Abstract
A membrane structure consisting of an aligned array of open ended carbon nanotubes (~ 7 nm i.d.) spanning across an inert polymer matrix allows the diffusive transport of aqueous ionic species through CNT cores. The plasma oxidation process that opens CNTs tips inherently introduces carboxylic acid groups at the CNT tips, which allows for a limited amount of chemical functional at the CNT pore entrance. However for numerous applications, it is important to increase the density of carboxylic acid groups at the pore entrance for effective separation processes. Aqueous diazonium based electro-chemistry significantly increases the functional density of carboxylic acid groups. pH dependent dye adsorption-desorption and interfacial capacitance measurements indicate ~ 5–6 times increase in functional density. To further control the spatial location of the functional chemistry, a fast flowing inert liquid column inside the CNT core is found to restrict the diazonium grafting to the CNT tips only. This is confirmed by the increased flux of positively charged Ru(bi-py)3+2 with anionic functionality. The electrostatic enhancement of ion diffusion is readily screened in 0.1(M) electrolyte solution consistent with the membrane pore geometry and increased functional density.
1. Introduction
Transport processes modulated by electro-statics are central to several separation processes including charged based nano-filtration1, adsorption2, ion-exchange3 and electro osmosis4. Interest is also in ionic transport through pores with dimensions close to the Debye screening length which causes overlap of the electrical double layer. Ion-permselective membranes5 and energy conversion devices6 rely on high charge density in geometrically well defined pore structures.
A membrane structure7 consisting of an array of open ended carbon nanotubes (~ 7 nm i.d.) spanning across an inert polymer matrix offers possibilities of extremely fast mass transport through the non-interacting and atomically planar graphitic inner core.8 A plasma oxidation step, inherent to the membrane fabrication process, introduces carboxylate functionality at the CNT entrances. This allows for facile covalent attachment of functional ‘gate-keeper’ molecules of different size, charge, hydrophilicity, and hydrophobicity at the CNT tips. Initial studies showed that ionic transport (flux and size selectivity) through the membrane structure can be modulated by this ‘gate-keeper’ functionality9. However the observed separation factors were modest indicating less than ideal density of functional molecules tethered to CNT core. For numerous applications including ionic-separations and engineering a membrane with ‘voltage-gated’ control of transport10 it is imperative to increase surface charge density of the functional molecules on the carbon nanotubes.
Various methods for increasing functional density on the relatively chemically inert carbon nanotubes have been reported in the literature.11 A key attribute of the CNT-membrane structure is that they are conducting and hence electro-chemically active. Aryl diazonium salts are known to react covalently with glassy-carbon12, graphite13, Carbon Nano-Fibers (CNF) and Carbon Nano-Tubes (CNT)14,15, by an electro-reduction mechanism. In this report, we demonstrate an enhancement of functional density by an aqueous diazonium grafting electro-chemistry. A fast flowing inert liquid column can also shield the inner core and restrict the reaction to the CNT tips only. Increase in functional density is confirmed by dye adsorption-desorption tests and interfacial capacitance measurements. The ionic transport through the diazonium grafted membrane is also significantly altered by introducing charged functional groups at the CNT surface.
2. Experimental
2.1 CNT Membrane Fabrication
The synthesis of multiwall carbon nanotube membrane are described in detail elsewhere7. Briefly, multiwalled carbon nanotubes were grown on quartz substrates by chemical vapor deposition (CVD) at 700°C using a ferrocene and xylene as a source of catalyst and carbon, respectively. The typical MWCNT mat thickness determined by SEM and profilometry measurements was ~ 60µm, whereas inner and outer nanotube diameter determined from TEM were 7nm and 40 nm, respectively. Initially, the CNTs were impregnated in polystyrene matrix by spin coating (50 weight % polystyrene in toluene) and dried at 80 °C in a vacuum oven. The resulting CNT-polymer matrix was then removed from the quartz substrate by HF etching. Water vapor plasma oxidation was used to remove the excess polymer from the CNT surface, open the CNTs and remove the iron catalyst particles at the tips of the CNTs.16 Finally, the membrane was soaked in conc. HCl solution in order to further decrease the amount of residual Fe catalyst inside the carbon nanotubes and rinsed with distilled water.
2.2 Synthesis of 4- carboxy phenyl diazonium tetrafluoroborate
Synthesis of the diazonium compound from p-aminobenzoic acid was carried out following a method of Belanger et al.17,18 2.74g (0.02mol) p-aminobenzoic acid (Aldrich) was dissolved in 20ml water, which was heated at about 50 °C until it was dissolved. 0.044 moles of concentrated HCl was added dropwise to the solution, followed by cooling of the solution to −3°C. 0.022 mol NaNO2 (Sigma) in 10ml water at 0 °C was added slowly to the solution. The reaction was allowed for 1 hour. The solution was filtered, then 0.022 mole of NaBF4 (Aldrich) solution was added to the filtrate at −3°C. A light yellow precipitate was formed. The precipitate was filtered and washed with ice water and cold ether. The product was dried in vacuum and preserved in dessicator at 4°C. NMR H1 (CDCl3): (4-carboxyphenyl)-diazonium tetrafluoroborate, two doublet, 8.87-8.83 and 8.46-8.42 ppm.
