Abstract
‘Masked hypertension’ is defined as having non-elevated clinic blood pressure (BP) with elevated out-of-clinic average BP, typically determined by ambulatory BP monitoring. Approximately 15–30% of adults with non-elevated clinic BP have masked hypertension. Masked hypertension is associated with increased risks of cardiovascular morbidity and mortality compared to sustained normotension (non-elevated clinic and ambulatory BP), which is similar to or approaching the risk associated with sustained hypertension (elevated clinic and ambulatory BP). The confluence of increased cardiovascular risk and a failure to be diagnosed by the conventional approach of clinic BP measurement makes masked hypertension a significant public health concern. However, many important questions remain. First, the definition of masked hypertension varies across studies. Further, the best approach in the clinical setting to exclude masked hypertension also remains unknown. It is unclear whether home BP monitoring is an adequate substitute for ambulatory BP monitoring in identifying masked hypertension. Few studies have examined the mechanistic pathways that may explain masked hypertension. Finally, scarce data are available on the best approach to treating individuals with masked hypertension. Herein, we review the current literature on masked hypertension including definition, prevalence, clinical implications, special patient populations, correlates, issues related to diagnosis, treatment, and areas for future research.
Keywords: masked hypertension, review, ambulatory blood pressure
INTRODUCTION
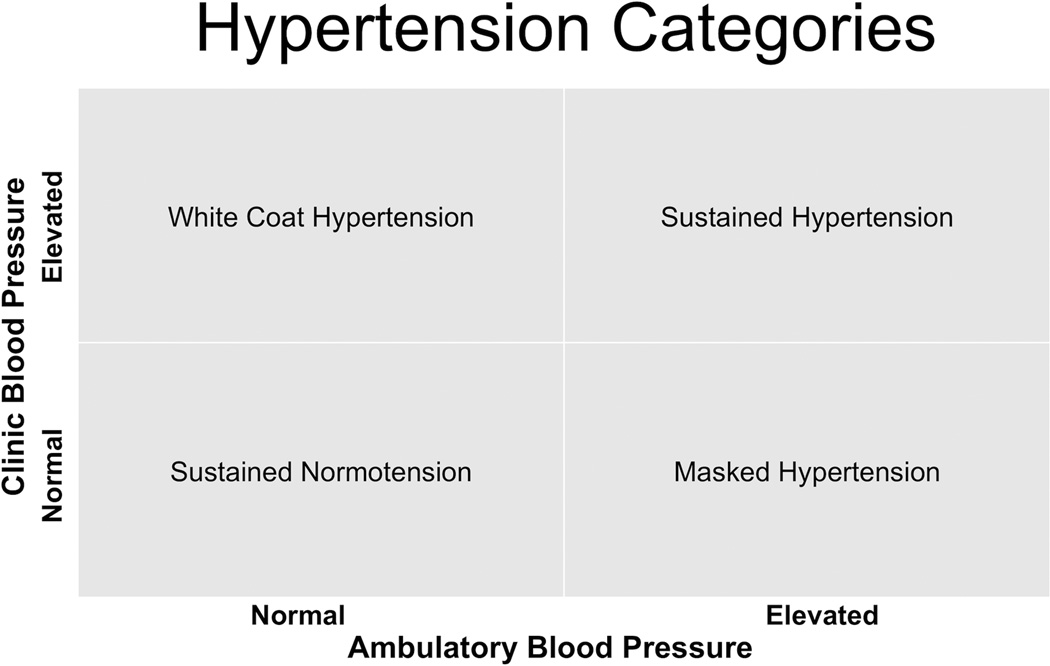
Traditionally, the diagnosis of hypertension is based on clinic blood pressure (BP), but the use of ambulatory blood pressure monitoring (ABPM) along with clinic BP has made the classification of hypertension more complex. The correlation between BP taken during routine clinic visits and ambulatory BP is only moderate1. Thus, many individuals exhibit a large discrepancy between these two measures. Figure 1 shows the four possible BP categorizations derived from the combination of clinic and average ambulatory BP. For two of the categories – sustained hypertension and sustained normotension – the classification is concordant by the two methods of BP measurement. In the other two categories – white coat hypertension and masked hypertension – the classification by the two methods is discordant. Pickering et al2 first introduced the concept of white coat hypertension more than 25 years ago, which is defined as having high clinic BP but normal ambulatory BP. White coat hypertension occurs in 15–25% of people who are thought to be hypertensive by clinic measurement3. The risk of cardiovascular disease (CVD) events in patients with white coat hypertension is relatively low compared to patients with sustained hypertension, although there has been some dispute about whether white coat hypertension is a benign phenotype3,4.
