Summary
The objective of this study was to compare the predictive ability of potential tissue biomarkers to known prognostic factors that predict renal cell carcinoma (RCC) recurrence using an automated system of immunohistochemical analysis. After institutional review board approval, a tissue microarray was constructed using tissue from patients who had partial or radical nephrectomy for RCC. Patients with metastatic disease were excluded. Immunohistochemical staining of the tissue microarray for Ki-67, C-reactive protein, carbonic anhydrase 9, and hypoxia-inducible factors 1α and 2α was analyzed using automated image analysis. Univariable and multivariable analyses were performed to evaluate the association of putative biomarkers and known prognostic factors. Of 216 patients who met the entrance criteria, 34 (16%) patients developed metastatic recurrence within a median follow-up interval of 60.9 (interquartile range, 13.9–87.1) months. RCC morphotypes analyzed in this study include clear cell (n = 156), papillary (n = 38), chromophobe (n = 16), and collecting duct/unclassified (n = 6). Univariate analysis identified that only increased Ki-67 was predictive of RCC recurrence among the proteins evaluated, in addition to other known clinicopathological prognostic factors. After multivariate analysis, Ki-67 was identified as an independently predictive risk factor for RCC recurrence (hazard ratio [HR], 3.73 [confidence interval {CI}, 1.60–8.68]). Other independent predictors of RCC recurrence included tumor diameter (HR, 1.20 [CI, 1.02–1.41]) and perinephric fat invasion (HR, 4.49 [CI, 1.11–18.20]). We conclude that Ki-67 positivity is independently predictive of RCC recurrence after surgery in nonmetastatic patients. Automated analysis of tissue protein expression can facilitate a more objective and expedient investigation of tissue biomarkers for RCC.
Keywords: Multispectral imaging, Immunohistochemistry, Renal cell carcinoma, Biomarkers, Recurrence
1. Introduction
It is estimated that more than 65 000 men and women in the United States will be diagnosed as having kidney cancer in 2013 [1], with renal cell carcinoma (RCC) accounting for most of those cases [2]. Despite complete surgical excision of the primary tumor, a significant number of patients will develop metastatic RCC. Clinicopathological variables have traditionally been used to stratify patients for increased surveillance regimens or as inclusion criteria for adjuvant therapy trials. However, the ability to predict which patients are at highest risk for RCC recurrence is limited. Unlike other cancers, tissue biomarkers are not currently used clinically to predict recurrence or individualize treatment or surveillance regimens after surgery.
Several studies have suggested using the expression of certain proteins in RCC tissue as a method to evaluate the risk of future metastasis [3]. However, studies using protein expression to measure recurrence risk may be difficult to reproduce or validate in separate patient populations. One obstacle that limits efficient comparison of tissue protein expression among large numbers of patient samples is the conventional method of immunohistochemical (IHC) analysis. Although IHC staining is a straightforward and commonly performed laboratory technique to evaluate protein expression, the subsequent interpretation and quantification of the level of protein expression are subjective and labor intensive with individual analysis and grading of each image by a pathologist. The subjective nature of the analysis does not allow detection of subtle differences in tissue expression to be evaluated, and variation in interpretation is common when analyzing hundreds of samples. Computerized image analysis of IHC offers the potential advantages of increasing objectivity of analysis by decreasing variability in interpretation [4,5]. When applied to pathologic tissue microarrays (TMAs), automated analysis of IHC may provide an efficient method to investigate and validate putative tissue biomarkers. The objective of this study was to compare the predictive ability of potential tissue biomarkers with clinicopathological variables to predict RCC recurrence using an automated system of IHC analysis.
2. Materials and methods
After institutional review board approval was obtained for this study, clinicopathological information was reviewed for patients with RCC who were treated with nephrectomy or partial nephrectomy at The University of Wisconsin Hospital from January 2000 to December 2005. Patients were not eligible for this study if there was lymph node or distant metastatic disease at the time of surgery, or if there were positive surgical margins. Histologic morphotypes reported were based on the 2013 International Society of Urological Pathology (ISUP) consensus classification [6], and T stage was assigned using the American Joint Committee on Cancer staging, seventh edition [7]. Tumor necrosis was noted at the time of surgery or upon microscopic evaluation at the time of TMA construction. Five tissue proteins were chosen for investigation because of prognostic significance suggested in prior studies using conventional IHC [3,8–12].
2.1. Tumor TMA construction
A Manual Tissue Arrayer (Beecher Instruments, Sun Prairie, WI; model MTA-1) was used to construct a TMA with 0.6-mm cores arranged 0.8 mm apart center to center. The pathologic stage and ISUP grade of the adjacent tissue were reviewed by an expert genitourinary pathologist. Duplicate samples were included for each patient. Benign kidney samples for 54 patients from nephrectomy specimens were also included for paired analysis.
