | NA | NA | NA | NA | NA | NA | - |
| BDE-28 | 100 | ND | NA | NA | NA | NA | NA | NA | - |
| BDE-47 | 100 | 35 | 126 | 558 | 1,068 | 1.3 | 5.6 | 10.7 | Excellent, BG Correction |
| BDE-99 | 100 | ND | NA | 142 | 277 | NA | 1.4 | 2.8 | Excellent |
| BDE-100 | 100 | ND | 24 | 121 | 239 | 0.2 | 1.2 | 2.4 | Good |
| BDE-153 | 50 | ND | 31 | 214 | 431 | 0.6 | 4.3 | 8.6 | Good |
| BDE-154 | 50 | ND | NA | 14 | 28 | NA | 0.3 | 0.6 | Poor |
(1) NA: not reported in NHANES.
It is not surprising to find trace levels of PCBs and PBDEs on unused cards since these chemicals have been manufactured in large quantities, have been used indoors, and are persistent. Contamination can arise throughout the card's lifecycle, e.g., manufacturing, packaging, handling, labeling, transportation, processing, and storage. The sources, types and concentrations of contaminants may vary over time, e.g., an analysis of cards manufactured from 1987 to 2009 showed significant declines of BDE-47, PCB-153 and PCB-194 concentrations, but constant levels of other PBDEs and PCBs [26]. This study reports levels of BDE-47 and PCB-194 on unused cards from 1987 (7,000 and 11,000 ng/L, respectively) that are two to three orders of magnitude higher than measured in the present study. Since lot-to-lot variation is likely, sampling and analysis of a punch taken close to the archived DBS is recommended, using areas at least 1 mm from the edge of the blood spot [5, 26]. If background contamination is consistent across the card, and small relative to levels expected in the population, then background corrections can be used to avoid overestimating concentrations. Such corrections may be important for some PCB and BDE congeners.
The background contamination in blank cards is compared to contaminant levels expected in the population as estimated using NHANES, which is probably the best population-oriented study for the U.S. population although POPs were measured in only adults. As shown in Table 2, background levels of PCBs-170+190, 105 and 194 approach or exceed median blood levels, while the BDE-47 background level represents 28% of the median level. Thus, uncorrected DBS measurements may have limited utility for these three PCBs, although more exposed individuals (above 75th to 90th percentiles) are easily identified. These results do not reflect card-to-card or lot-to-lot variation in background contamination levels.
Analysis of smaller punches within the DBS, as discussed below, may lower levels of background contamination. This is possible since the perimeter area of the 15 mm dia punch used to sample the roughly 10 mm dia DBS included card material with little or no blood. However, differences are not expected to be large.
2.2 Volunteer tests
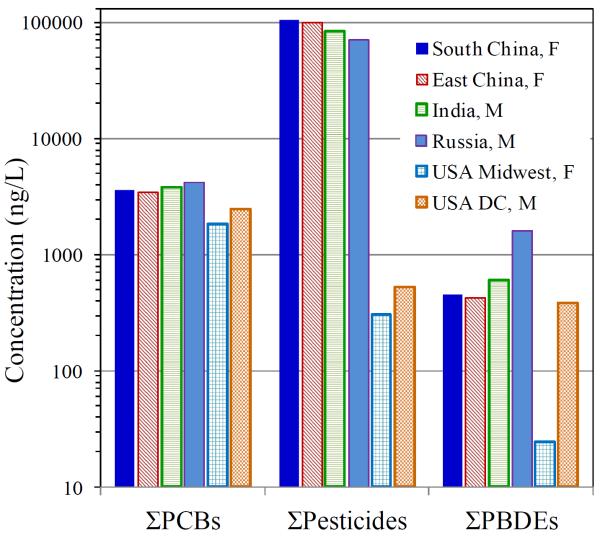
Figure 1 contrasts levels of PCBs, pesticides and PBDEs measured on DBS across six volunteers. All target compounds were detected. This small sample showed considerable variation. For Σ11PCB, factor of two differences were seen, and the Russian and Indian males had the highest levels (3,800 – 4,200 ng/L), well above US norms (shown later in Table 2), while the US male and female had the lowest levels (1,900 – 2,500 ng/L). Pesticides showed larger differences: the two Chinese females had very high levels of p,p'-DDE (84,000 – 104,000 ng/L); the US male and female had the lowest levels (300 – 500 ng/L). Concentrations of nonachlor, HCH and HCB were much lower but followed similar trends. Again, the variation and range are high compared to US norms. Σ6BDE levels ranged from 1,600 ng/L (Russian male with tri-, tetra, penta- and hexa-congeners detected) to only 50 ng/L (US female, only BDE-47 was detected near the background level). For the first time, we report levels of PBB-153 and TBBP-a in DBS (120 and 940 ng/L, respectively), which were found in one individual (64 year-old Russian male); these compounds were not detected in other volunteers (all below 40 years of age). PBB is an older flame retardant that was phased out in the 1970s following a contamination event in Michigan; TBBP-a remains widely used in circuit boards and other applications [42].
