Abstract
Aims
Carbon nanotube (CNT) membranes offer an exciting opportunity to mimic natural protein channels due to 1) a mechanism of dramatically enhanced fluid flow 2) ability to place ‘gatekeeper’ chemistry at the entrance to pores 3) the ability for biochemical reactions to occur on gatekeeper molecules and 4) an ability to chemically functionalize each side of the membrane independently.
Main methods
Aligned CNT membranes were fabricated and CNT pore entrances modified with gatekeeper chemistry. Pressure driven fluid flow and diffusion experiments were performed to study the mechanisms of transport through CNTs.
Key findings
The transport mechanism through CNT membranes is primarily 1) ionic diffusion near bulk expectation 2) gas flow enhanced 1–2 orders of magnitude primarily due to specular reflection 3) fluid flow 4–5 orders of magnitude faster than conventional materials due to a nearly ideal slip-boundary interface. The transport can be modulated by ‘gatekeeper’ chemistry at the pore entrance using steric hindrance, electrostatic attraction/repulsion, or biochemical state. The conformation of charged tethered molecules can be modulated by applied bias setting the stage for programmable drug release devices.
Significance
The membrane structure is mechanically far more robust than lipid bilayer films, allowing for large-scale chemical separations, delivery or sensing based on the principles of protein channels. The performance of protein channels is several orders of magnitude faster than conventional membrane materials. The fundamental requirements of mimicking protein channels are present in the CNT membrane system.
Keywords: Drug delivery, Biomimetic, Nanostructure, Gatekeeper, Membrane, Transdermal, Nanoporous
Introduction
Biological protein channels are remarkable systems that have the ability to selectively pump necessary chemicals and signals through cell walls at rates orders of magnitude faster simple diffusion. Mimicking this function in large-area robust man-made structures can have broad application in chemical separations, drug delivery and sensing. In the case of drug delivery, chemical conditions or outside signaling can open the pore to initiate a controlled dosing of drug. Sophisticated dosing regiments, such as addiction treatments, can be enabled by such technology. The key to Nature’s protein channels are 1) selective receptor chemistry at the pore entrance, 2) a mechanism for fast fluid flow or mass transport and 3) signal chemistry at the exit side of protein to activate the channel (Hille 2004; Murata et al. 2000). Recent efforts towards this goal have focused on well ordered nanoporous monoliths with minimal path tortuosity that have an ability for surface chemical modification (Jirage et al. 1997). Fine control of pore size has been achieved with track-ion etch, where polymers are exposed to high energy particles that leave a uniform track of broken bonds that can be easily etched. Typically they have initial pore dimensions of ~20–30 nm. These can be reduced to ~1 nm by an electrode-less plating of gold that can further be chemically functionalized to form an affinity membrane (Jirage et al. 1997). This approach demonstrates molecular size exclusion, but suffers from a low areal density (108/cm2), subsequent low transport rate, and high fabrication expense. Porous Al can be formed by the electrochemical oxidation of foils and has well ordered aligned pores (20–50 nm diameter). These are commercially produced, have high areal density (~1010/cm2), high porosity (30–50%), and can have a wide variety of surface functionalization (Steinle et al. 2002). Controlled sol–gel plating can further reduce the pore diameter and subsequent surface functionalization results in the demonstration of enantiomeric separations (Lee et al. 2002). However all the approaches of ordered nm-scale pores with selective chemical transport suffer from the common problem of slow flux rates. Slow flux rates are a result of the inherent limitations of Fickian diffusion and slow fluid flow in nm-scale pores for Newtonian fluids.