2.2. Immunohistochemistry
The unstained TMA slides were cut 4 µm thick. Xylene was used for de-paraffinization, followed by graded alcohols for hydration. Diva Decloaker 10× (Ki-67, C-reactive protein [CRP] slides) or Borg Decloaker (carbonic anhydrase 9 [CAIX], hypoxia-inducible factor [HIF] 1α, HIF2α slides) was used with a digital electric pressure cooker (Biocare, Concord, CA, USA) for antigen retrieval, and Background Punisher (Biocare) was used to block any nonspecific background staining. Monoclonal mouse anti–Ki-67 (Leica Biosystems, Buffalo Grove, IL, USA; 1:200 in BCM Renoir Red) and monoclonal rabbit anti-CRP (Millipore, Billerica, MA, USA; 1:200 in BCM Renoir Red) primary antibodies were applied to one slide. Polyclonal rabbit anti-HIF2α (Epitomics; 1:200 in Renaissance), monoclonal rabbit anti-HIF1α (Epitomics, Burlingame, CA, USA; 1:200 in Renoir Red), and monoclonal rabbit anti-CAIX (Epitomics; 1:200 in Renaissance) were each applied to 1 of 4 individual slides. Mach 2 Mouse AP-Polymer and Mach 2 Rabbit HRP-Polymer (Biocare) were used as secondary antibodies. Betazoid DAB (Biocare) was used to detect CRP, HIF1α, and HIF2α, and Warp Red (Biocare) was used for detection of Ki-67 and CAIX. CAT Hematoxylin and Tacha's Bluing Solution (Biocare) were used for counterstaining and better visualization, respectively.
2.3. Image acquisition and analysis
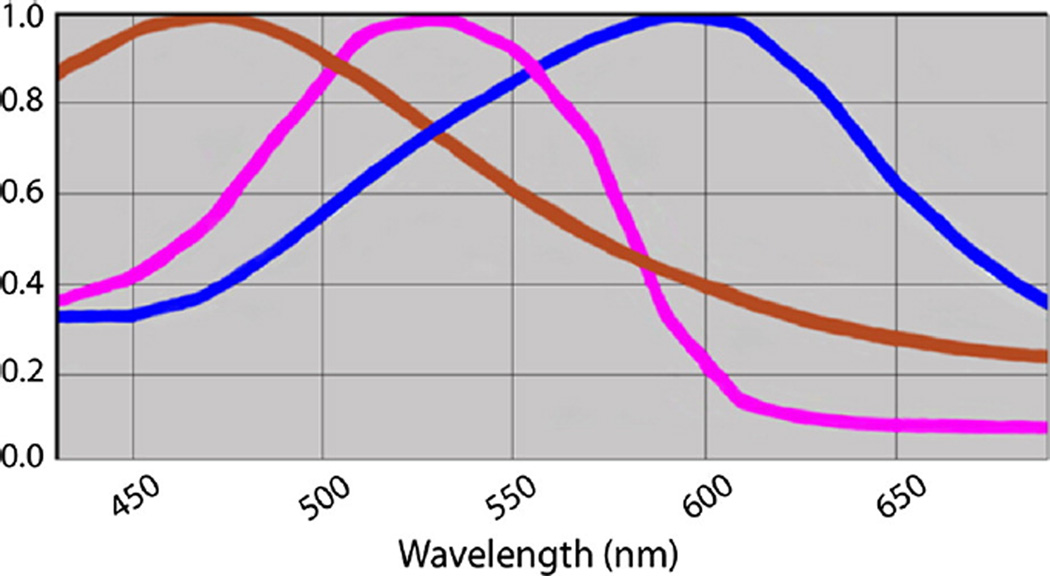
Analysis using the Vectra system was performed as described in previous studies [5,13]. Stained slides were loaded into the Vectra slide scanner (PerkinElmer, Waltham, MA, USA), and a scanning protocol was produced. Eight-bit Nuance multispectral image cubes were created with the ×20 lens. Nuance software (version 3.0.0; PerkinElmer) was then used to build the spectral library (Fig. 1). Three control slides with 1 chromogen (3,3′-diaminobenzidine, hematoxylin, and Warp Red) were scanned, and spectral curves were defined to unmix signals (Fig. 2).
Fig. 1.
The spectral curve is defined and enables separation and quantification of different chromogens.