Figure 1.
Summary results for PCBs, pesticides and PBDEs using DBS prepared from 6 volunteers. Nationality and gender of volunteer indicated in legend.
DBS and whole blood concentrations showed high correlation (Deming regression correlation = 0.80), however, DBS measurements were biased downwards by an average of 11±9% (n=124) from whole blood measurements (see Bland-Altman plot, Supplemental Figure 1). While most measurements were within 20% and are thus deemed acceptable, the relative differences tended to increase at low (<250 ng/L) concentrations, a trend suggestive of detection limit issues and imperfect integration of smaller peaks. Other possible biases, e.g., incomplete extraction from the card, or chromatographic drift beyond the 15 mm punch, appeared minimal. However, further evaluation using larger samples is suggested since our samples came from only six individuals. Such analyses might evaluate the performance of different analytical procedures for specific compounds or classes of compounds, as well as effects associated with variation in hematocrit levels. [8, 47]
These results demonstrate the value of DBS for assessing current exposures. The results demonstrated large differences in exposure in a small set of individuals. While most uses of the target POPs are now restricted, these chemicals have been applied widely in household, agricultural and other applications. Exposures can be attributed to the large reservoirs of “legacy” contaminants (e.g., DDT and PCBs) remaining in soils and sediments, the large quantities of PBDEs and other flame retardants in building materials and furnishings that accumulate in dust and elsewhere, the slow breakdown of these compounds, and the tendency to accumulate in the food chain [48–51]. The use of DBS may be especially valuable in estimating exposures among individuals potentially exposed from about 1960 to 2005 when many POPs were heavily applied, and for countries where persistent pesticides are still used.
2.3 Storage stability
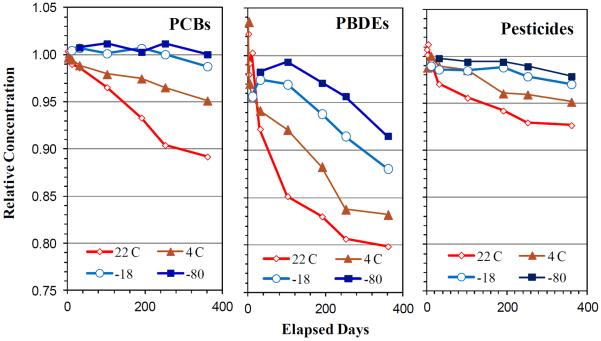
Trends of concentrations in DBS samples stored at −80, −18, 4 and 20 °C for one year are shown in Figure 2. At −80 and −20 °C, concentrations of each POP group showed flat or only very slowly declining trends, indicating high sample integrity. In these samples, losses were minimal for most of the target PCBs and persistent pesticides. Several compounds in these groups showed small losses, e.g., 4 to 9% loss per year for PCB-28+31, PCB-187+182 and HCB. Linearized loss rates across the compound groups at each temperature are listed in Table 3, and loss rates for individual compounds are in Supplemental Table 2. All PBDEs (except BDEs-99 and 100) showed faster losses, 9±7% loss per year at −18 °C and 8±5% at −80 °C across the six congeners (average and standard deviation). At 4 °C, loss rates increased to an average of 17±17% for the PBDEs. Again, BDEs-99 and 100 had the lowest loss (4–5% per year). Pesticides and PCB losses remained below 5% per year (on average). Loss rates increased to 12±7% per year for the PCBs, 9±6% for the pesticides, and 23±15% for the PBDEs. Loss rates of compounds within a POP group tended to increase with the compound's volatility, lower molecular weight, and lower octanol-water partition coefficients (Kow), e.g., r=0.61 between loss rates and log Kow for the 11 PCBs stored at −18 °C. At both −4 and 20 °C, the chromatograms showed peaks believed to include hydroxy-BDE, which suggests PBDE breakdown products. At −20 °and −80 °C, no such peaks were observed, and the abundance of the target compounds remained unchanged.