2.3 Electro-chemical Grafting
In most literature precedence for diazonium grafting, acetonitrile was used as the solvent. However, suspected mechanical instability of the polymer (polystyrene) in acetonitrile required us to use an aqueous recipe similar to Corgier et al.19 Details of the experimental set-up for the grafting reaction are reported elsewhere.10 Briefly, the grafting reaction (Figure 1) was carried out at −0.6 V in a 3 electrode cell, with Ag/AgCl as the reference electrode, a Pt wire as the counter electrode and the CNT membrane as the working electrode. The electrolyte was 0.1 (M) in KCl, 0.1 (M) in HCl and 5 mM in 4-carboxy phenyl diazonium tetrafluoroborate. For flow grafting (FG), a perforated glass plate was covered with a perforated Cu tape and a column of water (0.1 M KCl) ~ 10 cm higher than the diazonium grafting solution was provided on the feed side in a u-tube permeation cell. The positive flow of the inert water column inside the CNT core allowed the reaction to be localized on the top surface of the permeate side of the cell that contained the diazonium salt. After the grafting reaction the membranes were rinsed with water, 0.1(M) KCl and iso-propyl alcohol to dissolve any unwanted byproducts.
Figure 1.
(a) Schematic of the aligned multiwalled CNT membrane structure and (b) Scanning Electron Micrograph (SEM) of the cross-section of the CNT membrane. The CNT membranes have significant in-plane conduction due to modest tortuosity allowing CNTs to cross. (c) Schematic of electro-chemical reduction of (4-carboxyphenyl)-diazonium tetrafluoroborate on the Carbon Nanotube membrane leading to covalent attachment of benzoic acid groups to the conducting graphitic surface.
2.4 Dye Adsorption-Desorption Assay
CNT membrane was incubated in Toluidine Blue O (TBO, Sigma) solution at pH 10 and room temperature for 5 hours to coordinate the cationic dye with anionic carboxylate groups. The membrane was then rinsed with deionized water and kept in 0.1 (M) NaOH solution for 5 minutes to removed non-complexed TBO. The complexed TBO was desorbed in 50 % (v/v) acetic acid solution. The concentration of desorbed TBO in acetic acid solution was determined by the absorbance at 632 nm using UV-vis Spectroscopy (USB-ISS-UV-vis, Ocean Optics Inc.). The calculation of carboxyl functional density was based on the assumption that positively charged TBO binds with carboxyl groups of CNT membrane at 1:1 ratio.20
2.5 Electro-chemical Impedance Spectroscopy Measurements
Electrochemical impedance measurements (EIS) were carried out in the frequency range of 100kHz–0.2Hz with sinusoidal amplitude modulation of 10mV using a Model 263A Potentiostat and FRD 100 Frequency Response Analyzer from Princeton Applied Research. The electrochemical cell consisted of as prepared CNT membrane or diazo-grafted CNT membrane on platinum support plate as a working electrode Ag/AgCl (sat. KCl) as a reference electrode, and platinum wire as a counter electrode. In order to obtain the data about interfacial capacitance, the non-Faradaic EIS measurements were performed at various potentials in aqueous 0.1M KCl and 0.01M KCl solutions with 10mM K2CO3 (pH=10.8). The pH of the solution was altered by adding small quantities of HCl or KOH in solution. The stabilization time was found experimentally and the CNT membrane was soaked for at least 6 hours in electrolyte solution before starting the measurements. All reported values are average of at least 10 measurements. The capacitance was calculated from the slope of linear part in log|Z| vs log (ω) plot at intermediate frequencies, corresponding to electrode/electrolyte interface electric double layer capacitance.21
2.6 Transport Measurements
The experimental set-up (U-tube permeation cell) for transport measurements is shown elsewhere.9 An aqueous 5mM solution in Ruthenium bipyridine hexahydrate (Aldrich) and Methyl Viologen dichloride hydrate was used as a feed solution, whereas the permeate cell was filled with distilled water. For the transport measurements in electrolyte the source was 5 mM in Ruthenium bipyridine hexahydrate and Methyl Viologen dichloride hydrate and 0.1 (M) in KCl while the permeate was 0.1 (M) KCl. Concentration of diffused Ru-(bipy)32+ through the CNT membrane into the permeate was measured using UV-Vis Spectrophotometer (Ocean Optics Inc.) at 286 nm and converted into transport rate from appropriate calibration curve and volume of the permeate.