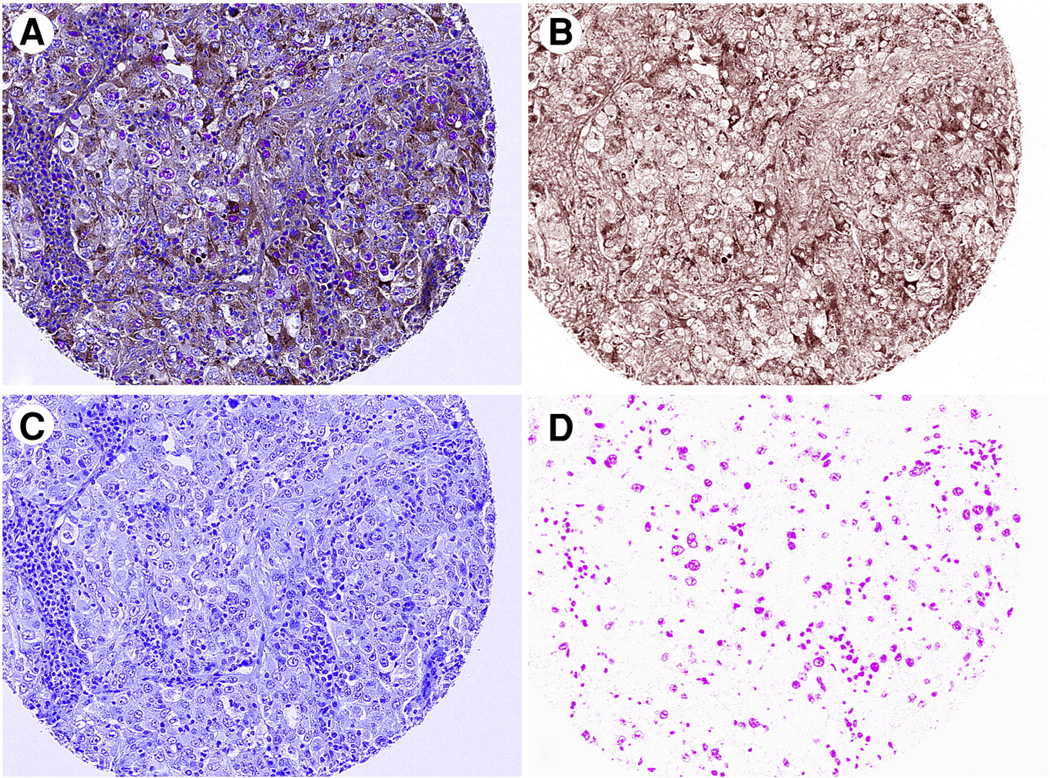
Fig. 2.
Separation from the composite image (A) of 3,3′-diaminobenzidine (B), Warp Red (C), and hematoxylin (D) chromogens using Nuance software. Composite image was acquired using the ×20 objective lens on the Nuance microscope.
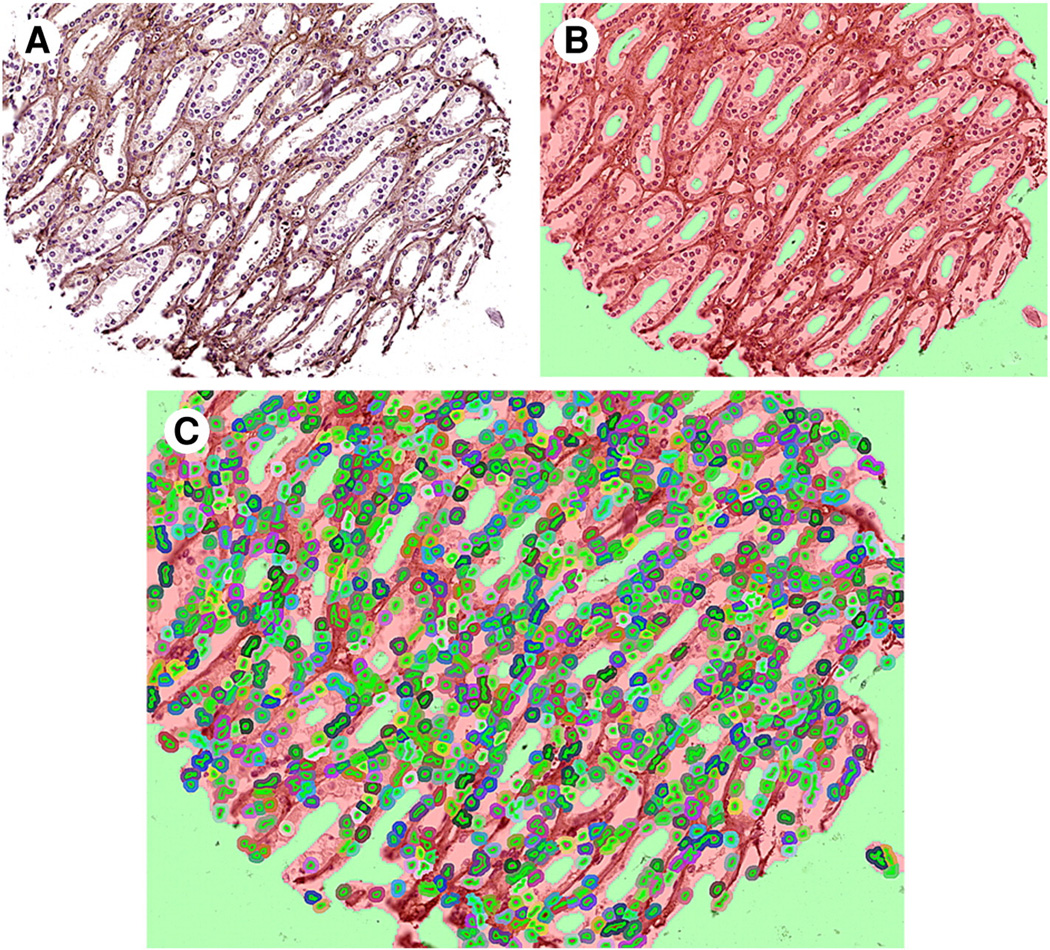
Images were imported using inForm software (version 1.4; PerkinElmer). Initially, 18% of cores from the TMA were used to set up an algorithm for differentiation, assuring 97% acceptable tissue segmentation [5]. Target signals were then quantified in selected tissues and cellular compartments of interest (Fig. 3). For HIF1α and HIF2α, nuclear expression was used in the analysis because these transcription factors are active when present in the nuclear compartment. For Ki-67, the percentage of cells with positive nuclear staining was used because Ki-67 is a cellular marker for proliferation located in nuclei. For CRP, the global cellular expression was used in the analysis. Core images with less than 5% epithelial component, significant folding, or loss of tissue were excluded.