Fluid flow through the cores of carbon nanotubes (CNTs) is predicted to show enhanced transport of hydrocarbon gases and water (Ohba et al. 2005). For instance, one pioneering Molecular Dynamics (MD) study (Hummer et al. 2001) predicted that water flow through ‘hydrophobic’ single-walled CNTs (SWCNTs) should be not only possible but dramatically enhanced. This is because the process of water entering CNT can induce H-bond ordering in a chain of water molecules. This can make-up for the energy cost to lose 2 of the 4 weak hydrogen bonds as the water molecules separate from the bulk water into the hydrophobic CNT core. In order to preserve the H-bond ordering of the water chain, it is critical to have nearly frictionless interaction with the CNT graphite sheets to not scatter or even rotate the flowing water. The large van der Waals distance (~2 Å) and flat ordering of graphite sheets were predicted to allow this. Using the study’s theoretical volume-rate (which is comparable to that of the protein channel aquapourin-1) divided by the CNT cross sectional area, a water flow velocity of ~90 cm/s is predicted, which is 5 orders of magnitude faster than would be for conventional materials of similar pore size (0.6 nm). High flow velocities of molecules through CNTs were also predicted due to the nearly frictionless nature of the CNT walls (Sokhan et al. 2002) as well as the fast diffusion rates for hydrocarbons (Skoulidas et al. 2002; Mao and Sinnott 2001). In the latter case, favorable interactions of the methane with the CNT wall predict the molecule will ‘skate’ down the tube wall and not scatter in random directions, as would conventionally happen in the case of Knudsen diffusion. The flow velocity of gaseous methane is predicted to be ~260 cm/s at 1 bar (Skoulidas et al. 2002). In all models, the atomically flat nature of graphite sheets inherent to the CNT microstructure makes the enhanced flow possible over long length scales. In fact, if an atomic step edge is placed inside CNT core, MD simulation predicts a dramatic drop in flow rates due to scattering (Joseph and Aluru 2008). Thus CNT cores, with atomically smooth and inert surfaces are theoretically predicted to have dramatic flow enhancements rivaling that of protein channels.
Approach to making MWCNT membranes
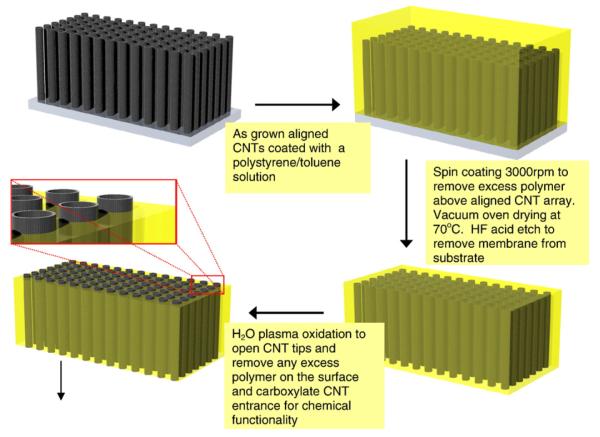
Aligned growth of dense arrays of multiwalled CNTs has been demonstrated at the University of Kentucky (Andrews et al. 1999; Sinnott et al.1999) and elsewhere (Ren et al.1998; Merkulov et al. 2002; Zhang et al. 2000). Although the outer diameters have significant variance (30±10 nm), the hollow inner core diameter is well controlled to 7 nm. Since this is a thermal chemical vapor deposition (CVD) process using readily available xylene/ferrocene, it is an industrially scalable process with an estimated cost of $0.60/m2. The primary goal is to form a membrane structure taking advantage of the as-deposited alignment of multiwalled CNTs to form a well-controlled nanoporous membrane structure (Hinds et al. 2004). If the space between CNTs could be filled with a polymer barrier, then a membrane with a rigid pore structure, high porosity, and small dispersion could be synthesized as diagrammed in Fig. 1(a).
Fig. 1.
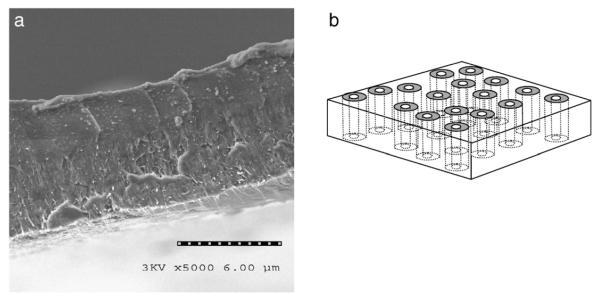
CNT membrane microstructure. (a) Cross sectional SEM image of aligned polymer film with CNTs aligned across film thickness. Shown is cleaved film (lower bright area is bottom membrane surface) with aligned CNTs slightly pulled out from surface. The polymer matrix is polystyrene. (b) Schematic of target membrane structure. With a polymer impregnation between CNTs, a viable membrane structure can be readily produced with the pore being the rigid inner tube diameter of the CNT.
