Abstract
Alcohol exposure can reduce adult proliferation and/or neurogenesis, but its impact on the ultimate neurogenic precursors, neural stem cells (NSCs), has been poorly addressed. Accordingly, the impact of voluntary consumption of alcohol on NSCs in the subventricular zone (SVZ) of the lateral ventricle was examined in this study. The NSC population in adult male C57BL/6J mice was measured after voluntary alcohol exposure in a two-bottle choice task using the neurosphere assay, while the number of NSCs that had proliferated two weeks prior to tissue collection was indexed using bromodeoxyuridine (BrdU) retention. There was a significant decrease in the number of BrdU-retaining cells in alcohol consuming mice compared to controls, but no difference in the number of neurosphere-forming cells that could be derived from the SVZ of alcohol consuming mice compared to controls. Additionally, PCNA-labeled cells in the SVZ tended to be lower but there was no difference in BrdU labeling in the dentate gyrus following alcohol exposure. To determine alcohol’s direct impact on NSCs and their progeny, neurospheres derived from naïve mice were treated with alcohol in vitro. Neurosphere formation was reduced by 100 mM alcohol without reducing cell viability. These findings are the first to assess the impact of moderate voluntary alcohol consumption on selective measures of adult NSCs and indicate that such exposure alters NSC proliferation dynamics in vivo and alcohol has direct but dissociable effects on the expansion and viability on NSCs and their progeny in vitro.
Keywords: Neural stem cells, Adult neurogenesis, Animal models, Neurosphere culture, Proliferation
Introduction
Alcohol is widely consumed by humans, with high rates of dependence and abuse (Grant et al., 2004). Excessive alcohol exposure causes widespread brain damage, particularly in limbic system structures, as observed in alcoholics (for reviews, Harper and Matsumoto, 2005; Mann et al., 2001; Mukamal, 2004) and animal models (e.g. Collins et al., 1996; Obernier et al., 2002). Alcohol also impacts the production of new cells in the adult brain, an event which has been assessed using a combination of thymidine analog (i.e. bromodeoxy uridine; BrdU) incorporation into new DNA during S-phase and labeling with immature cell markers (e.g. Crews et al., 2004; Hansson et al, 2010; Herrera et al., 2003; Nixon and Crews, 2002; Pawlak et al., 2002). However, limited attention has been directed at assessing the impact of alcohol exposure on the earliest precursors in the neurogenic process, the adult neural stem cells (NSCs). In particular, it is not known whether moderate levels of alcohol consumption can adversely impact the NSC population or some aspect of NSC function. This question is particularly significant as long-term moderate alcohol consumption is far more common in the human population than the extremely high consumption levels that produce the severe effects seen in the alcoholic brain (WHO, 2004), and it is only high alcohol exposure levels that are modeled in nearly all animal studies examining adult NSCs and/or their progeny. To understand the risk to NSCs in the vast majority of drinkers, it is necessary to model the alcohol consumption level of the majority of drinkers.
NSCs are the ultimate precursors for neurogenesis in the adult olfactory bulbs (OB) and dentate gyrus (DG) of the hippocampus (Gage, 2000; van der Kooy and Weiss, 2000). NSCs in the subventricular zone (SVZ) of the anterior lateral ventricles proliferate slowly (cell cycle of 15 days), last throughout life (Chiasson et al., 1999; Morshead et al., 1994) and give rise to constitutively proliferating progenitors (cell cycle of 12 h) that transiently reside within the SVZ before dying (Morshead et al., 1998) or migrating out of the SVZ to the OB where they become interneurons (e.g. Lois and Alvarez-Buylla, 1994; Luskin, 1994; Lledo and Saghatelyan, 2005; van Praag et al., 2002). Alcohol exposure might alter this adult neurogenic system in several ways including through effects on NSC pool numbers, NSC proliferation rate, or the proliferation and/or fate of NSC progeny.
The present study provides the first assessment of the impact of alcohol exposure on selective measures of NSC function in vivo. Adult male C57BL/6J mice were allowed moderate voluntary alcohol consumption (under two-bottle access to alcohol or water) and then NSC proliferation and number was determined. The number of SVZ NSCs that proliferated during alcohol consumption was indexed using long-term (14 day) BrdU retention, and the number of NSCs residing in this region was measured using a primary neurosphere assay. In addition, the direct effect of alcohol on the proliferation and viability of NSCs and their progeny in vitro was determined using established neurosphere cultures.
Methods
Subjects and General Design
Adult male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used as this strain exhibits voluntary moderate alcohol intake (Crabbe et al., 1994; Dudek and Underwood, 1993; McBride, 2002) and their intoxication is sufficient to reduce the proliferation of unspecified precursors in the SVZ and DG (Crews et al., 2004). The design of the voluntary alcohol consumption experiments are illustrated in Fig 1a; the BrdU-retention experiment comprised of 6 weeks of two-bottle choice (n=13 alcohol, n=10 controls) and the neurosphere assay experiment comprised of 4 weeks of two-bottle choice (n=15 alcohol, n=15 controls). Mice were 8 weeks of age at the start of the study. The alcohol exposed mice were single housed and given one bottle containing water and another containing 15% alcohol (vol./vol.; from dilution of 95% ethanol stock) in water. No sucrose fading or gradual alcohol increases were employed, yet mice reached the desired moderate consumption levels for the alcohol solution. For controls, both bottles were filled with water. For all mice, bottle locations were alternated and bottles refreshed each time the bottle weights were recorded (at least 3 times weekly). The average start weight for mice was 22.3 g with an average end weight of 27.8 g. While bottles did not leak when stationary, mouse activity was at times sufficient to cause blockage and/or leakage, so bottles were monitored for tampering and no data from the day of tampering was included in later analyses.