Fig. 3.
Manual training of software from the composite image (A) by tissue segmentation (B) and cell segmentation (C).
2.4. Statistical analysis
Protein expression of each putative biomarker was evaluated initially as a continuous variable using Cox proportional hazards analyses. Further analysis was performed using the upper quartile of values for each protein, except Ki-67, for which 1.00% positivity was chosen. Univariate and multivariate Cox proportional hazards analyses were used to evaluate the risk of RCC recurrence based on known clinicopathological variables [3] or tissue protein expression. Benign and cancer samples were compared using a paired Student t test. The relationship between clinicopathological variables and protein expression was compared using either an unpaired Student t test or 1-way analysis of variance. Statistical analysis was performed using Statistical Analysis System (version 9.3; SAS Institute, Cary, NC), and a 2-sided P value less than .05 was considered significant.
3. Results
An institutional database identified 216 patients with RCC who had surgery with curative intent and no evidence of metastatic disease. Of these, 34 (16%) patients developed metastatic recurrence within the study period. Patient and disease characteristics and mean staining densities are shown in Table 1. Median follow-up time was 60.9 (interquartile range, 13.9–87.1) months.
Table 1.
Patient and disease characteristics and biomarker expression
| n (%) | Ki-67 (%) | CAIX | HIF1α | HIF2α | CRP | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 139 (64.4) | 0.63 | 0.190 | 0.096 | 0.111 | 0.167 |
| Female | 77 (35.6) | 0.50 | 0.182 | 0.065 | 0.094 | 0.166 |
| P | .513 | .661 | .027 | .010 | .954 | |
| RCC morphotype | ||||||
| Clear cell | 156 (72.2) | 0.35 | 0.234 | 0.072 | 0.099 | 0.167 |
| Papillary | 38 (17.6) | 0.67 | 0.059 | 0.154 | 0.133 | 0.154 |
| Chromophobe | 16 (7.4) | 0.02 | 0.033 | 0.066 | 0.082 | 0.181 |
| Collecting duct | 1 (0.5) | – | – | – | – | – |
| Unclassified | 5 (2.3) | 2.38 | 0.211 | 0.021 | 0.128 | 0.226 |
| P | .012 | <.0001 | <.0001 | <.0001 | .869 | |
| ISUP grade | ||||||
| 1 | 60 (30.6) | 0.32 | 0.245 | 0.083 | 0.099 | 0.165 |
| 2 | 103 (52.6) | 0.56 | 0.190 | 0.094 | 0.106 | 0.164 |
| 3 | 23 (11.7) | 0.84 | 0.144 | 0.078 | 0.122 | 0.140 |
| 4 | 10 (5.1) | 2.81 | 0.186 | 0.041 | 0.122 | 0.302 |
| P | <.0001 | .003 | .445 | .170 | .145 | |
| Pathologic T stage | ||||||
| 1 | 139 (64.4) | 0.38 | 0.187 | 0.092 | 0.104 | 0.142 |
| 2 | 25 (11.6) | 0.78 | 0.170 | 0.090 | 0.090 | 0.219 |
| 3a | 43 (19.9) | 0.98 | 0.184 | 0.069 | 0.115 | 0.217 |
| 3b | 8 (3.7) | 1.37 | 0.275 | 0.040 | 0.102 | 0.151 |
| 4 | 1 (0.5) | – | – | – | – | – |
| P | .026 | .227 | .356 | .204 | .068 | |
| Sarcomatoid de-differentiation | 7 (3.2) | |||||
| Venous thrombus present | 31 (14.4) | |||||
| Perinephric fat invasion | 34 (16.7) | |||||
| Tumor necrosis | 11 (5.1) |
3.1. Comparison of tumor tissue versus normal kidney
In 54 patients, tissue from tumor and benign kidney was available for analysis (42 clear cell, 11 papillary, and 1 chromophobe RCC). Fig. 4 shows benign and malignant samples from matched patients. Ki-67 positivity was found to be significantly higher in malignant versus benign samples (0.04% versus 0.35%; P = .004), respectively. For CRP expression, tumor tissue levels of CRP were significantly decreased compared with benign tissues (P = .01). For HIF1α, HIF2α, and CAIX, the mean expression of proteins was increased in the cancerous tissue compared with benign renal tissue from the same patients but failed to reach statistical significance (P = .08, .22, and .08, respectively).