The aligned CNTarray then has a 50 wt.% solution of polystyrene (PS) and toluene spun coat over the surface. PS is known to have high wet ability with CNTs and readily impregnates into a CNT array (Mitchell et al. 2002; Qian et al. 2000). Due to high viscous drag within the CNT array, only excess polymer on top of structure is removed during the spin coating process. The film is dried in vacuum at 70 °C for 4 days. HF acid is then used to remove CNT/PS composite from the quartz substrate. To form an open membrane structure, one needs to remove a thin layer of excess polymer on the top surface and open the normally closed CNT tips (Fig. 2). This is done using an H2O plasma enhanced oxidation process (Huang and Dai 2002) at 600 mTorr H2O pressure and 2.5 W/cm2 for 7 min, which removes the Fe nanocrystal catalyst from the tips of CNTs. Importantly, the plasma process opens the initially closed CNTs and leaves the tips CNTs functionalized with carboxylic acid that are readily functionalized with a selective receptor (Wong et al. 1998). Fig. 1(b) shows the cleaved edge of the freestanding membrane structure with CNT alignment from top to bottom with the polymer film intact.
Fig. 2.
Processing steps involved in aligned CNT membrane fabrication process.
Major mechanisms of mass transport through CNTs
With such dramatic theoretical predictions, it was an imperative to confirm the fast transport phenomena experimentally. In addition, the unique geometry of the cut CNTs allows chemistry to be placed precisely at the pore entrances, allowing for gate keeper activity. The term gate keeper is used here as a chemical layer only at the pore entrance that selectively allows chemicals to pass into and through the pores of the membrane, much how natural protein channels work. Both enhanced transport and gate keeper selectivity are key elements for mimicking natural protein channels. Success in this approach would allow for fast transport and high selectivity, which is not attainable for conventional membrane systems at the performance level of natural protein channels.
Ionic diffusion and gatekeeper activity
Due to the plasma oxidation process to open CNTs, there are carboxylate groups at the tips (and pore entrances) that are readily reacted to form carboimide linkages. That is, most any ligand with a primary amine can be covalently attached to the tips of CNTs and act as a chemically selective gatekeeper. The first systematic study (Majumder et al. 2005a) to prove that ‘gatekeeper’ chemistry can be formed on CNT membranes was with a series of four chemically distinct gatekeepers: short chained alkane, long chained alkane, long polypeptide, and highly charged dye molecule. In this study, IR data was consistent with the formation of carboimide linking chemistry at the surface which was confirmed by later electrochemical studies (Majumder et al. 2008). The simultaneous diffusional transport of ionic dyes through the membrane was measured. To demonstrate gatekeeper activity we looked at the ratio of small dye methyl viologen2+ (MV) and large dye (Ru) permeation through the membrane, where the larger dye should be more hindered. With longest molecule (polypeptide) covalently tethered to entrance of the 7 nm diameter pore, the ratio of small to large molecule (MV/Ru) passing through the membrane was ~3.6. This is significantly higher than the ~1.6 to ratio of bulk water diffusivity hence showing modest gatekeeper activity. With the long and short alkanes, only modest separations were seen but overall flux of aqueous ionic dyes decreased due to the tip becoming hydrophobic. The observed transport effects were a combination of both steric bulk and hydrophobic/phylic nature of the CNT tip chemistry. The observed separations were modeled with hindered diffusion in small pores (Renkin equation) occurring only at the tip gatekeeper region and with bulk diffusivity through the long length of the CNT core. This is consistent with the fabrication process that cut CNT tips and places functional chemistry at the pore entrances. An interesting point in this study was that the negatively charge dye molecule tethered to CNT entrance increased the flux (5 fold) of the positively charged dyes by electrostatic attraction. The effect could be screened by spectator ions (KCl) in solutions of high ionic strength. Thus electrostatics can be a strong force in enhancing flow rates or manipulating charged gatekeepers.