Figure 2.
Trends of PCB, PBDE and pesticide concentrations measured in DBS and stored at four temperatures over one year. Relative concentration is referenced to the concentration measured on the first day. Average loss among compounds in each target group is shown.
Table 3.
Summary of linearized loss rates (percent per year) for DBS measurements storage at four temperatures. Average rate across compounds in each group. Standard deviation in parentheses.
| Storage Temperature |
||||
|---|---|---|---|---|
| Group | 22 C | 4 C | −18 C | −80 C |
| PCBs | −11.8 (6.6) | −4.5 (5.3) | −1.5 (3.5) | −0.7 (3.7) |
| PBDEs | −22.7 (14.8) | −17.1 (17.3) | −9.1 (7.3) | −7.9 (4.9) |
| Pesticides | −8.7 (5.8) | −4.6 (3.1) | −1.7 (5.7) | −2.0 (3.0) |
The laboratory stability tests indicate that many POPs can be measured with minimal or acceptable error (<20%) in refrigerated or frozen DBS samples that have been stored for a year and potentially much longer periods. However, this does not apply to PBDEs for DBS samples stored at room temperature. These tests also show smooth and gradual changes, indicating that a correction factor might be used to compensate for these losses. For many target compounds, losses remained small after one year and curvature in concentration trends (Figure 2) could not be discerned. For storage at room temperature, however, exponential-type models appear appropriate. A two compartment exponential model, representing fast and slow loss processes, was applied:
| (1) |
This model was estimated for the average change in the six PBDEs at 20 °C. In this case: Ct and C0 = BDE concentration measured at time t (days); fitted weight α = 0.8325; and fitted loss rate coefficients β1 and β2 = 0.000118 and 0.0161 days−1, respectively. The fast process (represented by β2) accounted for the initial loss (about 17%) with a half-life of 0.12 years; the slow process accounted for the remaining loss with a half-life of 16 years. Model fit was excellent (R2 = 0.97). Eq. (1) predicts that across the six BDE congeners, the concentration on a DBS stored at 20 °C for 2 years will be 76% of the initial concentration, and 54% after 10 years. For the 11 PCBs at 20 °C, model parameters were α = 0.870; β1 = 0.00009232 days−1, β2 = 0.00285 days−1, and R2 = 0.99; and the fast process accounted for 13% of the loss with a half-life of 0.67 years; the slow process had a half-life of 21 years; and storage for 2 and 10 years would retain 83 and 62% of the initial concentration, respectively. For the four pesticides at 20 °C, α = 0.9545, β1 = 0.00008629 days−1, β2 = 0.01832 days−1, and R2 = 0.97; the fast process accounted for 5% of the loss with a half-life of 0.10 years; the slow process had a half-life of 22 years; and storage for 2 and 10 years would retain 90 and 70% of the initial concentration, respectively. The linear and exponential models might be combined to represent multiple phases of storage, e.g., PBDE analyses on DBS stored at room temperature for 2 years and then refrigerated for 2 years might be anticipated to have 68% of the initial sample remaining (83% using the exponential model multiplied by 100 – (2 × 9.1% per year) from the linear model, see Table 3). While demonstrating the use of such models, such extrapolations can involve considerable uncertainty, especially because our data extends to only one year of storage and a single test concentration was used.