Figure 1.
Four hypertension categories based on clinic and ambulatory BP measurements. In sustained hypertension and sustained normotension, clinic BP and ambulatory BP categories agree. Sustained normotension is defined as non-elevated clinic and ambulatory BP. Sustained hypertension is defined as elevated clinic and ambulatory BP. White coat hypertension is defined as elevated clinic BP with normal ambulatory BP. Masked hypertension is defined as non-elevated clinic BP with elevated ambulatory BP. Elevated clinic BP is typically defined as ≥140/90 mmHg. Elevated ambulatory BP is typically defined as mean awake ambulatory BP ≥135/85 mmHg, although other cutpoints have been proposed (see review).
In 2002, Dr. Pickering, Dr. Schwartz and colleagues coined the term ‘masked hypertension’ to describe the condition of non-elevated clinic BP with elevated average ambulatory BP5. Unlike white coat hypertension, masked hypertension has consistently been shown to be associated with a greater risk of CV target-organ damage, CVD, and mortality, compared with sustained normotension. Although masked hypertension is increasingly being recognized as an important clinical entity, many questions remain. Herein, we review the current literature on masked hypertension including definition, prevalence, clinical implications, issues with diagnosis, correlates, treatment, and areas for future research.
References for this review were identified through searches of PubMed as of July 2013, using the key words “masked hypertension”, “isolated ambulatory hypertension”, “reverse white coat hypertension” or “white coat normotension”. Searches were limited to publications in English. We reviewed the articles identified from these searches (612 published papers) and relevant references cited in these articles. We focused our review on masked hypertension in individuals not taking antihypertensive medications. However, where appropriate, we do briefly discuss masked hypertension in individuals taking antihypertensive medications.
DEFINITION
For the diagnosis of masked hypertension, non-elevated clinic BP is typically defined as <140/90 mmHg6. However, the cutpoint for elevated out-of-clinic BP varies more than clinic BP in the published literature. Studies differ on whether mean awake ambulatory BP or mean 24-hour ambulatory BP is used to define masked hypertension4,7. The most widely used definition, by our group and other investigators8,9, for elevated ambulatory BP has been a mean awake ambulatory BP ≥135/85 mmHg. However, other definitions such as mean 24-hour ambulatory BP ≥125/79 mmHg4 or ≥130/80 mmHg10 have been used.
Nighttime (or sleep) BP has also been used as a criterion for masked hypertension given the independent predictive value of nighttime BP for cardiovascular events11. The European Society of Hypertension position paper12 incorporates elevated nighttime BP (≥120/70 mmHg) as part of the definition of masked hypertension: non-elevated clinic BP with elevated mean awake ambulatory BP and/or elevated mean 24-hour ambulatory BP, and/or elevated mean nighttime ambulatory BP. Individuals with non-elevated clinic BP and elevated mean nighttime ambulatory BP have masked nocturnal hypertension12. Individuals with non-elevated clinic BP and elevated mean nighttime ambulatory BP with normal mean awake ambulatory BP have isolated (masked) nocturnal hypertension12.
The term masked hypertension was originally used to describe individuals not taking antihypertensive medications5. However, many prevalence and outcome studies4,5,7,13 have also included participants on antihypertensive medications, which effectively combines two distinct masked hypertension populations (those not taking and those taking antihypertensive medications). The term ‘masked uncontrolled hypertensives’ has been used to describe treated individuals with non-elevated clinic but elevated ambulatory BP whereas “masked hypertension” has been used to describe untreated individuals12,14. A recent European Society of Hypertension position paper12 suggested that masked hypertension and masked uncontrolled hypertension be separately defined entities.