3. Results and Discussions
3.1 Estimation of COOH density by pH dependent dye adsorption-desorption
The understanding and manipulation of surface chemistry density at the entrance to CNT pores is critical for engineered separation applications. Figure 2(a) describes the mechanism for estimation of carboxylic acid density. In basic pH, the carboxylic acid groups are deprotonated. TBO is positively charged and forms an electro-static complex with the charged COOH groups. The experiments were carried out at varying concentration of TBO (from 1.2× 10−4 (M) to 2.5 × 10−3 (M)) to insure a monolayer coverage corresponding to charge density and physisorption. Figure 2(b) shows a lack of dependence of absorbed TBO dye on the concentration of incubation solution which is expected for Langmuir type isotherms for adsorption. The functional density is based on the planar area of the membrane and assuming a 1:1 correspondence between the amount of dye desorbed and the number of functional groups. It is important to point out that the true surface area of membranes are significantly higher than the planar area. In this membrane system, the CNTs protrude about 70nm above the PS surface22 increasing the area of exposed CNTs. Also, this estimation will not discriminate between carboxylate groups on the polymer and the CNTs as both form carboxylic acid groups in the plasma oxidation process. The conductive CNTs however are the only active site for diazonium grafting. Dye assay experiments indicated ~ 4–5 times increase in functional density of COOH groups by electro-chemical diazonium grafting to ~1×1015 /cm2 (planar area). These experiments also demonstrate the feasibility of membrane adsorbers based on CNTs with facile regeneration by pH dependent desorption.
Figure 2.
(a) Chemical assay for the estimation of carboxylic acid density of CNT membranes by pH dependent adsorption/desorption of charged dye molecule; (b) Functional density (#/cm2) of CNT membrane as a function of the concentration of the adsorbing dye solution. Increase by 4–5 times in functional density is observed with electrochemical diazonium (static) grafting. Area used for functional density calculation is the geometric planar area of the sample. Lines are added to aid visual clarity.
3.2 Electro-chemical Impedance Spectroscopy Measurements
The typical Electrochemical Impedance Spectroscopy (EIS) plots for as prepared CNT membrane and diazonium grafted CNT membrane measured at 0V vs. Ag/AgCl in 0.1M KCl (pH=10.8) are shown in Figure 3. The Nyquist plot (Figure 3(a)) indicate an electrochemical circuit consisting of a resistance (Rel) from the electrolyte and a capacitor corresponding to the electrical double layer (Cdl) in parallel.23 Presence of a single semicircle is attributed to the kinetics of charging and discharging of the electrochemical double layer at the CNT electrode/electrolyte interface with a RC time constant of ~ 8 msec.24 A typical Nyquist plot for an array of CNT (not impregnated with a polymer) showed a semicircle (i.e. a capacitor and a resistor in parallel) and a vertical tail at lower frequencies (from 10 Hz to 0.1 Hz)..25 Such a vertical tail was not observed for the membrane electrodes in this study. The Bode plots of log|Z| vs log(ω) demonstrate the plateaus at low frequencies associated with the sum of the resistance of the electrolyte solution and the contact resistance, while the straight declining line at intermediate frequencies characterizing the contact resistance(Figure 3(b)). For the CNT membrane the calculated capacitance from the intermediate frequency region of the Bode plot was estimated to 1.86×10−8 F. After the diazonium grafting, the capacitance has increased to 4.2×10−8 F, i.e. an increase by a factor of 2.2 at pH 10.8. This is consistent with ~ 3 times increase in capacitance for surface (COOH) functionalized carbon nanotubes.26 The small decrease in arc amplitude, characterized by charge transfer resistance (Rel), upon diazonium grafting of the membrane is also observed. This is consistent with the increase in the interfacial capacitance and indicates the enhanced electro-static attraction of the counter ions to form the electrical double layer.
Figure 3.
(a) Nyquist and (b) Bode Plots for as-made CNT membrane and diazonium (static) grafted CNT membrane electrode. The electrolyte is 0.1(M) KCl in aqueous 10mM K2CO3 buffer. A semicircle in the Nyquist plot indicate an electro-chemical circuit consisting of a resistor (Rel) and double layer capacitor (Cdl) in parallel. Apparent is a slight decrease in Rel after diazonium grafting due to the increased space-charge density at the electrode surface. An increase in total capacitance of ~ 2 times is also observed
Capacitance increase due to increase in functional group density on carbon nanotubes has been demonstrated earlier using a cyclic voltammetry measurements and is explained by the redox reactions of carboxylic groups.27 Also in our study, the presence of large density of carboxylic groups can be shown by cyclic voltammetry, where oxidation/reduction of surface carboxylic groups appear as two distinct broad peaks at potentials around – 0.3 to – 0.4 V. It is important to point out the EIS measurements in our study are carried out in a potential range (−200mV to 200mV) where no surface redox reactions would occur.