Information on DBS storage integrity as a function of storage time and temperature is limited [5, 7]. Many analytes are considered stable in DBS stored at ambient temperatures for several days, long enough for samples to be sent through the ordinary mail. A recent report indicates that 14 PCB and 5 PBDE congeners were stable for 30 days (within ±15% of values measured on day 0) when stored at room temperature [26]. No other literature regarding longer term storage was located. Other data gaps include stability at elevated temperatures, which may be encountered during transport [7], and the effect of repeated freeze/thaw cycles. The present study was limited to storage at a constant temperature, but this remains a key requirement for DBS used to estimate retrospective exposures.
2.4 Suitability determination
The suitability of DBS for measuring specific contaminants depends on the combined effects of method sensitivity, storage stability, background contamination, extraction/analysis variability and other factors, all relative to the levels and variation of contaminant in newborns or other populations of interest. To identify analytes for which DBS analyses may provide useful information, DBS measurement performance is compared to contaminant levels expected in the population. Table 2 lists concentrations reported in NHANES for target compounds. (In NHANES, nonachlor was not measured; PBDE were reported using lipid-adjusted concentrations which were converted to ng/L blood using the median ratio of lipid-adjusted to serum concentrations of 152.4, which was very consistent across all POPs; concentrations were reported as ng/g, and the minor density difference between serum and water was ignored.) NHANES levels are compared to the limit of quantitation (LOQ), estimated using a signal/noise ratio of 10 (3.33 times the IDL shown earlier in Table 1). In all cases, blank concentrations fell below LOQs (although blank levels exceeded IDLs for PCBs 118, 105 and 194 and BDE-47). Table 2 lists ratios of NHANES levels to LOQs. Higher ratios are desirable and mean that the compound can be reliably detected. Table 2 also provides a summary rating of the suitability of DBS measurements, where “excellent,” “good” and “fair” respectively indicate that the median, 90th or 95th percentile concentration exceeded the LOQ, and “poor” indicates that all three concentrations fell below the LOQ. Thus, an “excellent” rating suggests that the analyte can be measured reliably in most samples; “fair” means it will be detected reliably in only samples with the highest concentrations. Such ratings could use other criteria as well incorporate storage integrity and other factors that are relevant to the study design and purpose.
These ratios and the overall suitability ratings in Table 2 show that DBS can be used for most of the PCBs (except PCB-28); PCBs 105, 180 and 194 may need background corrections. For pesticides p,p'-DDE and β-HCH, DBS samples will provide excellent and good performance, respectively; HCB may be measurable in at least a fraction of the population. For the BFRs, BDE-47 and 153 can be quantified, as can higher concentrations of BDE-100 and 153. These results pertain to the DBS extraction and analysis protocols described in the methods and blank levels in new cards.
The US NHANES measured about 2,000 individuals aged 14 years and older in 2003–4 using a population-based sampling strategy, and is one of the largest biomarkers studies. While NHANES may not be an entirely relevant comparison for newborns, the range of POP concentrations in NHANES approximately matched (factor of two) PCB, BFR and pesticide levels reported in several recent studies [52, 53]. Notably, pesticide exposures in some countries can considerably exceed levels found in the USA (as suggested by results for the six volunteers discussed earlier). With these caveats, the NHANES data have relevance for the evaluation of the suitability of DBS measurements.
2.5 Improving sensitivity
For most target compounds, detection limits and not the background contamination is the limiting factor. Our GC/MS method used 2 μL injections from sample extracts reduced to 250 μL, representing analysis of a 0.4 μL blood sample (assuming a 50 μL, 10 mm dia blood spot). While the extraction volume could be further reduced to improve detection limits, e.g., available autosamplers can handle volumes down to 10 μl using conical tube inserts, this may adversely affect precision and limit opportunities for replicate and other analyses. Alternative methods, such as microextraction by packed sorbent or other means [54], can provide analysis of essentially the entire extracted sample, providing up to a 125-fold increase in mass to the detector with the simplifying assumption that sensitivity, selectivity, noise, and other factors affecting detection limits are comparable. While these assumptions are not completely realistic and they do not account for replicates, such methods might substantially increase sensitivity, as well as greatly simplify sample cleanup and preparation. In addition, the use of new instrumentation, such as magnetic sector mass spectrometers, can attain much lower detection limits and might be applicable for DBS analyses.