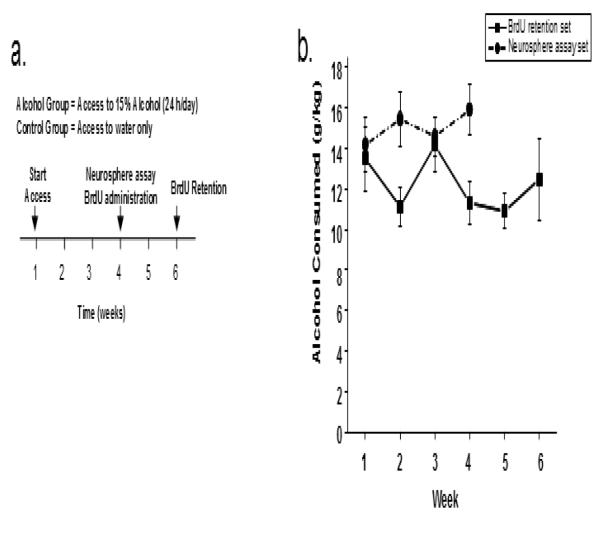
Fig 1.
Impact of voluntary alcohol consumption on the adult SVZ and DG neurogenic systems a. Schematic of experimental time line. Mice were allowed access to two drinking bottles, one contained water for all mice and the other contained either 15 % alcohol in water (Alcohol groups) or water (Control groups). After four weeks of access, mice were sacrificed or received injections of bromodeoxyuridine (BrdU) and sacrificed 2 weeks later b. Alcohol intake for the mice with access to alcohol (g/kg per day averaged across weekly intervals). All data expressed as Mean ± S.E.M.; mice used for examining BrdU-retention are squares and for neurosphere assay are circles. There were no significant differences in drinking in groups used for BrdU retention versus neurosphere assay.
A separate group of 16 male mice were allowed alcohol access under the same two-bottle choice conditions and blood alcohol levels were determined repeatedly at 2 hours following lights out, a time of high fluid consumption (Dole & Gentry, 1984; Rhodes et al., 2005). For blood alcohol concentration (BAC) determination, blood samples were collected from the submandibular vein, centrifuged, the plasma supernatant was extracted and stored in 0.5 ml microcentrifuge tubes at -80°C until determination of BAC in mg/dl using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA). During the first day of alcohol exposure (i.e. after having 24 hours access to alcohol), mice exhibited alcohol intake of 10.64 g/kg and bBACs of 18.8 ± 2.6 mg/dl. After 2 weeks of access mice exhibited an average daily alcohol intake of 11.40 ± 1.89 g/kg and BACs of 21.0 ± 3.5 mg/dl on the first day of that week, and after 4 weeks of access mice exhibited an average daily alcohol intake of 14.53 ± 1.25 g/kg and BACs of 20.0 ± 2.4 mg/dl on the first day of that week. Thus in mice under the same conditions as those used to determine the effects of alcohol on NSCs, we saw a rapid initiation of alcohol intake with detectable and relatively stable BACs persisting during the period of voluntary consumption. To prevent unnecessary stress, blood samples were not taken from the mice used to assess the NSC population because stress itself negatively impacts some facets of the adult neurogenic system (e.g. Schoenfeld and Gould, 2012). Finally, since tissue collection for examination of NSCs was performed during the light phase of their cycle when mice consume less fluid (Dole & Gentry, 1984), BAC measured from trunk blood samples were not analyzed as they would not be expected to correlate with the full extent of alcohol intake through the experiment. A subset of the in vivo results derived from these mice was briefly discussed in Campbell and Kippin (2011). All procedures were approved by the University of California at Santa Barbara Institutional Animal Care and Use Committee and conducted in accordance with the National Institute of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23, 8th edition 2011).
Bromodeoxyuridine Labeling and Detection
BrdU incorporation labels mitotic cells in the S phase and the number of SVZ NSCs labeled with BrdU was assessed 14 days after administration. The employment of the retention period prior to tissue collection is crucial to capturing NSCs exclusively: In contrast to the NSCs (which permanently reside in the SVZ), constitutively proliferating progenitor cells of the SVZ migrate out of the area via the rostral migratory stream or die during the retention period, resulting in clearance of BrdU-labeled non-NSCs from the SVZ (Luskin, 1993; Morshead and van der Kooy, 1992). Mice received BrdU (60 mg/kg, i.p. 5 injections, once every 3 h). This BrdU regimen labels the proliferating cells in the SVZ (Morshead & van der Kooy, 1992; Burns & Kuan, 2005) while also minimizing the labeling of non-proliferative cells and BrdU toxicity (Taupin, 2007). The series of injections were initiated just before the start of the lights-on portion of a 12:12 cycle, capturing a time after the nightly peak in alcohol consumption (Dole & Gentry, 1984). Fourteen days later, mice were euthanized (100 mg/kg sodium pentobarbital, IP) followed by transcardial perfusion with cold 0.9% saline and 4% paraformaldehyde. Brains were extracted and postfixed in 4% paraformaldehyde for 4 hours followed by cryoprotection in 30% sucrose in phosphate-buffered saline (PBS). Brains were sectioned on a cryostat at 20 μm and sections from the anterior lateral ventricle and hippocampus regions at 100 μm intervals were mounted onto Superfrost Plus (Fisher) slides.