Biological gating
With the relatively large pore diameters of 7 nm, the MWCNT membranes are a natural choice for examining large biological molecules to be gate keepers. In the first report of CNT membranes (Hinds et al. 2004), biotinwas tethered to CNTentrances and irreversibly bound to streptavidin protein to block the pore. In a subsequent study (Nednoor et al. 2005) reversible desthiobiotin was covalently tethered to CNT entrances. In the presence of large streptavidin protein, the pore was blocked with a corresponding drop in flux of a dye across the membrane. With the addition of the much stronger biotin molecule to the solution, the tethered desthiobiotin is displaced from the protein by solution phase biotin, opening the membrane pores and returning to original flux values. This is an important experiment on several levels. The first is that the streptavidin protein is of well defined size~4 nm that can block only CNT pores (radius) but not large macroscopic cracks. The fact that that blockage was removed by chemistry to displace binding sites (desthiobiotin) shows that this was not a physisorption phenomena, but selective surface chemistry binding. On a practical level, this system provides two sensor routes: 1) large proteins selectively blocking CNT pores or 2) proper release chemistry opening pores. In another biological gating study, the primary hypothesis was to see if known biocatalytic (enzymatic) activity could occur at the tips of CNTs and affect the mass transport through membrane structures (Nednoor et al. 2007). A peptide sequence G-R-T-G-R-R-N-S-I-NH2, specific to Protein Kinase, was covalently bound to CNT tip. The serin was phospholated by Protein Kinase A/ATP and subsequently dephospholated by Alkaline Phosphatase. The state of the tethered peptide ligand (phosphylated or not) was detected by monoclonal anti-phosphoserine antibody binding to the tethered peptide in the phospylated state. The diffusional flux through the CNT membrane was modulated by these events showing that enzymatic catalysis (ATP cycle) could be performed at the tips of CNTs. This has important implications for drug delivery where natural biological process can open pores for drug delivery when the chemistry requires it. Sensors can also be developed by monitoring ionic diffusion to an electrode through CNT membrane in the presence or absence of bound bio-chemistry at CNT tips.
Gas and fluid flow (and the puzzle)
Molecular dynamics simulation had predicted very rapid fluid and gas flow within CNTs due to a nearly atomically flat surface with minimal scattering or chemical attraction. The phenomena were experimentally observed with pressure induced solvent flow (Majumder et al. 2005b) and gas flow (Holt et al. 2006). In the initial report, flow through the aligned CNT membrane was measured in a syringepump pressure cell apparatus (Majumder et al. 2005b), where mass (hence volume) of the solvent passing through the membrane is directly measured on a pan balance as a function of time. Flow data are summarized in Table 1. The flow rates are incredibly fast. There is a remarkable 4–5 orders of magnitude increase in water flow over what would be seen in conventional nanoporous structures. For gas flow there is a 1–2 order of magnitude increase in flux over Knudsen diffusion (Holt et al. 2006) in 2 nm pores. For our larger multiwalled CNTs with 7 nm inner diameter, the gas flow enhancement is about 20. Both studies are consistent with specular reflection, where gas molecules keep their forward momentum (i.e. no back scattering). The result of such fast transport of fluids has important practical applications since less than one ten thousandth the membrane area will be needed for the same amount of chemical separation from conventional membranes. The enhanced flow is also one of the three critical components for mimicking protein channels.
Table 1.
Pressure fluid flow through CNT membranes as a function of solvent.
| Liquid | Flow velocity normalized at 1 bar (cm/s) |
Calculated Newtonian flow velocity at 1 bar |
Enhancement factor |
Slip length (cm/s) (micron) |
|---|---|---|---|---|
| Water | 26 | 5.7×10−4 | 4.5×104 | 54 |
| EtOH | 4.5 | 1.4×10−4 | 3.2×104 | 28 |
| Hexane | 5.6 | 5.16×10−4 | 1.1×104 | 9.5 |
| Decane | 0.67 | 1.72×10−4 | 3.9×103 | 3.4 |
| IPA | 1.12 | 7.7×10−4 | 1.5×103 | 13 |
The flow flux rate of liquids (J) through conventional porous membranes can be predicted using the well known Haagen–Poiseuille equation (Mulder 1994) given by:
In this formula, ε is the relative porosity, r is pore radius (7 nm for our system), P is pressure applied, μ is dynamic viscosity, τ is tortuosity (1.1) and L is the length of the pore. The basic assumptions of this equation are laminar flow and ‘no-slip’ at the boundary layer, i.e. the velocity of the fluid at the CNT wall is zero. This zero velocity at pore walls is the physical origin of two flow velocities in conventional membrane pores. For Newtonian fluids in pores the velocity goes from zero at the wall to a maximum ‘core’ velocity at the pore center based on the viscous shear from applied pressure. However in nm-scale pores this core velocity is exceedingly small. Needed are high velocities along pore walls, referred to as slip conditions. Protein channels have ideal slip conditions with single file pumping of solvent or permeate. A useful convention for slip boundary is slip length that can be calculated from the equation (Lauga et al. 2005):
In this formula, V(λ) is the experimentally observed flow velocity (cm/s), Vns is the ‘no-slip’ flow velocity calculated from the Haagen–Poiseuille Equation, λ is the slip length and r is the radius of the nanotube. For water dramatic slip lengths of 50–100 μm, compared to the CNT radius of 3.5 nm, are experimentally seen for water. This means that the surface velocity on the CNT wall is nearly identical to that of the pore center, or nearly ideal slip. The longest slip lengths were observed for the polar molecules (i.e. water and MeOH) that are expected to have the weakest interactions with hydrophobic CNTs. Both for the polar and non-polar liquids, the slip lengths decrease with the longer hydrocarbon chain length. This is consistent with concept of more interaction with the CNT wall will decrease slip lengths and flow enhancement. However, even for long chain alkanes, flow rates are still dramatically enhanced compared to conventional materials.