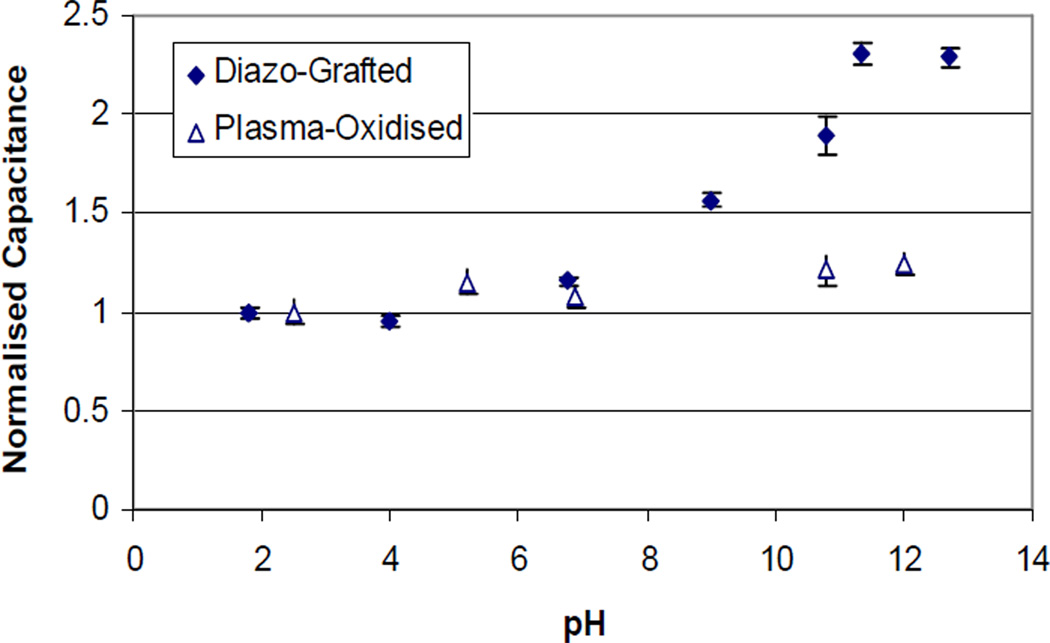
In the dye adsorption-desorption experiments, it was shown that the surface carboxylic acid groups could be deprotonated at high pH and protonated at low pH. It was potentially interesting to study the changes in interfacial capacitances with progressive deprotonation of the carboxylic acid groups. Also pertinent was to estimate the functional density of the surface carboxylic acid groups due to mild plasma oxidation conditions and strong diazonium grafting chemistry. The dye assay described in preceding section would not specifically estimate the functional density on the CNTs, since the plasma oxidized polystyrene polymer would also adsorb and desorp the dye molecule. Figure 4 shows the measured capacitances of the CNT membranes normalized to the capacitance at pH ~2. The interfacial capacitance of the diazonium grafted CNT membrane increases with increasing pH by ~ 128 % compared to 20 % for the as made (plasma oxidized membrane), indicating the presence of charged ionisable groups on the CNT surface. The total interfacial capacitance (CT) at the CNT surface is a function of the film capacitance (CF), the diffuse double layer capacitance (Cs) and the capacitance from the degree of protonation (C(f)), represented by the equation28:
Figure 4.
Interfacial Capacitance as a function of pH. The electrolyte is 0.1(M) KCl in aqueous 10mM K2CO3 buffer and pH is controlled by addition of KOH or HCl. The estimated charge density of fully deprotonated -COOH groups of diazonium grafted CNT membrane is 4 ×10−2 C/m2 compared to ~ 6.4 ×10−3 C/m2 for as made CNT membrane, indicating ~ 6 times increase in carboxylic group density.
The presence of carboxylic acid groups, which can be ionized at higher pH leads to increase in C(f) and hence CT. The presence of larger amount of carboxylic acid groups via diazonium grafting leads to the greater increase in pH dependent capacitance compared to the as made membrane. At low pH, the contribution of ionized groups is significantly less, hence, both C(f) and its corresponding Cs should be small. The total capacitance measured at low pH should be the film capacitance. The differential capacitance of highly ordered pyrolytic graphite in contact with a 0.2M of 1:1 electrolyte was measured to be ~ 3 µF/cm2.29 Assuming that the graphitic nanotube in contact with 0.1M of another 1:1 electrolyte exhibits similar behavior,one can estimate the active electrode area, Ae (cm2), from the measured capacitance at pH 2 using the equation:
The difference in capacitances at pH 2 and 12 (in Farad), should represent the degree of protonation with change in pH. The increase in capacitance will therefore be the amount of excess surface charge which can be converted to the the number of surface sites from the charge carried by a COO− group. Hence, the # sites/cm2 can be estimated from the equation:
where, e is the electronic charge.
It is important to note that the measurement of charge density from the capacitance measurements accounts for actual electrically active CNT area and is independent of functional groups on the oxidized polystyrene surface while the dye assay is not. Comparison of the capacitance-based measurement of functional sites on CNT surfaces are shown in Table 1 with the carboxyl density increasing from 4 × 1012 to 2.4 × 1013 after diazonium grafting. The maximum possible functional sites on the edge planes of a cut graphite surface are on the order of 1015/cm2. Also for comparison, commonly used strong acidic oxidation to cut and functionalize CNTs result in ~ 14 % of oxidized carbon atoms (~ 1014sites/cm2)30 but that process damages the polymer matrix thus is generally unsuitable for this membrane system. The plasma oxidation process used in the membrane fabrication process, is limited to the exposed graphite surfaces near the CNT tips only. This is because high reactivity of the free radicals generated by the plasma process precludes its entrance to the meso- or micropores.31 Electro-chemical grafting of diazonium salts is found to be a facile technique for increasing the functional density of the CNT membranes without compromising the integrity of the membrane structure.