Immunohistochemistry was performed as previously described (e.g. Kippin et al., 2005b) using the following antibody combinations. BrdU detection was performed by antigen retrieval with 2 M HCl at 60° C for 30 min followed with rat monoclonal anti-BrdU (1:200 in 20% NGS in PBS overnight at 4°C; AbD Serotec) visualized by Alexa Fluor 488 goat anti-rat (1:400 in 0.3% Triton-X in PBS for 2 hours at RT; Invitrogen). For labeling of all cycling cells, mouse monoclonal anti-proliferating cell nuclear antigen (PCNA; 1:200 in 20% NGS in PBS overnight at 4°C; Zymed) visualized by Alexa Fluor 555 goat anti-mouse (1:400 in 0.3% Triton-X in PBS for 2 hours at RT; Invitrogen) was used. For labeling neuronal nuclei, mouse monoclonal anti-neuronal nuclei (NeuN; 1:200 in 20% NGS in PBS overnight at 4°C; Millipore) visualized by Alexa-Fluor 555 goat anti-mouse (as above) was used in order to facilitate determination of the borders of the DG. PBS washes were performed between each step. Slides were coverslipped with Fluorogel (Electron Microscopy Sciences).
Labeled cells were counted under epi-fluorescence on a Nikon E800 microscope with BrdU-retaining cells/SVZ section (6 sections/subject minimum) representing the average number of NSCs undergoing the S-phase at the time of BrdU administration, and the PCNA-expressing cells representing the number of all cells proliferating at or near sacrifice. The average number of BrdU-positive cells in the DG through the extent of the hippocampus (10 sections/subject minimum) represented the average number of surviving progeny in this region.
Neurosphere Assay
The number of NSCs in the SVZ were measured using a primary neurosphere assay as described previously (Hitoshi et al., 2011; Kippin et al., 2004; 2005a,b; Morshead et al., 2003). Briefly, mice were sacrificed by decapitation, brains removed, placed in oxygenated artificial cerebrospinal fluid, and the anterior portion of the lateral ventricle SVZ was isolated and dissociated enzymatically (in 1.33 mg/ml trypsin, 0.66 mg/ml hyaluronidase, 0.13 mg/ml kynurenic acid; all from Sigma) and mechanically (using fire-polishing Pasteur pipettes) into single cells. The viable cells were counted using trypan blue exclusion and cells were plated at 10 cells/μl density in uncoated 24-well plates (Sarstedt) in 0.5 ml of serum-free media (SFM) supplemented with murine growth supplement (Stem Cell Technologies) and containing 20 ng/ml of epidermal growth factor (EGF; mouse submaxillary: Sigma), 10 ng/ml of fibroblast growth factor-2 (FGF; human recombinant: Sigma), and 2 μg/ml of heparin (Sigma). The number of neurospheres >100μm in diameter was determined after 7 days. Under these conditions, primary neurosphere colonies are derived from single cells and serve as an index of the number of in vivo NSCs (Coles-Takabe et al., 2008; Morshead et al., 2003). To assess initial NSC self-renewal capability, primary neurospheres were dissociated into single cells and cultured as above and the number of secondary neurospheres was determined after 7 days.
In vitro alcohol exposure of neurospheres
Neurosphere-forming cells were isolated as above from alcohol-naïve mice but expanded in uncoated 75 cm2 flasks (BD Bioscience) in SFM with growth factors (EGF, FGF, heparin) and passaged every 7 days (via mechanical dissociation and resuspension in fresh media) until initiation of experiments. All in vitro experiments were conducted on cells generated following 3-5 passages.
To determine the effects of administration of alcohol over the duration of colony growth, cells were passaged at 5 cells/μl into uncoated 24-well plates (Sarstedt) in 0.45 ml of SFM with growth factors with 50 μl of a water+alcohol added to achieve final in-media concentrations of 1 μM - 1 M or no alcohol (control). The 1 M dose was included to complete the dose-response curve, although it is well beyond potential consumption levels. After 7 days the number of neurosphere colonies of >100μm in diameter in each well were counted.
To determine the effects of alcohol on cell viability, dissociated cells were seeded into black 96-well plates (Greiner Bio One) at 500 cells/μl in SFM with growth factors or supplemented with 1% fetal bovine serum (FBS) containing 1 μM – 1 M or 0 M alcohol concentration. Cells were grown for 24, 48, or 72 hours and 4 hours prior to assessment Cell Titer-Blue (Promega) was added to each well. The resazurin component of the Cell Titer-Blue is metabolized by living cells into the red, highly fluorescent product resorufin which can be detected by fluorometer providing a measure of living cells.