In general, the transport through the CNT core shows nearly no enhancement in ionic diffusion, about a factor of 20–100 for gas flow, and a remarkable enhancement (4–5 orders of magnitude) of fluid flow. In the former case of ionic diffusion, the non-interacting CNTs offer no advantages for transport; essentially acting like a mirror for ions and molecules to bounce off of. Brownian scattering of ions within the solvent inside of CNTcores would dominate and thus follow Fick’s law of diffusion. Experimental measurements of ionic transport across the CNT membrane closely correlates with bulk diffusivity. For gasses, scattering is only off of the smooth CNT walls and the retention of forward momentum gives significant enhancement. However the rate limiting step becomes the gas molecule entering the pore entrances that are a very low percent of surface area. Since pore size cannot be controlled to sub-angstrom precision, chemical selectivity is given only by differences in gas velocity (v a mass1/2). Placing highly selective chemistry at pore entrances (i.e. gatekeeper) would, by necessity, slow the gas molecule to zero velocity and thus completely negates the fast flow phenomena of forward scattering off of CNT wall. In the case of the much denser fluid flow, dramatic enhancement is observed because of the nearly frictionless interface of the fluid and the CNT. This allows for very high wall velocity (~10 cm/s at 1 atm) whereas in conventional materials (atomically rough and short van der Waals distances) these have zero net velocity at pore walls. However a significant intellectual puzzle emerges: How selectively let chemicals into CNT cores while maintaining enhanced flow rates that are based on a nearly ideal non-interaction phenomenon. Adding selective chemistry to the pore entrances or along CNT cores ruins the enhanced fluid effect that is based on atomically flat non-interacting CNT wall. Thus it is not possible to get both high selectivity and high flow rate by simple chemical functionalization at the pore entrance. What is needed is a pumping mechanism at the location of selective chemistry. The velocity or momentum developed at this region can be transmitted down the nearly frictionless CNT core. Promising methods to induce pumping at the CNT tip region are by molecular motion or by electric field induced electro-osmosis and electrophoresis.
Electrostatic gatekeeping and nicotine delivery
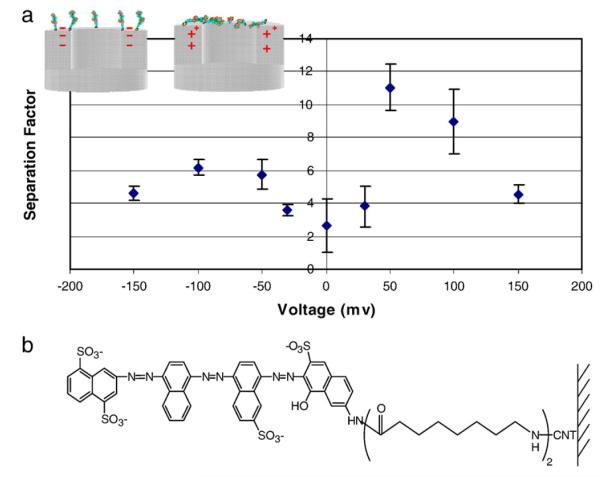
The concept of an active ‘gatekeeper’ that can be pulled into and out of the pore entrance can be the basis of a pump or the active element of a drug delivery system. In particular it is of interest to have small voltages, which can be provided by watch batteries, open and close membrane pores and regulate drug flow from a reservoir. This would allow for programmed dosing regiments adaptable to patient needs or provide a security protocol to prevent addiction to narcotic treatments. To prove the concept of an electrostatically actuated gatekeeper, a long quadra-charged dye molecule was tethered to the entrances of CNTs as shown in Fig. 3b (Majumder et al. 2007). Importantly, we needed a very high density of molecules on the CNT surface to be effective gatekeepers in the pore. To achieve