Table 1.
Comparison of functional site densities of Carbon Nanotube systems.
| Capacitance pH~2 (F) |
Capacitance pH~12 (F) |
Active Electrode Area (cm2) |
Functional site density (#/cm2) |
|
|---|---|---|---|---|
| Plasma Oxidized CNT Membrane | 5.4 (±0.2) × 10−8 | 6.7 (±0.03) × 10−8 | 0.018 | 4.4 (± 0.9) × 1012 |
| Diazo-Grafted CNT Membrane | 5.5 (±0.1) × 10−9 | 12.7 ((±0.07) × 10−8 | 0.00185 | 2.4 (± 0.07) × 1013 |
| Acidic Oxidation of CNTs28 | 1 – 2.5 × 1014 | |||
| Cleaved Graphite Edge Planes | 1.5 × 1015 |
Surface pKa of carboxylic acid groups on SWNT or MWNT by atomic force titration measurements was reported to be close to the bulk pKa of benzoic acid (4.5).32 Defining pKa as the pH at which 50 % of the groups are ionized, we observe the pKa of the surface bound carboxylic acid groups to be ~ 9. Shifts in pKa of surface bound benzoic acid groups on carbon surfaces are well known. As for e.g. benzoic acid groups on graphitic carbon synthesized by the same diazonium grafting chemistry have shown pKa shifts to pH~ 6.5.33 Also it is important to note that the determination of surface bound pKa depends strongly upon the experimental measurement technique and the higher surface pKa observed here is consistent with double layer capacitance measurements of mercaptoundecanoic acid on Au surface (pKa>10).34
It is important to note that the highly reactive electro-chemical grafting is not selective to the CNT tips and would also functionalize the interior core of the CNTs. Because CNTs show dramatically enhanced fluid flow through the cores of CNTs,8 it is thus possible to protect the inner core of CNTs from the highly reactive diazonium reagent with a fast flowing inert solution in the core. This is simply achieved during electro-chemical functionalization by using a u-tube electrochemical cell with a column of inert liquid (aqueous 0.1(M) KCl), 10 cm higher than the diazonium solution side of the membrane. Flow velocities are estimated to be 0.1 cm/s, which exceed the aqueous diffusion characteristic length by an order of magnitude at the time scale of the grafting experiment.
3.3 Voltage Dependent Capacitance Measurement
In the previous section we discussed the effect of pH dependent protonation, affecting the measured interfacial capacitance. We next turn our attention to two other important parameters affecting the interfacial capacitance measurements of the conducting CNT membranes: ionic strength and applied potential (Figure 5). It is well understood that the ion distribution at a charged surface depends on the ionic concentration of the electrolyte. The Debye screening length, often used to describe the double layer capacitor effects, is dependent upon the concentration of the electrolyte. At lower concentration of the electrolyte, the Debye length is large and consequently the interfacial capacitance is reduced. The observation of increase in capacitance with increasing concentration of electrolyte (from 0.01 (M) to 0.1(M) KCl) are consistent with capacitance measurements with varying electrolyte concentration in porous carbon materials.35 At higher ionic strength, diffuse double layer can be neglected, potential drop occurs within the Helmoltz layer and capacitance is large. Variation in capacitance with applied potential is a result of change in double layer structure. At negative potential, the interfacial capacitance increases as the charged electrode attracts the counter ions and compresses the double layer. A higher rate of increase in capacitance is observed with higher concentration of the electrolyte. At positive potential, the applied bias neutralizes the negative charge on the electrode and the double layer is large, hence the capacitance does not increase significantly with the potential.
Figure 5.
Interfacial Capacitance of the diazonium grafted CNT membrane as a function of applied potential, measured in aqueous 10 mM K2CO3 (pH =10.8) buffer solution with various KCl concentrations. A compact Debye length of ~ 0.9 nm at 0.1 (M) KCl compared to ~ 3 nm at 0.01(M) KCl increases the measured interfacial capacitance.
For homogeneous surfaces, the sharp minimum in differential capacitance versus potential is observed in dilute electrolyte solutions, where diffuse space-charge layer is formed. This minimum corresponds to an electro-statically discharged interface and is generally interpreted as potential of zero charge. The presence of such a minimum can be explained by general Gouy-Chapman theory. For heterogeneous systems with energetically different surfaces, the flat, broad minimum in capacitance could be obtained.36 For diazo-grafted CNT membranes, the broad but small decrease in differential capacitance is observed in the region of +12 to +25 mV.