Statistics
A repeated measures ANOVA was used to assess measures of mouse alcohol intake. T-tests and factorial ANOVAs were employed as appropriate for all other measures, with α = 0.05 for all comparisons. All data are represented as mean and standard error of the mean (SEM).
Results
C57BL/6J mice voluntarily consume moderate amounts of alcohol
Overall, the average daily g/kg alcohol consumption for all mice was 14.73 ± 0.54. Thus, the mice consumed a quantity typical of this strain under continuous access and no sweetening of the alcohol solution, and this fits well within the more general range of alcohol intake of the adult C57BL/6J mice (e.g. Crabbe et al., 1994; Dudek and Underwood, 1993; McBride, 2002; Wahlsten et al., 2006). In addition, to ensure experimental consistency, a repeated measures ANOVA was preformed, and it failed to detect any significant differences between weeks or groups of mice used for the the BrdU-retention and neurosphere assays on the four comparable weeks (Fig 1b; all ps > 0.05).
Voluntary chronic alcohol consumption reduces BrdU retention in the SVZ
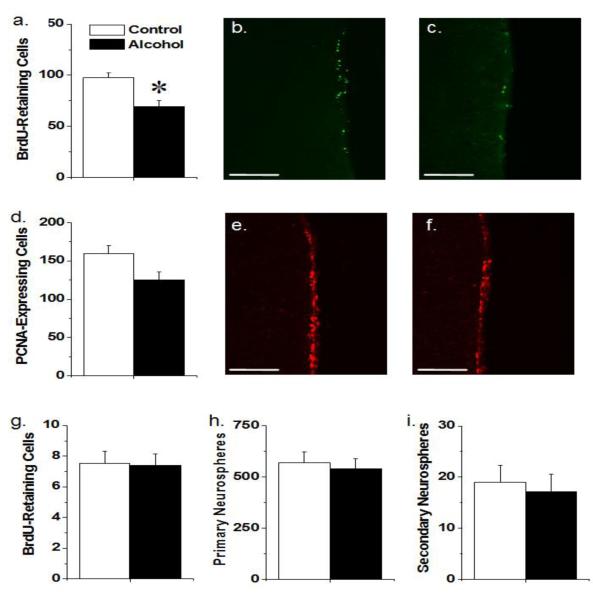
Alcohol and control mice were injected with BrdU two weeks prior to sacrifice to produce BrdU retention which highlighted BrdU incorporation into NSCs specifically. Voluntary chronic alcohol exposure resulted in significantly fewer BrdU-retaining cells in the SVZ (t21 = 3.41, p < 0.05; Fig 2a). There was also a trend toward a reduction in the total number of PNCA expressing cells in the SVZ at sacrifice (t21 = 1.82, p = 0.083; Fig 2d). Representative images of BrdU- and PCNA-labeled cells in the SVZ are presented in Fig 2b-c and 2e-f, respectively, for both control and alcohol-exposed mice.
Fig 2.
Impact of voluntary alcohol consumption on the adult SVZ and DG neurogenic systems a. Numbers of cells retaining BrdU for 2 weeks in the SVZ of alcohol and control mice. Mice allowed 4 weeks access to alcohol prior to BrdU administration show fewer BrdU-labeled cells than controls (p < 0.05) b. A photomicrograph displaying typical labeling of long-term BrdU-retaining cells in the SVZ of control mice c. A photomicrograph displaying typical labeling of long-term BrdU-retaining cells in the SVZ of alcohol-exposed mice d. Numbers of cells expressing proliferating cell nuclear antigen (PCNA) in the SVZ of alcohol and control mice. There was a trend towards a reduction in PCNA in mice allowed 6 weeks of alcohol access prior to tissue collection (p = 0.083) e. A photomicrograph displaying typical labeling of long-term PCNA-expressing cells in the SVZ of control mice f. A photomicrograph displaying typical labeling of long-term PCNA-expressing cells in the SVZ of alcohol-exposed mice g. Number of cells retaining BrdU in the DG. There were no significant differences between alcohol-exposed and control mice h. The total number of neurospheres derivable from the SVZ tissue of mice allowed 4 weeks of access to alcohol and controls. No significant difference was found between groups i. The number of secondary neurospheres per well in cultures derived from alcohol and control mice. No significant difference was found between groups. All data expressed as Mean ± S.E.M. All scale bars are 100 μm.
Examining the DG, there were no significant differences between the number of BrdU-retaining cells within the DG of alcohol exposed and control mice (ps > 0.05; Fig 2g).
Voluntary chronic alcohol consumption does not reduce the number of neurospheres-forming cells from the SVZ
SVZ tissue from alcohol and control mice was dissociated into a single cell suspension, plated at 10 cells/μl in SFM with growth factors and the number of neurospheres per well was determined 7 days later. Alcohol consumption in vivo did not alter primary neurosphere number (p > 0.05) as measured by either the average number of neurospheres per well (alcohol: 13.05 ± 1.18; control: 11.63 ± 1.13) or the total number of neurosphere-forming cells isolated from the total SVZ (Fig 2h). To assess initial self-renewal rate, the primary cultures were dissociated into a single cell suspension, re-plated in SFM with growth factors, and assessed after 7 days. No significant difference was detected between two conditions (p > 0.05; Fig 2i).