this, electrochemical grafting of highly reactive diazonium salt onto CNT surfaces (Bahr and Tour 2001) was performed. The diazonium salts are highly reactive and can react with the basal planes of CNT, not just the more reactive cut tips. To avoid this aggressive reaction inside of the CNT, a continuous flow of inert water, at the previously mentioned remarkably high velocities, is passed through the CNT core. This prevents the diazonium reaction inside the CNT core but allows the reaction only at the CNT tips exposed to the diazonium solution. This is referred to as ‘flow grafting’, which is a powerful tool to place chemistry only at the tips of CNTs to act as ‘gatekeepers’. Fig. 3a shows the separation coefficients (a=MV/Ru) between small and large permeates through CNT membranes as a function of bias applied to the conductive CNT membrane. At 0 V bias, selectivity between the two permeate molecules across the membrane is close to the ratio of bulk diffusivity (~1.6). After application of positive bias, the separation factor increases to about 10, significantly higher than what had been achieved in the earlier study (Majumder et al. 2005a) without bias applied. The applied bias evidently gives enough electrostatic force to the tethered charged gatekeeper molecule to effectively block the CNT pore entrance. In the case of ‘flow grafted’ chemistry at the top tip surface, positive bias attracts the negatively charged tethers into the pore blocking the larger permeate. With negative bias the tethers are repelled, opening the pore. The plasma oxidation step in the fabrication process can form carboxylate groups down the throat of the CNTs at a low density. In this case negative bias repels the tether and it blocks the pore, as is seen by the modest increase in separation factor. In the case where a high density of the tether was reacted inside of the core, negative bias produced a large increase in separation (data not shown; Majumder et al. 2007) of ~20. Thus the primary concept of an active membrane that can be modified by applied bias was demonstrated and has application for controlled drug delivery.
Fig. 3.
Separation factor of MV/Ru as a function of bias applied to CNT membrane with flow-grafted charged gatekeeper functionality (below).
Another important milestone is to demonstrate that therapeutically interesting drugs can be delivered through the CNT membrane system. The most practical approach at this time is to have the membrane be the active element in a transdermal device. As a first step, the transdermal approach has the benefit of avoiding many biocompatibility issues since the active region is protected by an adhesive polymer layer. A simple skin patch design was used with skin/commercial adhesive(medifilm)/CNT membrane/nicotine reservoir. The flux through the transdermal system is shown in Fig. 4. The flux is 8 μmol/cm2 h which is above the therapeutically required rate of 0.8–4 μmol/cm2 h. The dose through CNT membrane can be reduced by the electrostatic gatekeeper blocking of the pore as described in Fig. 3 and is the object of current research efforts.
Fig. 4.
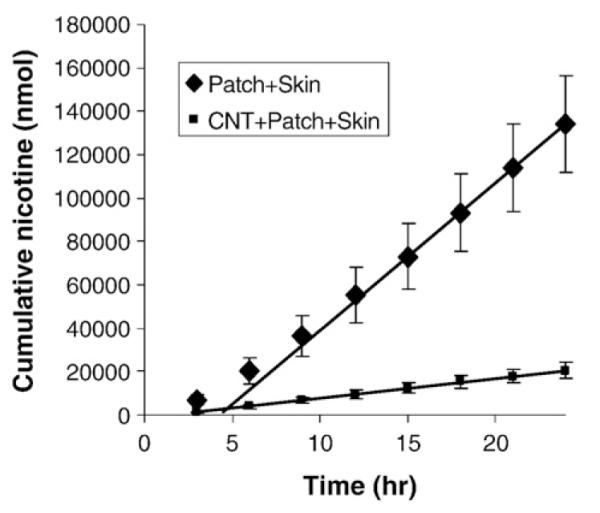
Nicotene flux through skin/patch (medifilm) assembly with and without CNT membrane barrier layer.