An estimate of the charge density from a PZC of 25 mV in 0.1(M) of a 1:1 electrolyte would lead to a charge density of 1.9 × 10−2 C/m2 (i.e. functional site density of ~ 1.2 × 1013 sites/cm2),37 which is consistent with the charge density estimates from pH dependent capacitance measurements. However, the absence of a sharp minimum in the potential vs. capacitance measurements introduces large uncertainties in this method. We believe the functional density estimates from the pH dependent capacitance measurements (Section 3.2) are more accurate. It is important to point out that the capacitance measurements were highly reversible i.e. 0 V capacitance were retained after measurements at ± 200 mV. It indicates the stability of the covalently grafted molecules on the Carbon Nanotube surface. An important observation from these measurements is the retention of electro-chemical activity of the CNT membrane structure after increased functional density, an important attribute for several applications of the CNT membrane.
3.4 Ionic Transport Measurements Through Carbon Nanotube Membranes
The porosity of the membranes used in this study was evaluated from the steady state MV2+ flux through the plasma oxidized membrane from the relations:
where, Ap (cm2) is the available pore area, J (moles/s) is the experimental steady state flux of MV2+, Δx (cm) is the thickness of the membrane evaluated from cross-sectional scanning electron micrographs, ΔC (moles/cc) is the difference of concentration between the feed and the permeate, ε is the porosity of the membrane, Am (cm2) is the membrane area exposed to the solution. For our case, the concentration of the feed is 5 mM and Am is 0.3 cm2. An implicit assumption in this estimation, is bulk diffusivity of MV+2 (D = 7.74 × 10−6 cm2/s) inside the CNTs with ~ 7 nm core diameter.8,9 The porosity (ε) of the membrane used for the diazonium grafting studies were ~ 9 × 10−4 % The low porosity is a result of a significant fraction of CNTs being blocked by Fe catalyst particles during the CNT growth process. The ionic transport measurements for the diazo-grafted membrane are shown in Figure.6. The fluxes shown are normalized with respect to their as-made (plasma-oxidized) flux of Ru(bi-py)32+ without the screening electrolyte (i.e. 0.1 (M) KCl), because of the sample-to-sample variation of porosity. For static-grafted CNT membrane the flux of positively charged Ru(bi-py)32+ increased ~ 2.3 times. This is consistent with our earlier observation of ~ 4–5 fold increase in the flux of Ru(bi-py)3+2, when the plasma oxidized membrane is functionalized with a large anionically charged (4e−) dye molecule.9
Figure 6.
Selective spatial functionalization of CNT membranes by static grafting (SG) and flow grafting (FG) and affect on Ru(bi-py)3+2 diffusion across membrane. The static grafted membrane shows > 2 fold increase in diffusive flux of positively charged Ru(bi-py)3+2 by the attraction to anionic carboxylate groups. The electrostatic attraction is screened in 0.1 (M) KCl solution.
When the diffusive transport measurements are performed in 0.1(M) KCl, the charged molecules on the membrane are screened and diffusive flux returns to near that of unmodified (plasma-oxidized) membranes. The transport measurements were carried out using a feed solution of 5mM Ru(bi-py)32+ and 5 mM MV2+. The Debye screening length for a 2:1 electrolyte of 10 mM concentration is ~ 1.7 nm, which decreases to 0.8 nm in presence of 0.1(M) KCl.38 A better parameter for understanding the double layer effects for small pores is the ratio of the Debye length to the pore radius. It is ~ 0.5 in the absence of the electrolyte and decreases to ~0.25 in presence of 0.1 (M) KCl, which explains that the fixed charges on the membranes are screened within a short distance of the pore walls. Thus, in ionic solutions with a very short a Debye length of <1 nm, the positively charged permeates fails to be electro-statically attracted to the anionic surface charge and the flux enhancement thus declines. However, for the case of flow grafting, where a fast fluid flow protects the inner core and restricts the grafting reaction the top tip surface, the number of surface carboxylic acid groups are significantly less. Also the charges are spatially located on the top surface and tips of the membrane, which will have significantly less electro-static influence than inside the pores.. Hence, the flux of Ru(bi-py)3+2 does not change significantly from the non-functionalized (plasma-oxidized) form. Likewise screening the charge does not show significant change on the transport of the charged ionic species, within the error of the experiment.
4. Conclusions
Electro-chemical diazonium grafting chemistry is a facile method to introduce functional groups onto the CNT membranes. The grafted carboxylic acid groups demonstrate a pH dependent protonation and deprotonation as confirmed by dye adsorption-desorption tests and interfacial capacitance measurements. Diazonium grafting increases carboxylate density on carbon nanotubes by about 5– 6 times (to 2.4 (± 0.07) × 1013/cm2) that of as produced the plasma oxidized CNT membranes. Ionic transport of a positively charged permeate is significantly increased by the presence of the anionic carboxylic acid groups at the entrance and along CNT core. The inner core of the CNTs can be protected by a positive flow of an inert water column and thus restrict the aggressive electro-chemical reaction to the CNT tip surfaces that are in contact with the diazonium salt. This allows the well defined placement of chemical ‘gate-keepers’ with high functional density at the entrances CNT cores, thus enabling further advances in membrane design.