Exposure to high doses of alcohol in vitro reduces neurosphere culture proliferation
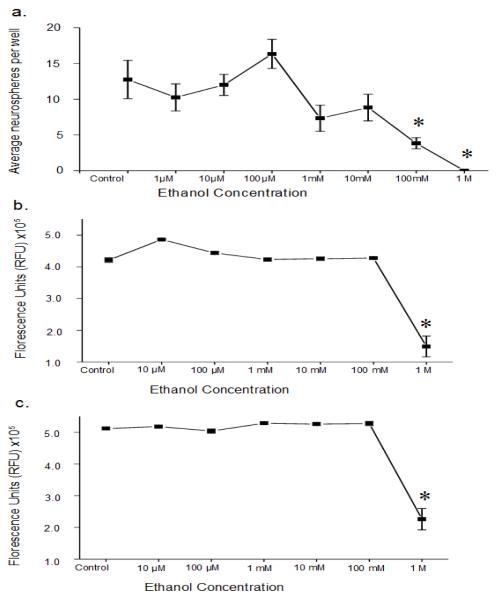
To determine the direct effects of alcohol on NSCs and/or their progeny, neurosphere cultures were dissociated and re-plated with alcohol (1 μM – 1 M) and the numbers of neurospheres were determined 7 days later. Alcohol reduced the average number of neurospheres per well (F7,34 = 10.32, p < 0.05) with significant decreases from controls occurring at doses of 100 mM and 1 M ethanol (ps < 0.05; Fig 3a).
Fig 3.
Impact of in vitro alcohol treatment on NSCs and progenitors derived from the adult SVZ. Alcohol can directly impact these cells, but only at high doses a. At high doses alcohol inhibits neurosphere formation in vitro when added at the start of passaging. Alcohol concentrations of 100 mM or 1 M reduced the formation of neurospheres (p < 0.05) b. The viability of cultures in serum-free growth media at 24h post-alcohol exposure. Only the supraphysiological 1 M alcohol dose negatively impacts viability (p < 0.05) c. Viability of cultures in media plus 1% fetal bovine serum at 24h post-alcohol exposure. Again, the only dose negatively impacting viability is 1 M (p < 0.05), suggesting that the effects of 100 mM alcohol are not due to the death of cells in the neuropshere. All data expressed as Mean ± S.E.M. * p < 0.05
To determine the effect of alcohol on cell viability under two media conditions, high-density cultures of neurosphere-derived cells grown in either SFM supplemented with growth factors or SFM supplemented with 1% FBS were exposed to alcohol (10 μM – 1 M) for 24, 48 or 72 hr. The Cell Titer-Blue assay was used to measure cell viability. The results were the same across media conditions with significant differences in cell viability (for 24, 48, 72 hr in SFM: F6,35 = 35.74, F6,35 = 45.24, F6,35 = 26.99, all ps < 0.05; and for 24, 48, 72 hr in 1% FBS: F6,35 = 33.88, F6,35 = 9.77, F6,35 = 97.15, all ps < 0.05) occurring only at 1 M alcohol dose compared to controls (ps < 0.05); Fig 3b and 3c illustrate the 24 hour time point for SFM with growth factors or 1% FBS, respectively, showing that viability was reduced only at 1 M. Additionally, these results show that the changes in neurosphere formation seen previously at the 100 mM alcohol concentration are not due to alterations in cell viability.
Discussion
This study is the first to demonstrate that chronic moderate alcohol consumption alters proliferation of NSCs in the subventricular zone in adult mice as indicated by reduced numbers of BrdU-retaining cells in the SVZ 14 days following BrdU administration. The difference in retention of BrdU-labeled cells over this time period may indicate that this reduction in BrdU-labeling is specific to NSCs, rather than due to reduced labeling of other mitotic cell types, since BrdU-labeled transient amplifying cells and committed progenitors would have migrated away from the SVZ or died (Luskin, 1993; Morshead and van der Kooy, 1992) while a daughter cell retaining the NSC fate should have stayed within the SVZ NSC niche. The decrease in BrdU-retaining NSCs could have been a direct consequence of alcohol-induced death of the SVZ NSC population, the inhibition of NSC proliferation during the alcohol intake, or some mechanism which changes the migration/behavior of daughter cells which should have otherwise remained in the SVZ as NSC. To exclude the first possibility, mice were exposed to alcohol and then the neurosphere assay was used to determine the total number of cells in the SVZ exhibiting NSC properties. There was no difference in the numbers of primary neurospheres that could be derived from SVZ tissue taken from alcohol consuming compared to control mice, indicating that alcohol consumption does not change the size of the NSC population under these conditions. Further, there was no significant difference in the alcohol intake of mice used in the BrdU-retention versus mice used in the neurosphere experiment, thus the differences in outcomes on the two assays cannot be explained by level of intake in the groups. Therefore a moderate level of alcohol consumption does not seem to cause loss in the NSC pool, but it does appear to reduce the proportion of NSCs proliferating near a particular point in time or potentially drive some daughter cells which should have become NSC out of the niche.
