Abstract
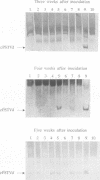
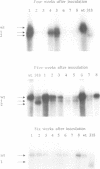
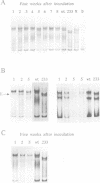
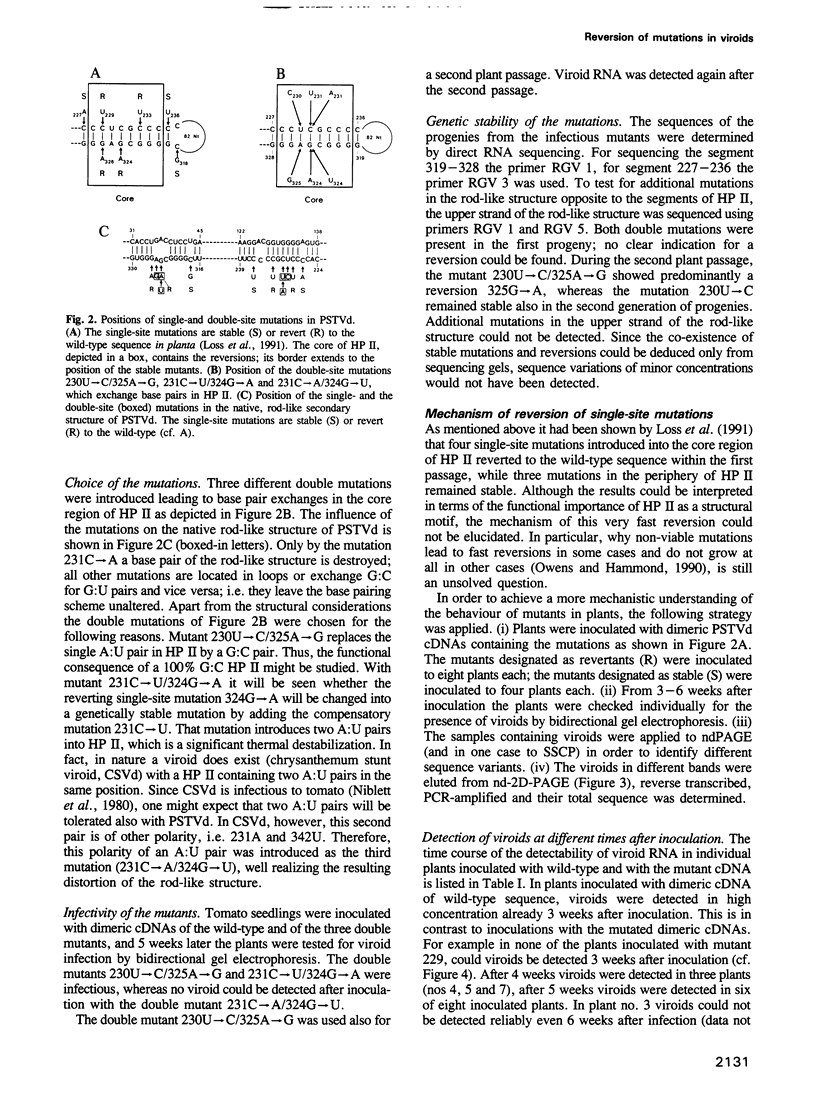
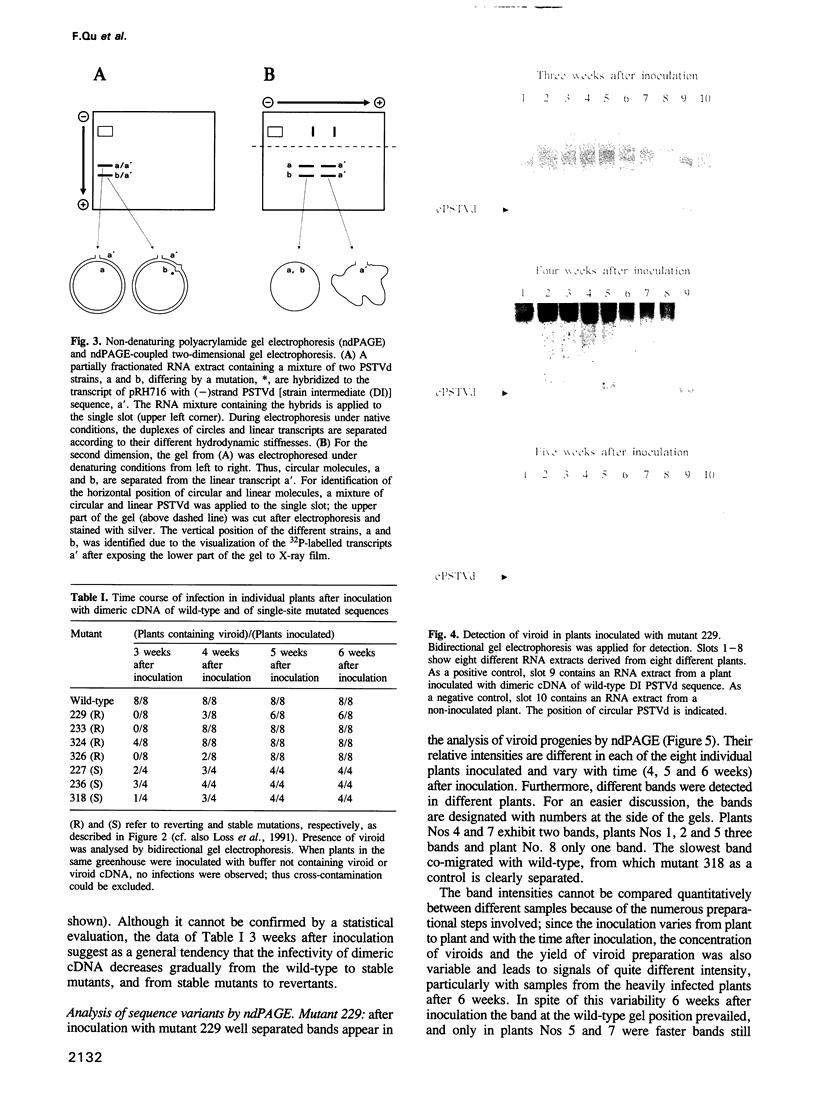
From site-directed mutagenesis of potato spindle tuber viroid (PSTVd) it had been concluded earlier that the formation of a thermodynamically metastable structure containing hairpin II (HP II) is critical for infectivity. In order to differentiate between structural and sequence effects, in the present work base pairs in HP II were exchanged by site-directed double mutations without significant alterations in the native rod-like structure of PSTVd. The mutants were viable and genetically stable in the first generation, but one of the two mutations reverted to the wild-type nucleotide in the second generation. Single-site mutations in the stem of HP II, which had been described as revertants to the wild-type sequence earlier, were analysed with respect to the time course of reversion and the sequence variation during reversion. All replicating sequence variants were separated by gel electrophoretic techniques and the sequences and their relative frequencies were determined. From both types of studies it can be concluded (i) that HP II is a functional element in the (-)strand replication intermediate, generated due to sequential folding during synthesis, and that it is essential for template activity of (+)strand synthesis; (ii) that G:U pairs are tolerated transiently in (-)strand HP II; the lower stability of such a HP II is compensated by additional mutations outside HP II which suppress the competition of a rod-like structure; and (iii) that the reversions are generated spontaneously during (-)strand synthesis. Furthermore, the double-stranded structure of HP II is the essential element for short term replication of PSTVd but the exact sequence of the wild-type proves to be superior with regard to fitness and replicability of PSTVd.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Colpan M., Schumacher J., Brüggemann W., Sänger H. L., Riesner D. Large-scale purification of viroid RNA using Cs2SO4 gradient centrifugation and high-performance liquid chromatography. Anal Biochem. 1983 May;131(1):257–265. doi: 10.1016/0003-2697(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Cress D. E., Kiefer M. C., Owens R. A. Construction of infectious potato spindle tuber viroid cDNA clones. Nucleic Acids Res. 1983 Oct 11;11(19):6821–6835. doi: 10.1093/nar/11.19.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Hecker R., Wang Z. M., Steger G., Riesner D. Analysis of RNA structures by temperature-gradient gel electrophoresis: viroid replication and processing. Gene. 1988 Dec 10;72(1-2):59–74. doi: 10.1016/0378-1119(88)90128-x. [DOI] [PubMed] [Google Scholar]