More sensitive methods also might permit the use of only a subsample of the DBS, which is particularly important for biorepositories unwilling to surrender the entire DBS. For example, a 3.2 mm dia punch taken from the DBS is commonly used for bioanalyses of newborn DBS. Assuming that a DBS from a typical 50 μL blood sample forms a homogeneous 10 mm dia spot (79 mm2), then a 3.2 mm dia punch will yield 10% of the area (8 mm2) and a sample equivalent to 5 μL blood. If the entire subsample can be utilized in a single analysis (e.g., using on-line concentration), then the 3.2 mm punch would provide up to a 12.5-fold increase in sensitivity compared to the method used in this paper assuming that other parameters remain unchanged. Further research is needed to address sensitivity issues.
2.6 Strengths and limitations
This paper used well-controlled and reproducible laboratory tests to measure storage stability, background contamination, and other factors affecting the suitability of DBS measurements for a wide range of organic compounds. In practice, laboratory conditions may not represent conditions used to transport and store DBSs, in particular, changing temperature and humidity, the possibility of contamination, and other factors that might affect sample integrity. Other limitations of the work include: the small sample size used to compare measurements in blood and DBS; a potential accuracy problem noted at lower concentrations; the use of adult (not newborn) blood to develop and evaluate the method (newborn blood will have lower levels of chemicals that do not cross the placental barrier as well as more variable hematocrit levels); and the need to extend tests of sample storage to multiple years, thus enabling evaluation of the integrity of DBS samples archived for a decade or more. However, potential issues related to accuracy or hematocrit seem unlikely to alter study results since entire adult blood spots were used and analyses focused on relative concentrations, e.g., ratios of measurements in blood levels to detection limits or background concentrations, and since temporal changes in the sample storage/integrity tests were expressed relative to initial measurements.
3 Conclusions
The GC/MS method developed to measure PCBs, PBDEs, PBBs and persistent pesticides using DBS performed well, as confirmed using certified SRMs and other analyses. All target compounds were detected in blood samples and DBS collected from six volunteers, and the two sample types showed good agreement. Cards used for DBS samples had background contamination of several target POPs, but samples were not further contaminated and sample integrity remained high in storage extending up to one year. Storage stability of target compounds excluding PBDEs was acceptable for DBS stored at room temperature for up to one month; refrigeration or freezing is needed for longer periods. Multiyear storage appears acceptable if samples are stored at −80 °C. A multicompartment model is presented that may be used to estimate (and possibly correct for) storage losses. Several analytes not previously measured in DBS were detected, including PBB-153, TBBP-a, and hydroxy-PBDE derivatives. Compounds potentially suitable for DBS measurements were identified by comparing experimental results to literature reports on POP exposures.
Epidemiology and exposure studies would benefit from biomarker measurements that are sufficiently accurate and precise to reduce the likelihood of exposure misclassification and bias. The use of DBS samples can be highly advantageous given their convenience, safety and low cost of sample collection and transport. DBS samples may be appropriate for certain persistent contaminants, as has been reported in a small number of studies. However, widespread application of DBS for the purpose of quantifying exposures requires an improved understanding of QA elements and methods that meet QA goals. This work addressed several research needs for DBS, specifically sample storage, background contamination, recovery and extraction variability, sensitivity and precision, information needed to obtain accurate measurements of toxicants using DBS.
Supplementary Material
Highlights.
DBS were used to measure PCBs, brominated flame retardants, and chlorinated pesticides
Sample DBS cards showed background contamination of several compounds.
Pesticides and PCBs, but not PBDEs were stable in DBS stored at room temperature.
When frozen, multiyear storage of DBS for many organic compounds is acceptable.
Multicompartment models may be used to estimate or correct for storage losses.
Archives of DBSs are kept by many states and represent a valuable exposure resource.
DBS sampling is simpler, more cost-effective, and safer than venipuncture.