The present finding extends a prior report of reduced BrdU labeling in the SVZ after alcohol consumption in C57BL/6J mice (Crews et al., 2004). In the study by Crews et al. (2004) BrdU was administered daily for 14 days and then tissue was collected for processing, resulting in assessment of cells at various stages of maturation. Our use of a time-constrained (instead of daily) BrdU pulse plus long-term BrdU-retention enables detection of only NSC proliferation, as non-NSCs either die or migrate out of the SVZ during the retention period (Morshead et al., 1998). Consistent with our findings that the NSC population is maintained despite reduced NSC proliferation, another study using a higher alcohol intoxication level reported no change in the numbers of cells expressing the putative NSC marker Sox2 within the SVZ despite a significant reduction in BrdU incorporation and PCNA expression (Hansson et al., 2010). Together, these studies indicate that although the NSC population is resilient to the effects of alcohol, ongoing production of neurogenic precursors is reduced.
The apparent resilience of the adult NSC pool to in vivo alcohol exposure contrasts in some respects with recent findings indicating that prenatal alcohol reduces the number of neurospheres in the neonatal SVZ (Akers et al., 2011; Rubert et al., 2006; Santillano et al., 2005). NSCs may be more sensitive to insults during embryonic expansion of the pool relative to the static population dynamics of adulthood, as has been demonstrated for other insults such as prenatal versus adult stress (Kippin et al., 2004). Several lines of research have also characterized the effects of alcohol on neurogenic precursors derived from various regions of the developing nervous system (for reviews, Costa et al., 2004; Crews et al., 2003; Luo and Miller, 1998). The most consistent finding of these studies is that alcohol inhibits the proliferation of a variety of neural precursors. Finally, alcohol is known to affect the size of fetal neurospheres, increasing them in a dose-dependent manner through increasing neurosphere coalescence (Vangipuram et al., 2008). In our adult neurosphere cultures, we only utilized a minimum size criterion during assessment, and so it is not known if cultures from alcohol-exposed mice had differences in mean neurosphere diameter. As this effect is coalescence-driven and there were no differences between adult control and alcohol-exposed mice in neurosphere numbers, a large increase in neurosphere size would only be possible if there were also an increase in neurosphere numbers pre-coalescence. Future, larger-scale in vitro studies are needed to determine if there are any changes in adult neurosphere sizes, and if not this also makes the adult NSC population distinct from its fetal counterpart.
Our proposed explanation for the observed reduction in BrdU incorporation in the SVZ NSC pool is that alcohol inhibited proliferation of NSC cells, resulting in fewer NSCs undergoing S-phase during the time of BrdU injection. However, a reduction in BrdU labeling may not always reflect a reduction in the number of proliferating cells as it has been found that alcohol can shorten the length of S-phase for hippocampal precursors in adolescents (McClain et al., 2011), which would also result in a reduction in the number of BrdU-retaining cells if over-proliferation diluted the signal. Yet the concurrent reduction in BrdU and trend towards a reduction in PCNA in the present experiments, which is supported by our in vitro data as well as similar findings in other studies (e.g. Hansson et al., 2010), is consistent with reduced NSC proliferation rather than with increased proliferation. Still, PCNA is not a particularly sensitive measure of proliferation. Even cells that have previously exited cell cycle still show PCNA expression, due to both the long half-life of PCNA and its role in DNA repair mechanisms (Bravo & Macdonald-Bravo, 1987; Toschi & Bravo, 1988). Also, a cell that is arrested in G1, as can happen with alcohol exposure (Mikami et al., 1997), would still express PCNA even while unable to incorporate BrdU and finish division. This provides another potential mechanism by which PCNA measures might be less sensitive to the effects of alcohol than others.
In vivo experiments cannot determine whether the observed reduction in NSC proliferation during alcohol consumption is due to the direct actions of alcohol or indirectly due to changes in other factors within an alcohol-exposed brain (e.g. alcohol-induced changes in neurotransmitters or growth factor levels). Thus, we tested the effects of alcohol on NSCs and their progeny in vitro. Only treatment with 1 M alcohol reduces the viability of these cells, which was expected at such a high dose. Treatment with 100 mM alcohol does not affect the viability of neurosphere-derived cells but results in the formation of fewer neurospheres, indicating that alcohol can directly reduce the proliferation of NSCs and/or their progeny. It is noteworthy that the present changes in vitro occurred at much higher alcohol concentrations than are reached in vivo during voluntary alcohol consumption in mice: C57BL/6J mice voluntarily achieved BAC around 20 mg/dl (approximately 4.3 mM) during two-bottle choice which is substantially lower than the lowest in vitro dose (100 mM) that significantly reduced neurosphere formation. The 100 mM level would also be rare in the human population outside of severely tolerant individuals. This apparent in vivo versus in vitro discrepancy in the dose-dependence of alcohol could indicate that facets of the culture conditions buffer the NSCs from low-dose alcohol-induced changes or perhaps that longer treatment durations are needed for lower alcohol doses to produce observable effects on NSCs. Alternatively, the impact of moderate doses of alcohol on NSC proliferation observed in vivo may be mediated through an indirect mechanism within the alcohol-exposed brain. In summary, while alcohol can have direct effects on NSC proliferation it is only seen at substantially higher doses than the BAC levels achieved during free-access to alcohol by C57Bl/6J mice, suggesting that the effect of moderate doses of alcohol on our mice was due to an indirect effect of alcohol.