Conclusions and future prospects
CNT membranes offer an exciting opportunity to mimic natural protein channels due to 1) a mechanism of dramatically enhanced fluid flow 2) ability to place ‘gatekeeper’ chemistry at the entrance to pores 3) the ability for biochemical reactions to occur on the gatekeeper 4) ability to chemically functionalize each side of the membrane independently (Chopra et al. 2005). Additionally, the structure is mechanically far more robust than lipid bilayer films and does not require expensive protein expression (and separations) to form the active channels. Furthermore the CNT membrane is also electrically conductive to either electrochemically or electrostatically modulate the CNT entrance with externally applied voltages. Although the currently used polystyrene and CNTs have limited material biocompatibility, a wide variety of FDA approved polymers (epoxies, silicone) can fill stabilized CNT arrays. The surface chemistry of both CNTs and polymer can be modified to improve biocompatibility as well. All these unique features allow for large-scale chemical separations, chemical delivery or chemical sensing based on the principles of protein channels.
The transport mechanism through CNT membranes is primarily 1) ionic diffusion near bulk expectation 2) gas flow enhanced 1–2 orders of magnitude primarily due to specular reflection off of smooth graphite core 3) fluid flow 4–5 orders of magnitude faster than conventional materials due an ideal slip-boundary interface. The transport can be modulated by ‘gatekeeper’ chemistry at the pore entrance that can block by steric hindrance, electrostatic attraction/repulsion, or biochemical state. The density of gatekeeper chemistry can be enhanced by electrochemical grafting at only the tip region. The conformation of charged tethered molecules can be modulated by applied bias setting the stage for programmable drug release devices. A more general intellectual puzzle is that the dramatic flow enhancement is negated if one places high density of selective chemistry along the CNT walls. What is important is to develop systems that actively pump selected chemicals through the region of chemical functionality so that they can continue down the length of CNTcores at dramatic flow rates. This would be truly following the elegant examples given by the natural protein channels.
Acknowledgements
The authors are grateful to Rodney Andrews and Dali Qian of the Center for Applied Energy Research (UKy) for providing aligned CNT mattes. Critical infrastructure was provided by the UKy Center for Nanoscale Science and Engineering. Gracious financial support was provided by NSF CAREER (0348544) and NIH NIDA (R01DA018822).
References
- Andrews R, Jacques D, Rao AM, Derbyshire F, Qian D, Fan X, Dickey EC, Chen J. Continuous production of aligned carbon nanotubes: a step closer to commercial realization. Chemical Physics Letters. 1999;303:467. [Google Scholar]
- Bahr JL, Tour JM. Highly functionalized carbon nanotubes using in situ generated diazonium compounds. Chemistry of Materials. 2001;13:3823. [Google Scholar]
- Chopra N, Majumder M, Hinds BJ. Bifunctional carbon nanotubes by sidewall protection. Advanced Functional Materials. 2005;15:858–864. [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sinauer Associates Inc; Sunderland, MA: 1984. [Google Scholar]
- Hinds BJ, Chopra N, Rantell T, Andrews R, Gavalas V, Bachas LG. Aligned multiwalled carbon nanotube membranes. Science. 2004;303:62. doi: 10.1126/science.1092048. [DOI] [PubMed] [Google Scholar]
- Holt JK, Park HG, Wang YM, Stadermann M, Artyukhin AB, Grigoropoulos CP, Noy A, Bakajin O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science. 312:1034–1037. doi: 10.1126/science.1126298. [DOI] [PubMed] [Google Scholar]; Experimental Fluid Dynamics. Springer; 2006. 2005. [Google Scholar]
- Huang SM, Dai LM. Plasma etching for purification and controlled opening of aligned carbon nanotubes. Journal of Physical Chemistry B. 2002;106:3543. [Google Scholar]
- Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- Jirage KB, Hulteen JC, Martin CR. Nanotubule-based molecular-filtration membranes. Science. 1997;278:655. [Google Scholar]