Fig. 4.
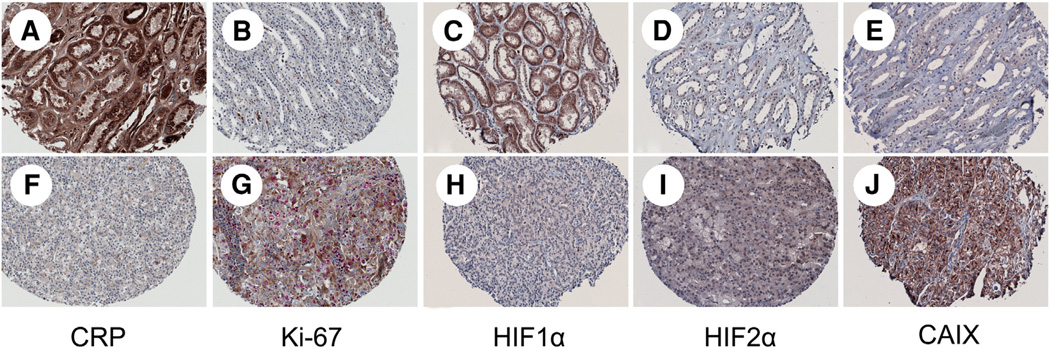
Benign (A–E) and malignant (F–J) tissue samples from patients with clear cell RCC demonstrating the expression of CRP, Ki-67, HIF1α and HIF2α, and CAIX. Images were acquired using the ×20 objective lens on the Nuance microscope.
3.2. Tissue protein expression evaluated as a continuous variable
Initial univariate analysis of tissue protein expression was performed, considering each putative biomarker as a continuous variable using optical density units of 0.01. Only Ki-67 expression was significantly predictive of RCC recurrence in this analysis with a hazard ratio (HR) of 1.60 (95% confidence interval [CI], 1.18–2.17; P = .003). Other putative biomarkers failed to reach significance to predict RCC recurrence, including CAIX (HR, 1.01 [0.99–1.03; P = .20]), HIF1α (HR, 0.96 [0.92–1.00; P = .05]),HIF2α (HR, 1.02 [0.97–1.08; P = .46]), and CRP (HR, 1.00 [0.99–1.02; P = .58]).
3.3. Clinicopathological prognostic factors
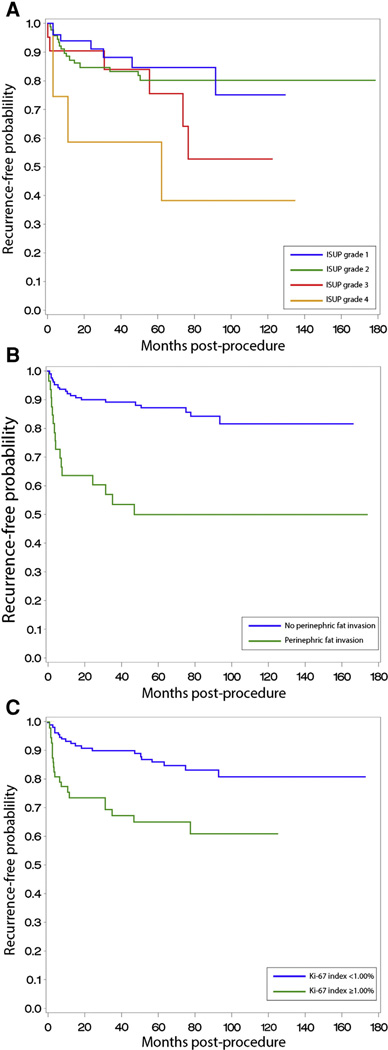
Univariate analysis identified ISUP grade 4, T stage, tumor diameter, RCC morphotype, sarcomatoid de-differentiation, venous thrombus, perinephric fat invasion, and increased expression of Ki-67 as prognostic factors for RCC recurrence (Table 2). Kaplan-Meier analysis of recurrence-free survival based on ISUP grade, perinephric fat invasion, and Ki-67 positivity is shown in Fig. 5.
Table 2.