We also assessed the impact of alcohol consumption on the DG but failed to detect changes in this brain region. Comparing across studies there appear to be dose-dependent effects of alcohol on neurogenic processes in the DG in C57BL/6 mice: minimal intake (6 g/kg/day) increases proliferation and survival (Aberg et al., 2005), moderate intakes (14-18 g/kg/day) produce no changes on these measures (Stevenson et al., 2009; present study), and binge or high intake (2-4 g/kg/2 hr; 26 g/kg/day) decreases both of these measures in the DG (Contet et al., 2013; Crews et al., 2004). Similarly, studies in rats indicate that a similar effect, with reductions in hippocampal proliferation/early survival of precursors being proportional to level of intake (Anderson et al., 2012; Richardson et al., 2009). Since there are no established methods to efficiently isolate and grow adult DG precursor cells in vitro (see e.g. Seaberg and van der Kooy, 2002; Clarke and van der Kooy, 2011) it was not possible to assess direct effects of alcohol on neurogenic precursors in this region.
The in vivo decrease in BrdU-labeled NSCs was a statistically significant but modest effect, as would be expected from a modest insult such as a month of moderate alcohol consumption. The even more modest decrease in PCNA at the time of sacrifice suggests that 6 weeks of moderate alcohol consumption produces only minor impacts in a healthy adult male mouse, as also shown by the finding that there were no differences in 2 week new cell survival in the DG of these same mice. Still, this underscores that even moderate alcohol consumption in healthy adults decreases the functioning of some aspects of the adult neurogenic system; individuals with less resilient neurogenic systems due to illness or age may be unable to compensate for even this modest disruption due to alcohol. It is known that among alcoholics, older men are more susceptible to reductions in both gray and white matter compared to younger men, and that this effect is not attributable to length of alcohol use (Pfefferbaum et al., 1992; Pfefferbaum et al., 1997). This was also recently shown in a mixed gender study of non-treatment seeking alcoholics, where there was a greater gray matter volume decline in older individuals than could accounted for by alcohol intake or age alone (Fein et al., 2010) – that is, the aged brain has increasing vulnerability to alcohol’s effects. The impact of moderate alcohol consumption on the aged brain, both structurally and behaviorally, are less clear (Kim et al., 2012). Determining the effects of age and moderate alcohol consumption on the neural stem cell population in humans could lead to new insights on the safest alcohol consumption level at each age, so that any vascular benefits of alcohol can be gained without adversely impacting brain function.
Together, these findings indicate that moderate voluntary consumption of alcohol has effects on some aspects of the adult neurogenic system while sparing others. Specifically, in male C57BL/6J mice, the proliferation of NSCs in the SVZ is decreased without reducing the total NSC population. Alcohol treatment also decreased neurosphere formation in vitro, but only at extremely high doses. Thus, the present study extends existing findings by demonstrating that moderate alcohol consumption reduces SVZ NSC proliferation without altering the NSC population. In addition the in vitro studies extend previous findings in proposing that the moderate voluntary consumption of alcohol reduces proliferation of NSCs in the SVZ, possibly through an indirect mechanism. Further studies will be necessary to establish if and how moderate alcohol levels alter the SVZ environment to cause reduced NSC proliferation.
Acknowlegements
This work was supported by National Institute on Drug Abuse grant (DA-027115 and DA-027525) and an Alcoholic Beverage Medical Research Foundation grant to Professor Tod E. Kippin.
Footnotes
Present address: Center for Neuropharmacology and Neuroscience, Albany Medical College, MC-136, 47 New Scotland Avenue, Albany, NY 12208. Present phone: 1-518-262-8626
References
- Aberg E, Hofstetter CP, Olson L, Brene S. Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. Int J Neuropsychopharmacol. 2005;8:557–567. doi: 10.1017/S1461145705005286. [DOI] [PubMed] [Google Scholar]
- Akers KG, Kushner SA, Leslie AT, Clarke L, van der Kooy D, Lerch JP, Frankland PW. Fetal alcohol exposure leads to abnormal olfactory bulb development and impaired odor discrimination in adult mice. Mol Brain. 2011;4:29. doi: 10.1186/1756-6606-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ML, Nokia MS, Govindaraju KP, Shors TJ. Moderate drinking? Alcohol consumption significantly decreases neurogenesis in the adult hippocampus. Neuroscience. 2012;224:202–209. doi: 10.1016/j.neuroscience.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Kuan CY. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur. J. Neurosci. 2005;21:803–807. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987;105(4):1549–54. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JC, Kippin TE. The interaction of neural stem cells and chronic alcohol consumption. In: Olive MF, editor. Drug Addiction and Adult Neurogenesis. Research Signpost; Kerala, India: 2011. pp. 121–138. [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, van der Kooy D. The adult mouse dentate gyrus contains populations of committed progenitor cells that are distinct from subependymal zone neural stem cells. Stem Cells. 2011;29:1448–58. doi: 10.1002/stem.692. [DOI] [PubMed] [Google Scholar]