ACKNOWLEDGMENT
Funding support was provided by NSF CAREER award (CTS), NIH (NIDA) and ARO Advanced Carbon Nanotechnology Program. Aligned Arrays of MWCNTs were generously provided by Rodney Andreews and Dali Qian at the Center for Applied Energy Research. Critical infrastructure provided by Center for Nanoscale Science and Engineering, University of Kentucky. The authors acknowledge Uma Prasad Mullick for chemical synthesis advice, Wendy Satterwhite for supporting experiments and D. Bhattacharyya for helpful discussions. MM acknowledges final year dissertation fellowship from the University of Kentucky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lefebvre X, Palmeri J, David P. Nanofiltration Theory: An Analytic Approach for Single Salts. Journal of Physical Chemistry B. 2004;108(43):16811–16824. [Google Scholar]
- 2.Hollman AM, Bhattacharyya D. Pore assembled multilayers of charged polypeptides in microporous membranes for ion separation. Langmuir. 2004;20(13):5418–5424. doi: 10.1021/la049688+. [DOI] [PubMed] [Google Scholar]
- 3.Xu T. Ion exchange membranes: State of their development and perspective. Journal of Membrane Science. 2005;263(1–2):1–29. [Google Scholar]
- 4.Miller SA, Young VY, Martin CR. Electroosmotic flow in template-prepared carbon nanotube membranes. Journal of the American Chemical Society. 2001;123(49):12335–12342. doi: 10.1021/ja011926p. [DOI] [PubMed] [Google Scholar]
- 5.Nishizawa M, Menon VP, Martin CR. Metal Nanotubule Membranes with Electrochemically Switchable Ion-Transport Selectivity. Science. 1995;268(5211):700–702. doi: 10.1126/science.268.5211.700. [DOI] [PubMed] [Google Scholar]
- 6.Daiguji H, Yang P, Szeri AJ, Majumdar A. Electrochemomechanical Energy Conversion in Nanofluidic Channels. Nano Lett. 2004;4(12):2315–2321. [Google Scholar]
- 7.Hinds BJ, Chopra N, Rantell T, Andrews R, Gavalas V, Bachas LG. Aligned Multiwalled Carbon Nanotube Membranes. Science. 2004;303(5654):62–65. doi: 10.1126/science.1092048. [DOI] [PubMed] [Google Scholar]
- 8.Majumder M, Chopra N, Andrews R, Hinds BJ. Nanoscale hydrodynamics: Enhanced flow in carbon nanotubes. Nature. 2005;7064;438:44. doi: 10.1038/43844a. [DOI] [PubMed] [Google Scholar]
- 9.Majumder M, Chopra N, Hinds BJ. Effect of Tip Functionalization on Transport through Vertically Oriented Carbon Nanotube Membranes. J. Am. Chem. Soc. 2005;127(25):9062–9070. doi: 10.1021/ja043013b. [DOI] [PubMed] [Google Scholar]
- 10.Majumder M, Zhan X, Andrews R, Hinds BJ. Voltage Gated Carbon Nanotube Membranes. Langmuir. doi: 10.1021/la700686k. (in-press) [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, H-B T, Wong SS. Covalent Surface Chemistry of Single-Walled Carbon Nanotubes. Advanced Materials. 2005;17(1):17–29. [Google Scholar]
- 12.Combellas C, Kanoufi F, Pinson J, Podvorica FI. Time-of-Flight Secondary Ion Mass Spectroscopy Characterization of the Covalent Bonding between a Carbon Surface and Aryl Groups. Langmuir. 2005;21(1):280–286. doi: 10.1021/la048106l. [DOI] [PubMed] [Google Scholar]
- 13.Allongue P, Delamar M, Desbat B, Fagebaume O, Hitmi R, Pinson J, Saveant J-M. Covalent Modification of Carbon Surfaces by Aryl Radicals Generated from the Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1997;119(1):201–207. [Google Scholar]
- 14.Bahr JL, Yang J, Kosynkin DV, Bronikowski MJ, Smalley RE, Tour JM. Functionalization of Carbon Nanotubes by Electrochemical Reduction of Aryl Diazonium Salts: A Bucky Paper Electrode. Ibid. 2001;123(27):6536–6542. doi: 10.1021/ja010462s. [DOI] [PubMed] [Google Scholar]
- 15.Lee C-S, Baker SE, Marcus MS, Yang W, Eriksson MA, Hamers RJ. Electrically Addressable Biomolecular Functionalization of Carbon Nanotube and Carbon Nanofiber Electrodes. Nano Lett. 2004;4(9):1713–1716. [Google Scholar]
- 16.Huang SM, Dai LM. Plasma etching for purification and controlled opening of aligned carbon nanotubes. Journal of Physical Chemistry B. 2002;106:3543–3545. [Google Scholar]
- 17.D'Amours M, Belanger D. Stability of substituted phenyl groups electrochemically grafted at carbon electrode surface. Journal of Physical Chemistry B. 2003;107(20):4811–4817. doi: 10.1021/jp027223r. [DOI] [PubMed] [Google Scholar]