PREVALENCE IN THE GENERAL POPULATION
Table 1 lists large (>500 participants) prospective cohort studies of masked hypertension in individuals recruited from the general population. As Table 1 shows, the overall prevalence in the general population ranges from 8.5 to 16.6%, and the prevalence ranges from 14.7 to 30.4% when restricted to participants with non-elevated clinic BP. The variability in prevalence estimates is attributed to the heterogeneous definition of masked hypertension, and differences in the sample characteristics and populations across studies.
Table 1.
Large population cohort studies (> 500 participants) of masked hypertension: cardiovascular morbidity and mortality outcomes, and prevalence.
| Author | Population | N | Follow-up | Anti- hypertensive meds (%) |
Out-of-clinic BP measure |
Cutpoint for Ambulatory HTN* |
Prevalence † |
Outcome | Adjusted HR (95% CI)‡ |
|---|---|---|---|---|---|---|---|---|---|
| Bjorklund et al (2003)15 | 70 years, men and women from Sweden (ULSAM) | 578 | 5.9 yrs (mean) | No | Daytime ABPM | ≥135/85 mmHg | 12.0% (30.4%) | Death from CHD, stroke, and PVD, and nonfatal CHD and stroke | 2.77 (1.15 to 6.68) for MHT, 2.94 (1.49 to 5.82) for SHT |
| Ohkubo et al (2005)9 | ≥40 years, Japanese men and women from Ohasama, Japan | 1332 | 10.2 yrs (mean) | Yes (30%) | Awake ABPM | ≥135/85 mmHg | 16.6% (23.0%) | CV mortality and stroke morbidity CV mortality Stroke morbidity |
2.13 (1.38–2.29) for MHT, 2.26 (1.49–3.41) for SHT. 1.88 (0.95–3.72) for MHT, 1.94 (1.04–3.61) for SHT. 2.17 (1.31–3.60) for MHT, 2.83 (1.77–4.54) for SHT. |
| Mancia et al (2006)4 | 25–74 years, Italian men and women from Monza (PAMELA study) | 2024 | 12.3 yrs (average) | Yes (not stated) | 24-hr ABPM and HBPM |
≥125/79 mmHg (ABP) ≥135/83 mmHg (home) |
8.5% (14.7%) | CV death All-cause mortality |
Linear trend from WCT, MHT to SHT (P=0.0142) using ABPM. Linear trend from WCT, MHT to SHT (P=0.0084) using HBPM. Linear trend from WCT, MHT to SHT (P=0.1332) using ABPM. Linear trend from WCT, MHT to SHT (P=0.0560) using HBPM. |
| Hansen et al (2006)7 | 41–72 years, Danish men and women (MONICA 1 survey) | 1700 | 9.5 yrs (mean) | Yes (9%) | Daytime ABPM | ≥135/85 | 12.4% (19.7%) | CV mortality, ischemic heart disease, and stroke | 1.52 (0.91–2.54) for MHT, 2.10 (1.45–3.06) for SHT |
| Hanninen et al (2012)46 | 44–74 years, Finnish men and women (Health 2000 study) | 2046 | 7.5 yrs (mean) | Yes (23%) | HBPM | ≥135/85 | 9.2% (17.9%) | CV events All-cause mortality |
1.00 (0.60–1.67) for MHT, 1.88 (1.32–2.68) for SHT 1.28 (0.72–2.29) for MHT, 1.39 (0.90–2.16) for SHT |
Cutpoint for elevated clinic blood pressure was the same for all studies (i.e. ≥140/90 mmHg).
Prevalence in the study sample (prevalence in participants with non-elevated clinic blood pressure).
The referent group is participants with sustained normotension. Also, the most adjusted model is presented. In the PAMELA study4 exact HRs (95% CI) were not provided.