Acknowledgements
Lei Huang and Mareisha-Ann Lala provided laboratory assistance. Financial support was provided by the Michigan Bloodspot Environmental Epidemiology Project under grant “Investigation and assessment of the use of blood spots for retrospective exposure estimation of persistent organic contaminants, including chlorinated and brominated compounds, in human blood.” The National Institute of Environmental Health Sciences, National Institutes of Health provided additional support in grant P30ES017885 “Lifestage exposures and adult disease.” The reviewers provided a number of helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5 References
- 1.Therrell BL, Jr., et al. Committee report: Considerations and recommendations for national guidance regarding the retention and use of residual dried blood spot specimens after newborn screening. Genet Med. 2011;13(7):621–4. doi: 10.1097/GIM.0b013e3182147639. [DOI] [PubMed] [Google Scholar]
- 2.Therrell BL, Jr., Hannon WH. Newborn dried blood spot screening: residual specimen storage issues. Pediatrics. 2012;129(2):365–6. doi: 10.1542/peds.2011-3416. [DOI] [PubMed] [Google Scholar]
- 3.Therrell BL, et al. Guidelines for the retention, storage, and use of residual dried blood spot samples after newborn screening analysis: Statement of the Council of Regional Networks for Genetic Services. Biochem Mol Med. 1996;57(2):116–24. doi: 10.1006/bmme.1996.0017. [DOI] [PubMed] [Google Scholar]
- 4.Déglon J, et al. Direct analysis of dried blood spots coupled with mass spectrometry: concepts and biomedical applications. Analytical and Bioanalytical Chemistry. 2012;402(8):2485–2498. doi: 10.1007/s00216-011-5161-6. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Tse FL. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed Chromatogr. 2010;24(1):49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- 6.Michigan Neonatal Biobank. 9/5/12]; Available from: http://www.mnbb.org/researchers.
- 7.Tanna S, Lawson G. Analytical methods used in conjunction with dried blood spots. Analytical Methods. 2011;3(8):179–1718. [Google Scholar]
- 8.De Vries R, et al. The effect of hematocrit on bioanalysis of DBS: Results from the EBF DBS-microsampling consortium. Bioanalysis. 2013;5(17):2147. doi: 10.4155/bio.13.170. [DOI] [PubMed] [Google Scholar]
- 9.Ma W-L, et al. Temporal trends of polybrominated diphenyl ethers (PBDEs) in the blood of newborns from New York State during 1997 through 2011: analysis of dried blood spots from the newborn screening program. Environmental science & technology. 2013;47(14):8015. doi: 10.1021/es401857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams SA, Snodgrass JJ, McDade TW. What a Drop Can Do: Dried Blood Spots as a Minimally Invasive Method for Integrating Biomarkers Into Population-Based Research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 11.Keevil BG. The analysis of dried blood spot samples using liquid chromatography tandem mass spectrometry. Clinical Biochemistry. 2011;44(1):110–118. doi: 10.1016/j.clinbiochem.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 12.McDade TW. Development and validation of assay protocols for use with dried blood spot samples. American Journal of Human Biology. 2014;26(1):1. doi: 10.1002/ajhb.22463. [DOI] [PubMed] [Google Scholar]
- 13.Stove CP, et al. Dried blood spots in toxicology: From the cradle to the grave? Critical Reviews in Toxicology. 2012;42(3):230. doi: 10.3109/10408444.2011.650790. [DOI] [PubMed] [Google Scholar]
- 14.Ma W, et al. Analysis of polyfluoroalkyl substances and bisphenol A in dried blood spots by liquid chromatography tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2013;405(12):4127. doi: 10.1007/s00216-013-6787-3. [DOI] [PubMed] [Google Scholar]
- 15.Langer EK, et al. Characterization of the elemental composition of newborn blood spots using sector-field inductively coupled plasma-mass spectrometry. J Expo Sci Environ Epidemiol. 2011;21(4):355–64. doi: 10.1038/jes.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhuri SN, et al. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J Expo Sci Environ Epidemiol. 2009;19(3):298–316. doi: 10.1038/jes.2008.19. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZW, et al. Further reduction in lead exposure in women in general populations in Japan in the 1990s, and comparison with levels in east and south-east Asia. Int Arch Occup Environ Health. 2000;73(2):91–7. doi: 10.1007/s004200050013. [DOI] [PubMed] [Google Scholar]