Univariate analysis of ability to predict disease recurrence
| HR | 95% CI for HR |
P | |
|---|---|---|---|
| Clinical | |||
| Sex | 1.92 | 0.98–3.77 | .06 |
| Age | 1.02 | 0.99–1.05 | .28 |
| Pathologic RCC morphotype | .02 | ||
| Clear cell | Reference | ||
| Papillary | 0.91 | 0.37–2.24 | |
| Chromophobe | 0.37 | 0.05–2.78 | |
| Unclassified | 4.87 | 1.68–14.13 | |
| ISUP grade | .05 | ||
| 1 | Reference | ||
| 2 | 1.23 | 0.51–3.00 | |
| 3 | 2.23 | 0.75–6.63 | |
| 4 | 4.61 | 1.35–15.79 | |
| Sarcomatoid de-differentiation | 7.01 | 2.69–18.26 | <.0001 |
| Pathologic T stage | <.0001 | ||
| 1 | Reference | ||
| 2 | 1.31 | 0.37–4.64 | |
| ≥3 | 5.21 | 2.50–10.85 | |
| Venous thrombus | 6.30 | 3.08–12.90 | <.0001 |
| Perinephric fat invasion | 6.03 | 2.95–12.35 | <.0001 |
| Tumor necrosis | 0.46 | 0.06–3.35 | .45 |
| Maximum diameter of tumor (per cm) | 1.16 | 1.06–1.27 | .001 |
| Tissue protein expression | |||
| CRP | |||
| <0.17 | Reference | .58 | |
| ≥0.17 | 1.52 | 0.74–3.11 | |
| Ki-67 | |||
| <1.00% | Reference | .004 | |
| ≥1.00% | 3.60 | 1.78–7.27 | |
| HIF1α | |||
| <0.14 | Reference | .06 | |
| ≥0.14 | 0.41 | 0.16–1.06 | |
| HIF2α | |||
| <0.18 | Reference | .76 | |
| ≥0.18 | 0.88 | 0.40–1.95 | |
| CAIX | |||
| <0.45 | Reference | .69 | |
| ≥0.45 | 1.17 | 0.54–2.50 |
Fig. 5.
Kaplan-Meier estimate of disease recurrence based on ISUP grade (A), perinephric fat invasion (B; P = .01), and Ki-67 expression (C; P = .01).
After multivariate analysis, only tumor diameter, perinephric fat invasion, and Ki-67 positivity were identified as independent risk factors for RCC metastatic recurrence (Table 3).
Table 3.
Multivariable analysis for risk of recurrence
| HR | 95% CI for HR |
P | |
|---|---|---|---|
| Pathologic | |||
| Maximum diameter of tumor (per cm) | 1.20 | 1.02–1.41 | .02 |
| Perinephric fat invasion | 4.49 | 1.11–18.20 | .04 |
| Pathologic T stage | .80 | ||
| 1 | Reference | ||
| 2 | 0.53 | 0.08–3.45 | |
| ≥3 | 0.84 | 0.16–4.47 | |
| ISUP grade | .76 | ||
| 1 | Reference | ||
| 2 | 0.99 | 0.40–2.49 | |
| 3 | 0.94 | 0.28–3.24 | |
| 4 | 2.33 | 0.40–13.59 | |
| Tissue protein expression | |||
| Ki-67 | |||
| <1.00% | Reference | .002 | |
| ≥1.00% | 3.73 | 1.60–8.68 |
3.4. Analysis in patients with clear cell morphotype
In the cohort of patients with clear cell morphotype, a Ki-67 index of at least 1.00% was significantly predictive of recurrence with an HR of 2.91 (1.20–7.07; P = .02). Other proteins investigated that failed to reach significance include CAIX(HR, 0.92 [0.38–2.23; P = .85]),HIF1α (HR, 0.42 [0.10–1.82; P = .25]), HIF2α (HR, 1.43 [0.56–3.63; P = .45]), and CRP (HR, 1.53 [0.65–3.62; P = .33]). Ki-67 was independently predictive of recurrence in multivariable analysis (HR, 4.17 [1.60–10.85; P = .003]). Analysis of clear cell–only patients is summarized in Supplementary Table.
4. Discussion
Almost 20% of patients undergoing surgery for localized RCC develop metastatic disease within 2 years of surgery [14], which continues to result in poor survival despite new therapies [15]. In this study, quantitative expression of several proteins was measured using automated image analysis of IHC staining and demonstrated that increased Ki-67 positivity in RCC tumors is independently predictive of progression to metastatic disease after surgery. If demonstrated in prospective controlled studies, these data suggest that Ki-67 staining may help identify which patients are at highest risk for recurrence and could be enrolled in adjuvant therapy clinical trials. This study demonstrates the use of automated analysis of IHC staining to evaluate tissue protein expression in RCC, providing a straightforward and high-throughput method to evaluate future prospective tissue biomarkers.
After attempted curative surgery, clinicians currently use clinicopathological variables to evaluate a patient's risk of recurrence. No prognostic tissue biomarkers are currently used clinically for patients with RCC, although several potential biomarkers have been described. One major obstacle in the development of tissue biomarkers is the lack of straightforward and efficient methods to identify and validate prospective tissue markers in separate populations. Computerized image analysis of IHC decreases variability in interpretation when compared with manual IHC analysis. Automated slide analysis facilitates high-throughput reproducible results, which could expedite research of RCC tissue biomarkers. Development of meaningful RCC biomarkers may also have significant clinical applications including individualization of adjuvant therapy regimens, similar to other cancers. In patients with breast cancer and with overexpression of the HER2 receptor, metastatic recurrence is decreased when patients are treated with adjuvant trastuzumab after surgery [16,17]. In addition, identifying proteins that are consistently expressed in certain RCC subtypes may have clinical applications similar to how alpha-methylacyl-CoA racemase (AMACR) is currently used clinically to aid the identification of prostate cancer [18]. Finally, if favorable prognostic markers could be identified, expensive and time-consuming follow-up surveillance regimens [19] could be minimized in low-risk patients.