- Henco K., Sänger H. L., Riesner D. Fine structure melting of viroids as studied by kinetic methods. Nucleic Acids Res. 1979 Jul 11;6(9):3041–3059. doi: 10.1093/nar/6.9.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Klein R. D., Wells R. D. Preparation of milligram amounts of 21 deoxyribonucleic acid restriction fragments. Biochemistry. 1981 Jun 23;20(13):3748–3756. doi: 10.1021/bi00516a013. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss P., Schmitz M., Steger G., Riesner D. Formation of a thermodynamically metastable structure containing hairpin II is critical for infectivity of potato spindle tuber viroid RNA. EMBO J. 1991 Mar;10(3):719–727. doi: 10.1002/j.1460-2075.1991.tb08002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbach H. P., Sänger H. L. Viroid replication is inhibited by alpha-amanitin. Nature. 1979 Mar 8;278(5700):185–188. doi: 10.1038/278185a0. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Schmitz M., Steger G. Base-pair probability profiles of RNA secondary structures. Comput Appl Biosci. 1992 Aug;8(4):389–399. doi: 10.1093/bioinformatics/8.4.389. [DOI] [PubMed] [Google Scholar]
- Schumacher J., Randles J. W., Riesner D. A two-dimensional electrophoretic technique for the detection of circular viroids and virusoids. Anal Biochem. 1983 Dec;135(2):288–295. doi: 10.1016/0003-2697(83)90685-1. [DOI] [PubMed] [Google Scholar]
- Steger G., Baumstark T., Mörchen M., Tabler M., Tsagris M., Sänger H. L., Riesner D. Structural requirements for viroid processing by RNase T1. J Mol Biol. 1992 Oct 5;227(3):719–737. doi: 10.1016/0022-2836(92)90220-e. [DOI] [PubMed] [Google Scholar]
- Steger G., Hofmann H., Förtsch J., Gross H. J., Randles J. W., Sänger H. L., Riesner D. Conformational transitions in viroids and virusoids: comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984 Dec;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Infectivity studies on different potato spindle tuber viroid (PSTV) RNAs synthesized in vitro with the SP6 transcription system. EMBO J. 1985 Sep;4(9):2191–2199. doi: 10.1002/j.1460-2075.1985.tb03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Tzortzakaki S., Tsagris M. Processing of linear longer-than-unit-length potato spindle tuber viroid RNAs into infectious monomeric circular molecules by a G-specific endoribonuclease. Virology. 1992 Oct;190(2):746–753. doi: 10.1016/0042-6822(92)90912-9. [DOI] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Sänger H. L. Ribonuclease T1 generates circular RNA molecules from viroid-specific RNA transcripts by cleavage and intramolecular ligation. Nucleic Acids Res. 1991 Apr 11;19(7):1605–1612. doi: 10.1093/nar/19.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]