- 18.Olshan AF. Meeting Report: The Use of Newborn Blood Spots in Environmental Research: Opportunities and Challenges. Environmental Health Perspectives. 2007;115(12):1767–1769. doi: 10.1289/ehp.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spector LG, et al. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1902–5. doi: 10.1158/1055-9965.EPI-07-0230. [DOI] [PubMed] [Google Scholar]
- 20.Otero-Santos SM, et al. Analysis of perchlorate in dried blood spots using ion chromatography and tandem mass spectrometry. Analytical chemistry. 2009;81(5):1931–1936. doi: 10.1021/ac802419n. [DOI] [PubMed] [Google Scholar]
- 21.Funk WE, et al. Hemoglobin adducts of benzene oxide in neonatal and adult dried blood spots. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(8):1896. doi: 10.1158/1055-9965.EPI-08-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard S, et al. Quantitation of Bisphenol A in Dried Blood Spots Using Liquid Chromatography Tandem Mass Spectrometry. Annual Meeting, American Association for Clinical Chemistry (AACC); Atlanta, GA, USA. 2011. [Google Scholar]
- 23.Burse VW, et al. Preliminary investigation of the use of dried-blood spots for the assessment of in utero exposure to environmental pollutants. Biochem Mol Med. 1997;61(2):236–9. doi: 10.1006/bmme.1997.2603. [DOI] [PubMed] [Google Scholar]
- 24.Shlosberg A, et al. Examination of Eurasian griffon vultures (Gyps fulvus fulvus) in Israel for exposure to environmental toxicants using dried blood spots. Archives of environmental contamination and toxicology. 2012;62(3):502–511. doi: 10.1007/s00244-011-9709-4. [DOI] [PubMed] [Google Scholar]
- 25.Dua VK, et al. Determination of HCH and DDT in Finger-Prick Whole Blood Dried on Filter Paper and Its Field Application for Monitoring Concentrations in Blood. Bulletin of Environmental Contamination and Toxicology. 1996;56(1):50–57. doi: 10.1007/s001289900008. [DOI] [PubMed] [Google Scholar]
- 26.Lu DS, et al. Measurements of polybrominated diphenyl ethers and polychlorinated biphenyls in a single drop of blood. Journal Of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2012;891:36–43. doi: 10.1016/j.jchromb.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Kato K, et al. Analysis of blood spots for polyfluoroalkyl chemicals. Anal Chim Acta. 2009;656(1–2):51–5. doi: 10.1016/j.aca.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Spliethoff HM, et al. Use of newborn screening program blood spots for exposure assessment: declining levels of perluorinated compounds in New York State infants. Environ Sci Technol. 2008;42(14):5361–7. doi: 10.1021/es8006244. [DOI] [PubMed] [Google Scholar]
- 29.Batterman AR, et al. Data quality objectives in environmental research planning. Quality assurance (San Diego, Calif.) 1999;7(4):181–194. doi: 10.1080/105294199750061290. [DOI] [PubMed] [Google Scholar]
- 30.US Environmental Protection Agency . EPA QA/G-4. 2006. Guidance on Systematic Planning Using the Data Quality Objectives Process. [Google Scholar]
- 31.Garcia Boy R, et al. Determination of morphine and 6-acetylmorphine in blood with use of dried blood spots. Therapeutic Drug Monitoring. 2008;30(6):733–739. doi: 10.1097/FTD.0b013e31818d9fdb. [DOI] [PubMed] [Google Scholar]
- 32.Spooner N, Lad R, Barfield M. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Analytical chemistry. 2009;81(4):1557–1563. doi: 10.1021/ac8022839. [DOI] [PubMed] [Google Scholar]
- 33.de Sain-van der Velden MG, et al. Differences between acylcarnitine profiles in plasma and bloodspots. Mol Genet Metab. 2013;110(1–2):116–21. doi: 10.1016/j.ymgme.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Kulik W, et al. Bloodspot assay using HPLC-tandem mass spectrometry for detection of Barth syndrome. Clin Chem. 2008;54(2):371–8. doi: 10.1373/clinchem.2007.095711. [DOI] [PubMed] [Google Scholar]
- 35.Fingerhut R, et al. Stability of acylcarnitines and free carnitine in dried blood samples: implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal Chem. 2009;81(9):3571–5. doi: 10.1021/ac8022235. [DOI] [PubMed] [Google Scholar]