Several prognostic systems have been developed to identify patients at highest risk for RCC recurrence after treatment of the primary tumor [20]. Recent studies have also evaluated panels of tissue markers for RCC demonstrating improved predictive ability compared with the available prognostic systems [21]. Prognostic ability was further improved when analysis of tissue biomarkers was combined with clinicopathological risk models for RCC [10,21]. However, before biomarkers become useful for clinical practice, the prognostic ability should be evaluated in separate patient populations. Validation of biomarkers can be challenging because of the lack of standardized conditions in whole tissue sections and the difficultly of producing objective IHC analysis from multiple tissue samples in a timely fashion [22]. The use of a TMA allows for greater standardization of assay conditions compared with whole specimens, but sampling may also be a source of error, especially in heterogeneous tumors, so TMA findings should be compared with whole-mount specimens [22].
Analysis of protein expression using IHC is a common laboratory technique, but quantification of the level of protein expression is difficult and there may be significant differences in interpretation between different pathologists, despite efforts to maintain objectivity. The traditional 4-tiered scoring system and semiquantitative H score [23] are both labor intensive and do not allow for evaluation of small differences in protein expression. Using automated slide scanning, unique pattern recognition (inForm software), and multispectral imaging technology (Nuance software), the Vectra system of image analysis removes interobserver variability and provides a high-throughput method of quantitative protein expression. The use of Vectra in proteomic and morphometric studies decreases labor time 10-fold while reliably producing results consistent with current studies [5]. In addition to creating more objective data from IHC staining, automated image analysis allows for separation of spectra to evaluate subcellular (nuclear) expression of transcription factors, some of which are active only when present in the nucleus (HIF1α, HIF2α, and Ki-67). The ability to evaluate which proteins are functionally active by virtue of their cellular location may also be evaluated for prognostic ability using this system.
Higher risk of recurrence was demonstrated in patients with increased Ki-67 staining, similar to prior studies [2,8,9,24,25]. Increased Ki-67 positivity is a typical marker of proliferating eukaryotic cells [26], which is a known poor prognostic marker for several cancers [27,28]. Other potential tissue biomarkers that were tested failed to predict RCC recurrence in our cohort. Although, serum CRP levels have been shown to be predictive for RCC outcomes in several studies [29,30], few studies have evaluated tissue expression of CRP [11]. In addition, HIF1α, HIF2α, and CAIX have shown variable ability to predict recurrence or survival in several studies [3,10,21,27]. Multiple explanations are possible to account for the inability to produce consistent results among different studies using IHC for protein analysis including the following: differences in reagents used, technique of staining, tissue heterogeneity, preparation of the tissue [22], age of the tissue [12], and variability in interpretation of the results [5]. Achieving meaningful results in future studies of RCC biomarkers is dependent on standardizing techniques and improving IHC interpretative ability with automated high-throughput systems, as described in this article.
Despite the advantage using automated image analysis with IHC to report quantitative tissue protein expression compared with conventional techniques, several limitations should be mentioned. Unstable proteins may not be accurately measured, and poorly stained or folded tissue is unable to be analyzed through Vectra, similar to manual techniques. In addition, although automated techniques decrease interobserver variability, it is impossible to remove all subjectivities because a pathologist manually trains the system initially. Furthermore, RCC is known to have significant tissue heterogeneity [31], which may increase the sampling error when evaluating subtly increased proteins in tissue samples. In future studies with novel biomarkers, increasing the number of samples analyzed per patient or comparing TMA findings to whole pathologic specimens should be performed to decrease the potential sampling bias.
5. Conclusions
The current study is the first study describing the Vectra image analysis system to evaluate tissue biomarkers in RCC, which improves the ability to evaluate prospective tissue biomarkers because of decreased interobserver variability and the ability to produce quantitative high-throughput results. In RCC, increased Ki-67 positivity is independently predictive of metastatic recurrence after surgery for localized disease.
Supplementary Material
Footnotes
Research support: There was no outside funding used in this project.
Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.humpath.2014.01.008.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Dudderidge TJ, Stoeber K, Loddo M, et al. Mcm2, Geminin, and Ki67 define proliferative state and are prognostic markers in renal cell carcinoma. Clin Cancer Res. 2005;11:2510–2517. doi: 10.1158/1078-0432.CCR-04-1776. [DOI] [PubMed] [Google Scholar]
- 3.Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–661. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Rimm DL. C-Path: a Watson-like visit to the pathology lab. Sci Transl Med. 2011;3:108fs8. doi: 10.1126/scitranslmed.3003252. [DOI] [PubMed] [Google Scholar]
- 5.Huang WH, Kenneth H, Drew S. A colorful future of quantitative pathology: validation of Vectra technology using chromogenic multiplexed immunohistochemistry and prostate tissue microarrays. Hum Pathol. 2012;44:29–38. doi: 10.1016/j.humpath.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A., III . AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 479–489. [Google Scholar]
- 8.Bui MHT, Visapaa H, Seligson D, et al. Prognostic value of carbonic anhydrase IX and Ki67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461–2466. doi: 10.1097/01.ju.0000116444.08690.e2. [DOI] [PubMed] [Google Scholar]
- 9.Tollefson MK, Thompson RH, Sheinin Y, et al. Ki-67 and coagulative tumor necrosis are independent predictors of poor outcome for patients with clear cell renal cell carcinoma and not surrogates for each other. Cancer. 2007;110:783–790. doi: 10.1002/cncr.22840. [DOI] [PubMed] [Google Scholar]
- 10.Parker AS, Leibovich BC, Lohse CM, et al. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115:2092–2103. doi: 10.1002/cncr.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson TV, Ali S, Abbasi A, et al. Intratumor C-reactive protein as a biomarker of prognosis in localized renal cell carcinoma. J Urol. 2011;186:1213–1217. doi: 10.1016/j.juro.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Biswas S, Charlesworth PJ, Turner GD, et al. CD31 angiogenesis and combined expression of HIF-1alpha and HIF-2alpha are prognostic in primary clear-cell renal cell carcinoma (CC-RCC), but HIFalpha transcriptional products are not: implications for antiangiogenic trials and HIFalpha biomarker studies in primary CC-RCC. Carcinogenesis. 2012;33:1717–1725. doi: 10.1093/carcin/bgs222. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson TM, Sehgal PD, Drew S, et al. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013;85:140–149. doi: 10.1016/j.diff.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chae EJ, Kim JK, Kim SH, et al. Renal cell carcinoma: analysis of postoperative recurrence patterns. Radiology. 2005;234:189–196. doi: 10.1148/radiol.2341031733. [DOI] [PubMed] [Google Scholar]
- 15.Eggener SE, Yossepowitch O, Pettus JA, et al. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101–3106. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 16.Roses RE, Paulson EC, Sharma A, et al. HER-2/Neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers & Prevent. 2009;18:1386–1389. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra A, Verma M. Cancer biomarkers: are we ready for the prime time? Cancers (Basel) 2010;2:190–208. doi: 10.3390/cancers2010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin MA, Zhou M, Dhanasekaran SM, et al. Alpha-methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. J Am Med Assoc. 2002;287:1662–1670. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui SA, Frank I, Cheville JC, et al. Postoperative surveillance for renal cell carcinoma: a multifactorial histological subtype specific protocol. BJUI. 2009;104:778–785. doi: 10.1111/j.1464-410X.2009.08499.x. [DOI] [PubMed] [Google Scholar]
- 20.Tan MH, Li H, Choong CV, et al. The Karakiewicz nomogram is the most useful clinical predictor for survival outcomes in patients with localized renal cell carcinoma. Cancer. 2011;117:5314–5324. doi: 10.1002/cncr.26193. [DOI] [PubMed] [Google Scholar]
- 21.Klatte T, Seligson DB, LaRochelle J, et al. Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Cancer Epidemiol Biomarkers & Prev. 2009;18:894–900. doi: 10.1158/1055-9965.EPI-08-0786. [DOI] [PubMed] [Google Scholar]
- 22.Di Napoli A, Signoretti S. Tissue biomarkers in renal cell carcinoma: issues and solutions. Cancer. 2009;115:2290–2297. doi: 10.1002/cncr.24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gayed BA, Youssef RF, Bagrodia A, et al. Ki67 is an independent predictor of oncological outcomes in patients with localized clear-cell renal cell carcinoma. BJU Int. 2014;113(4):668–673. doi: 10.1111/bju.12263. [DOI] [PubMed] [Google Scholar]
- 25.Aaltomaa S, Lipponen P, Ala-Opas M, et al. Prognostic value of Ki-67 expression in renal cell carcinomas. Eur Urol. 1997;31:350–355. doi: 10.1159/000474482. [DOI] [PubMed] [Google Scholar]
- 26.Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation–associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 27.Kroeze SG, Bijenhof AM, Bosch JL, et al. Diagnostic and prognostic tissue markers in clear cell and papillary renal cell carcinoma. Cancer Biomark. 2010;7:261–268. doi: 10.3233/CBM-2010-0195. [DOI] [PubMed] [Google Scholar]
- 28.Margulis V, Lotan Y, Karakiewicz PI, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009;101:114–119. doi: 10.1093/jnci/djn451. [DOI] [PubMed] [Google Scholar]
- 29.Ito K, Yoshii H, Sato A, et al. Impact of postoperative C-reactive protein level on recurrence and prognosis in patients With N0M0 clear cell renal cell carcinoma. J Urol. 2011;186:430–435. doi: 10.1016/j.juro.2011.03.113. [DOI] [PubMed] [Google Scholar]
- 30.Cho DS, Kim SJ, Lee SH, et al. Prognostic significance of preoperative C-reactive protein elevation and thrombocytosis in patients with nonmetastatic renal cell carcinoma. Korean J Urol. 2011;52:104–109. doi: 10.4111/kju.2011.52.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.