ABPM: ambulatory blood pressure monitoring, CHD: coronary heart disease, CV: cardiovascular, HBPM: home blood pressure monitoring, HTN: hypertension, HR: hazard ratio, MHT: masked hypertension, MONICA 1: MONItoring of trends and determinants in CArdiovascular disease, PAMELA: Pressioni Arteriose Monitorate e Loro Associazioni, PVD: peripheral vascular disease, SHT: sustained hypertension, ULSAM: Uppsala Longitudinal Study of Adult Men
In the U.S., population estimates of masked hypertension are scarce. In a study of adult employees conducted in New York, we found that the prevalence of masked hypertension among those with non-elevated clinic BP who were not taking antihypertensive medications and had no history of overt CVD was 15.2%8.
CLINICAL IMPLICATIONS
Bjorklund et al15 demonstrated an increased risk of cardiovascular events with masked hypertension in a study of 578 untreated 70-year old men in Sweden (Table 1). After a mean 5.9 years of follow up, compared to those with sustained normotension, the adjusted hazard ratio for cardiovascular morbidity was 2.77 (95% CI 1.15 to 6.68) in the masked hypertension group and 2.94 (95% CI 1.49 to 5.82) in the sustained hypertension group.
However, Hansen et al7 studied 1700 Danish men and women and demonstrated that compared with sustained normotension, only sustained hypertension and not masked hypertension had a statistically significant adjusted HR for cardiovascular mortality, ischemic heart disease and stroke: HR for sustained hypertension was 2.10 (95% CI 1.45–3.06) whereas the HR for masked hypertension was 1.52 (95% CI 0.91–2.54).
Meta-analyses have provided more consistent findings. Fagard and Cornelissen published a meta-analysis of seven studies involving 11,502 participants – recruited not only from the general population (4 studies), but also from primary care clinics (2 studies) and specialty clinics (one study) – on the occurrence of cardiovascular events in people with white coat hypertension, masked hypertension, and sustained hypertension3. In two studies, home BP monitoring (HBPM) was used instead of ABPM to define masked hypertension. Over a mean follow up of 8 years, compared to sustained normotension, the adjusted hazard ratios for CVD events were 1.12 (95% CI 0.84 to 1.50) for white coat hypertension, 2.00 (95% CI 1.58 to 2.52) for masked hypertension, and 2.28 (95% CI 1.87 to 2.78) for sustained hypertension. The results did not differ significantly across the studies (P=0.89).
Investigators from Denmark, Belgium, Japan and Sweden created the International Database of Ambulatory BP in Relation to Cardiovascular Outcomes or IDACO database using eligible data from four population-based cohort studies including ULSAM (Uppsala Longitudinal Study of Adult Men), Ohasama, MONICA 1 (Multinational Monitoring of Trends and Determinants in Cardiovascular Disease), and the Belgian Population Study16. Using individual-level data from this database, Hansen et al.16 examined differences in cardiovascular risk in participants with white coat hypertension, masked hypertension, and sustained hypertension in 7,030 participants. At a median follow up of 9.5 years (64,958 person years), compared to sustained normotension, the adjusted hazard ratios for cardiovascular events were 1.22 (95% CI 0.96–1.53) for white coat hypertension, 1.62 (95% CI 1.35–1.96) for masked hypertension, and 1.80 (95% CI 1.59–2.03) for sustained hypertension. The hazard ratios between masked hypertension and sustained hypertension were not significantly different (P=0.14).
Findings from most individual cohort studies, and results from two meta-analyses demonstrate that individuals with masked hypertension carry an increased cardiovascular risk compared to individuals with sustained normotension. The cardiovascular risk associated with masked hypertension approaches the risk associated with sustained hypertension.