- Coles-Takabe BLK, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM, van der Kooy D. Don’t look: Growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26(11):2938–44. doi: 10.1634/stemcells.2008-0558. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Contet C, Kim A, Le D, Iyengar SK, Kotzebue RW, Yuan CJ, Kieffer BL, Mandyam CD. μ-Opioid receptors mediate the effects of chronic ethanol binge drinking on the hippocampal neurogenic niche. Addict Biol. 2013 doi: 10.1111/adb.12040. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Guizzetti M. Signal transduction mechanisms involved in the antiproliferative effects of ethanol in glial cells. Toxicol Lett. 2004;149:67–73. doi: 10.1016/j.toxlet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ, Metten P. Use of recombinant inbred strains for studying genetic determinants of responses to alcohol. Alcohol Alcohol Suppl. 1994;2:67–71. [PubMed] [Google Scholar]
- Crews FT, Miller MW, Ma W, Nixon K, Zawada WM, Zakhari S. Neural stem cells and alcohol. Alcohol Clin Exp Res. 2003;27:324–335. doi: 10.1097/01.ALC.0000052581.46630.C5. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry T. Toward an analogue of alcoholism in mice: Scale factors in the model. PNAS. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek BC, Underwood KA. Selective breeding, congenic strains, and other classical genetic approaches to the analysis of alcohol-related polygenic pleiotropisms. Behav Genet. 1993;23:179–189. doi: 10.1007/BF01067423. [DOI] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naïve actively drinking alcohol-dependent sample. Alcohol Clin Exp Res. 2010;34(1):175–82. doi: 10.1111/j.1530-0277.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol. 2010;13:583–593. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Kippin TE, van der Kooy D. Culturing neural stem cells: Application to the study of neurodegenerative and neuropsychiatric pathology. In: Seki T, Sawamotot K, Parent JM, Alvarez-Buylla A, editors. Neurogenesis in the Adult Brain II: Clinical Implications. Springer; Toyko, Japan: 2011. pp. 189–208. [Google Scholar]
- Kim JW, Lee DY, Lee BC, Jung MH, Kim H, Choi YS, Choi IG. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8–16. doi: 10.4306/pi.2012.9.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Masum Z, Ralph MR. Neural stem cells show bidirectional experience-dependent plasticity in the perinatal mammalian brain. J Neurosci. 2004;24:2832–2836. doi: 10.1523/JNEUROSCI.0110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine Specifically Inhibits Forebrain Neural Stem Cell Proliferation, Suggesting a Novel Effect of Antipsychotic Drugs. J Neurosci. 2005a;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005b;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Neuronal cell lineage in the vertebrate central nervous system. FASEB J. 1994;8:722–730. doi: 10.1096/fasebj.8.10.8050671. [DOI] [PubMed] [Google Scholar]
- Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, et al. Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res. 2001;25:104S–109S. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McClain JA, Hayes DM, Morris SA, Nixon K. Adolescent Binge Alcohol Exposure Alters Hippocampal Progenitor Cell Proliferation in Rats: Effects on Cell Cycle Kinetics. J Comp Neurol. 2011;519:2697–2710. doi: 10.1002/cne.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K, Haseba T, Ohno Y. Ethanol induces transient arrest of cell division (G2 + M block) followed by G0/G1 block: dose effects of short- and longer-term ethanol exposure on cell cycle and cell functions. Alcohol Alcohol. 1997;32(2):145–52. doi: 10.1093/oxfordjournals.alcalc.a008248. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells butnot retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ. Alcohol consumption and abnormalities of brain structure and vasculature. Am J Geriatr Cardiol. 2004;13:22–28. doi: 10.1111/j.1076-7460.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals. 8th edition The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Pawlak R, Skrzypiec A, Sulkowski S, Buczko W. Ethanol-induced neurotoxicity is counterbalanced by increased cell proliferation in mouse dentate gyrus. Neurosci Lett. 2002;327:83–86. doi: 10.1016/s0304-3940(02)00369-5. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain Gray and White Matter Volume Loss Accelerates with Aging in Chronic Alcoholics: A Quantitative MRI Study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21(3):521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubert G, Miñana R, Pascual M, Guerri C. Ethanol exposure during embryogenesis decreases the radial glial progenitorpool and affects the generation of neurons and astrocytes. J Neurosci Res. 2006;84:483–496. doi: 10.1002/jnr.20963. [DOI] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validiation. Brain Res. Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Toschi L, Bravo R. Changes in cyclin/proliferating cell nuclear antigen distribution during DNA repair synthesis. J Cell Biol. 1988;107:1623–38. doi: 10.1083/jcb.107.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Weiss S. Why stem cells? Science. 2000;287:1439–1441. doi: 10.1126/science.287.5457.1439. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Grever WE, Parker GC, Lyman WD. Ethanol increases fetal human neurosphere size and alters adhesion molecule gene expression. Alcohol Clin Exp Res. 2008;32(2):339–47. doi: 10.1111/j.1530-0277.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc. Natl. Acad. Sci. USA. 2006;103(44):16364–9. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Global Status Report on Alcohol 2004. World Health Organization Department of Mental Health and Substance Abuse; Geneva: 2004. [Google Scholar]