- Joseph S, Aluru NR. Why are carbon nanotubes fast transporters of water? Nano Letters. 2008;8:452–458. doi: 10.1021/nl072385q. [DOI] [PubMed] [Google Scholar]
- Lauga E, Brenner MP, Stone HA. Handbook of Experimental Fluid Dynamics. Springer; 2005. The No-slip Boundary Condition — A Review. 2005. [Google Scholar]
- Lee SB, Mitchell DT, Trofin L, Nevanen TK, Soderlund H, Martin CR. Antibody-based bionanotube membranes for enantiomeric drug separations. Science. 2002;296:2198. doi: 10.1126/science.1071396. [DOI] [PubMed] [Google Scholar]
- Mao ZG, Sinnott SB. Separation of organic molecular mixtures in carbon nanotubes and bundles: molecular dynamics simulations. Journal of Physical Chemistry B. 2001;105:6916. [Google Scholar]
- Majumder M, Chopra N, Hinds BJa. Effect of tip functionalization on transport through vertically oriented carbon nanotube membranes. Journal of the American Chemical Society. 2005;127:9062–9070. doi: 10.1021/ja043013b. [DOI] [PubMed] [Google Scholar]
- Majumder M, Chopra N, Andrews R, Hinds BJb. Nanoscale hydrodynamics — enhanced flow in carbon nanotubes. Nature. 2005;438:44–44. doi: 10.1038/43844a. [DOI] [PubMed] [Google Scholar]
- Majumder M, Zhan X, Andrews R, Hinds BJ. Voltage gated carbon nanotube membranes. Langmuir. 2007;23:8624–8631. doi: 10.1021/la700686k. [DOI] [PubMed] [Google Scholar]
- Majumder M, Keis K, Zhan X, Meadows C, Cole J, Hinds BJ. Enhanced electrostatic modulation of ionic diffusion through carbon nanotube membranes by diazonium grafting chemistry. Journal of Membrane Science. 2008;316:89–96. doi: 10.1016/j.memsci.2007.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkulov VI, Melechko AV, Guillorn MA, Simpson ML, Lowndes DH, Whealton JH, Raridon RJ. Controlled alignment of carbon nanofibers in a large-scale synthesis process. Applied Physics Letters. 2002;80:4816. [Google Scholar]
- Mitchell CA, Bahr JL, Arepalli S, Tour JM, Krishnamoorti R. Dispersion of functionalized carbon nanotubes in polystyrene. Macromolecules. 2002;35:8825. [Google Scholar]
- Mulder M. Basic Principles of Membrane Technology. Kluwer Academic Publishers; 1994. 1994. [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Nednoor P, Chopra N, Gavalas V, Bachas LG, Hinds BJ. Reversible biochemical switching of ionic transport through aligned carbon nanotube membranes. Chemistry of Materials. 2005;17:3595–3599. [Google Scholar]
- Nednoor P, Gavalas VG, Chopra N, Hinds BJ, Bachas LG. Carbon nanotube based biomimetic membranes: mimicking protein channels regulated by phosphorylation. Journal of Materials Chemistry. 2007;17:1755–1757. [Google Scholar]
- Ohba T, Kanoh H, Kaneko K. Structures and stability of water nanoclusters in hydrophobic nanospaces. Nano Letters. 2005;5:227. doi: 10.1021/nl048327b. [DOI] [PubMed] [Google Scholar]
- Qian D, Dickey EC, Andrews R, Rantell T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Applied Physics Letters. 2000;76:2868. [Google Scholar]
- Ren ZF, Huang ZP, Xu JW, Wang JH, Bush P, Siegal MP, Provencio PN. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science. 1998;282:1105. doi: 10.1126/science.282.5391.1105. [DOI] [PubMed] [Google Scholar]
- Sinnott SB, Andrews R, Qian D, Rao AM, Mao Z, Dickey EC, Derbyshire F. Model of carbon nanotube growth through chemical vapor deposition. Chemical Physics Letters. 1999;315:25. [Google Scholar]
- Skoulidas AI, Ackerman DM, Johnson JK, Sholl DS. Rapid transport of gases in carbon nanotubes. Physical Review Letters. 2002:89. doi: 10.1103/PhysRevLett.89.185901. [DOI] [PubMed] [Google Scholar]
- Sokhan VP, Nicholson D, Quirke N. Fluid flow in nanopores: accurate boundary conditions for carbon nanotubes. Journal of Chemical Physics. 2002;117:8531. [Google Scholar]
- Steinle ED, Mitchell DT, Wirtz M, Lee SB, Young VY, Martin CR. Ion channel mimetic micropore and nanotube membrane sensors. Analytical Chemistry. 2002;74:2416. doi: 10.1021/ac020024j. [DOI] [PubMed] [Google Scholar]
- Wong SS, Woolley AT, Joselevich E, Cheung CL, Lieber CM. Covalently-functionalized single-walled carbon nanotube probe tips for chemical force microscopy. Journal of the American Chemical Society. 1998;120:8557. [Google Scholar]
- Zhang ZJ, Wei BQ, Ramanath G, Ajayan PM. Substrate-site selective growth of aligned carbon nanotubes. Applied Physics Letters. 2000;77:3764. [Google Scholar]