- 36.El-Hajjar DF, et al. Validation of use of annular once-punched filter paper bloodspot samples for repeat lead testing. Clin Chim Acta. 2007;377(1–2):179–84. doi: 10.1016/j.cca.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Keller JM, et al. Comparison of five extraction methods for measuring PCBs, PBDEs, organochlorine pesticides, and lipid content in serum. Analytical and Bioanalytical Chemistry. 2009;393(2):747–760. doi: 10.1007/s00216-008-2453-6. [DOI] [PubMed] [Google Scholar]
- 38.Peck HR, et al. A survey of apparent blood volumes and sample geometries among filter paper bloodspot samples submitted for lead screening. Clin Chim Acta. 2009;400(1–2):103–6. doi: 10.1016/j.cca.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Liang X, et al. Study of dried blood spots technique for the determination of dextromethorphan and its metabolite dextrorphan in human whole blood by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(8–9):799–806. doi: 10.1016/j.jchromb.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Slazyk WE, et al. Effect of lot-to-lot variability in filter paper on the quantification of thyroxin, thyrotropin, and phenylalanine in dried-blood specimens. Clin Chem. 1988;34(1):53–8. [PubMed] [Google Scholar]
- 41.Li F, et al. Liquid chromatography/tandem mass spectrometry sensitivity enhancement via online sample dilution and trapping: applications in microdosing and dried blood spot (DBS) bioanalysis. Rapid Commun Mass Spectrom. 2010;24(17):2575–83. doi: 10.1002/rcm.4670. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention . Fourth National Report on Human Exposure to Environmental Chemicals 2009. U.S. Department of Health and Human Services; 2009. [Google Scholar]
- 43.Schantz MM, et al. Milk and serum standard reference materials for monitoring organic contaminants in human samples. Analytical and Bioanalytical Chemistry. 2013;405(4):1203. doi: 10.1007/s00216-012-6524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batterman S, et al. Trends of brominated diphenyl ethers in fresh and archived Great Lakes fish (1979–2005) Chemosphere. 2007;69(3):444–57. doi: 10.1016/j.chemosphere.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 45.Hickey JP, Batterman SA, Chernyak SM. Trends of chlorinated organic contaminants in great lakes trout and walleye from 1970 to 1998. Arch Environ Contam Toxicol. 2006;50(1):97–110. doi: 10.1007/s00244-005-1007-6. [DOI] [PubMed] [Google Scholar]
- 46.Lee JH, et al. Ozone artifacts and carbonyl measurements using Tenax GR, Tenax TA, Carbopack B, and Carbopack X adsorbents. J Air Waste Manag Assoc. 2006;56(11):1503–17. doi: 10.1080/10473289.2006.10464560. [DOI] [PubMed] [Google Scholar]
- 47.Denniff P, Spooner N. The effect of hematocrit on assay bias when using DBS samples for the quantitative bioanalysis of drugs. Bioanalysis. 2010;2(8):1385. doi: 10.4155/bio.10.103. [DOI] [PubMed] [Google Scholar]
- 48.Miller MF, et al. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417–418:294–8. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batterman S, et al. Brominated flame retardants in offices in Michigan, USA. Environ Int. 2010;36(6):548–56. doi: 10.1016/j.envint.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller MF, et al. Polybrominated diphenyl ethers in human gestational membranes from women in southeast Michigan. Environ Sci Technol. 2009;43(9):3042–6. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batterman SA, et al. Concentrations and emissions of polybrominated diphenyl ethers from U.S. houses and garages. Environ Sci Technol. 2009;43(8):2693–700. doi: 10.1021/es8029957. [DOI] [PubMed] [Google Scholar]
- 52.Herbstman JB, et al. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect. 2008;116(10):1376–82. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fulara I, Czaplicka M. Methods for determination of polybrominated diphenyl ethers in environmental samples--review. Journal of separation science. 2012;35(16):2075. doi: 10.1002/jssc.201200100. [DOI] [PubMed] [Google Scholar]
- 54.Abdel-Rehim M. Microextraction by packed sorbent (MEPS): A tutorial. Analytica Chimica Acta. 2011;701(2):119. doi: 10.1016/j.aca.2011.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.