- 18.Marwan J, Addou T, Belanger D. Functionalization of glassy carbon electrodes with metal-based species. Chemistry of Materials. 2005;17(9):2395–2403. [Google Scholar]
- 19.Corgier BP, Marquette CA, Blum LJ. Diazonium-Protein Adducts for Graphite Electrode Microarrays Modification: Direct and Addressed Electrochemical Immobilization. J. Am. Chem. Soc. 2005;127(51):18328–18332. doi: 10.1021/ja056946w. [DOI] [PubMed] [Google Scholar]
- 20.Tesema Y, R D, Stubbs J., III Bone cell viability on collagen immobilized poly(3-hydroxybutrate-co-3-hydroxyvalerate) membrane: Effect of surface chemistry. Journal of Applied Polymer Science. 2004;93(5):2445–2453. [Google Scholar]
- 21.Application Note, AC-1. Princeton, NJ: EG&G Princeton Applied Research; 1985. [Google Scholar]
- 22.Chopra N, Majumder M, Hinds BJ. Bifunctional carbon nanotubes by sidewall protection. Advanced Functional Materials. 2005;15:858–864. [Google Scholar]
- 23.Monk PS. Fundamentals of Electroanalytical Chemistry. Wiley: West Sussex; 2001. [Google Scholar]
- 24.Evegenij Barasoukov JRM. Impedance Spectroscopy: Theory, Experiment and Applications. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 25.Zhang H, Cao GP, Yang YS. Using a cut&paste method to prepare a carbon nanotube fur electrode. Nanotechnology. 2007;18(19):195607. [Google Scholar]
- 26.Kim Y-T, Ito Y, Tadai K, Mitani T, Kim U-S, Kim H-S, Cho B-W. Drastic change of electric double layer capacitance by surface functionalization of carbon nanotubes. Applied Physics Letters. 2005;87(23):234106–234113. [Google Scholar]
- 27.Barisci JN, Wallace GG, Baughman RH. Electrochemical studies of single-wall carbon nanotubes in aqueous solutions. Journal of Electroanalytical Chemistry. 2000;488(2):92–98. [Google Scholar]
- 28.Bryant MAC, RM Determination of Surfcae pKa Values of Surface-Confined Molecules Derivatized with pH-Sensitive Pendant Groups. Langmuir. 1993;9:385–387. [Google Scholar]
- 29.Gerischer H, McIntyre R, Scherson D, Storck W. Density of the Electronic States of Graphite: Derivation from Differential Capacitance Measurements. J. Phys. Chem. 1987;91:1930–1935. [Google Scholar]
- 30.Ago H, Kugler T, Cacialli F, Salaneck WR, Shaffer MSP, Windle AH, Friend RH. Work Functions and Surface Functional Groups of Multiwall Carbon Nanotubes. Journal of Physical Chemistry B. 1999;103(38):8116–8121. [Google Scholar]
- 31.Domingo-Garcia M, Lopez-Garzon FJ, Perez-Mendoza M. Effect of Some Oxidation Treatments on the Textural Characteristics and Surface Chemical Nature of an Activated Carbon. Journal of Colloid and Interface Science. 2000;222(2):233–240. doi: 10.1006/jcis.1999.6619. [DOI] [PubMed] [Google Scholar]
- 32.Wong SS, Joselevich E, Woolley AT, Cheung CL, Lieber CM. Covalently functionalized nanotubes as nanometre- sized probes in chemistry and biology. 1998;394(6688):52–55. doi: 10.1038/27873. [DOI] [PubMed] [Google Scholar]
- 33.Abiman P, Crossley A, Wildgoose GG, Jones JH, Compton RG. Investigating the Thermodynamic Causes Behind the Anomalously Large Shifts in pKa Values of Benzoic Acid-Modified Graphite and Glassy Carbon Surfaces. Langmuir. 2007 doi: 10.1021/la7005277. [DOI] [PubMed] [Google Scholar]
- 35.Hou C-H, Liang C, Yiacoumi S, Dai S, Tsouris C. Electrosorption capacitance of nanostructured carbon-based materials. Journal of Colloid and Interface Science. 2006;302(1):54–61. doi: 10.1016/j.jcis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Oren Y, Tobias H, Soffer A. The electrical double layer of carbon and graphite electrodes: Part I Dependence on electrolyte type and concentration. Journal of Electroanalytical Chemistry. 1984;162(1–2):87–99. [Google Scholar]
- 37.Charge Density estimated was estimated from the Poisson-Boltzmann equation: , where is the reciprocal Debye length for a 1:1 electrolyte solution with dielectric constant εr, n0 is the number density of monovalent cations, e the electronic charge, ε0 is the permittivity of vacuum, ΔV is the potential of zero-charge, k is the Boltzmann Constant and T is the temperature.
- 38. where (1/K) is the Debye screening length, F is the Faraday constant, ε is the solvent dielectric permittivity, R is the Universal gas constant, T is the temperature, zi is the charge on the ions and Ci is the concentration.