MASKED HYPERTENSION IN SPECIAL PATIENT POPULATIONS
TREATED HYPERTENSION
The prevalence of masked hypertension may differ based on whether individuals are taking antihypertensive medications. For example, in a study by Franklin et al17 that examined 12,148 participants from the IDACO database, with masked hypertension defined as clinic BP < 140/90 mmHg and daytime ambulatory BP ≥135/85 mmHg, among non-diabetic, clinic normotensives not on antihypertensive medications, 18.8% had masked hypertension. In contrast, among individuals taking antihypertensive medications, 30.5% had masked uncontrolled hypertension. Masked uncontrolled hypertension is also associated with an increased risk of mortality. A study by Ben-Dov et al18 showed that among 2285 treated hypertensive patients, hazard ratios for all-cause mortality were 1.88 (95% CI 1.08–3.27) for masked uncontrolled hypertension and 2.02 (95% CI 1.30–3.13) for sustained hypertension compared to white-coat uncontrolled hypertension.
DIABETES
Patients with diabetes have a higher prevalence of masked hypertension than patients without diabetes17,19,20. Among 7,826 subjects from the IDACO database not taking antihypertensive medications, the prevalence of masked hypertension (clinic BP < 140/90 mmHg and daytime ambulatory BP ≥135/85 mmHg) was higher in the clinic normotensive participants with diabetes (29.3%) than without diabetes (18.8%)17. Over a median follow up period of 11.0 years, in untreated diabetics, the adjusted risk for cardiovascular events for masked hypertensives was higher than for sustained normotensives (HR 1.96, 95% CI 0.97–3.97, P=0.059), and was similar to untreated stage 1 hypertensives (HR 1.07, 95% CI 0.58–1.98, P=0.82) but less than stage 2 hypertensives (HR, 0.53, 95% CI, 0.29–0.99; P=0.048)17.
CHRONIC KIDNEY DISEASE
Few studies have examined the prevalence of masked hypertension in untreated individuals with chronic kidney disease (CKD). A study by Gorostidi et al.21 showed that among 5,693 hypertensive individuals from the Spanish ABPM Registry with CKD stages 1–5, the proportion of individuals with masked hypertension, defined by clinic BP < 140/90 mm Hg and mean 24-hour ambulatory BP≥ 130/80mm Hg, was 7.0% in all individuals, and 32.1% of patients with clinic normotension. 72.6% of the participants were on antihypertensive medications. Using data from a cross-sectional study of 617 treated hypertensive African Americans with CKD from the African American Study of Kidney Disease cohort study (AASK), Pogue et al.13 reported that the prevalence of masked uncontrolled hypertension, defined as having controlled clinic BP (<140/90 mm Hg) but elevated daytime (≥135/ 85 mm Hg) and/or elevated nighttime (≥120/70 mm Hg) ambulatory BP was 70%among individuals with controlled clinic BP. Elevated nighttime ambulatory BP with or without elevated daytime ambulatory BP was the most common phenotype (94.3%) of masked hypertension. Masked uncontrolled hypertension was associated with increased proteinuria and left ventricular hypertrophy compared to those with controlled clinic BP or white-coat hypertension. It is unclear what portion of the high prevalence of masked hypertension (70%) among individuals with controlled clinic BP was explained by antihypertensive medication use and/or by the presence of CKD.
OBSTRUCTIVE SLEEP APNEA
Baguet et al.22 examined 133 newly diagnosed obstructive sleep apnea patients, free of known cardiovascular disease and taking no medications, and found 39 patients (30.0%) exhibited masked hypertension defined as non-elevated clinic BP and a mean 24-hour ambulatory BP ≥125/80 mm Hg. Interestingly, in 61 male clinic normotensive individuals free of known cardiovascular disease and taking no medications, Drager et al.23 found that obstructive sleep apnea was also associated with masked hypertension using mean awake ambulatory BP, suggesting that sleep apnea also adversely affects ambulatory BP beyond the sleep period. In a randomized controlled trial of 36 patients with obstructive sleep apnea on no medications randomized to either continuous positive airway pressure (CPAP) or no CPAP, Drager et al.24 found that CPAP reduced clinic and ambulatory BPs, and the proportion of patients with masked hypertension fell from 39% to 5% (P = 0.04) only in the CPAP group.
OTHER CORRELATES OF MASKED HYPERTENSION
Masked hypertension shares many of the same correlates as clinic hypertension including increasing age, male gender, obesity, chronic kidney disease and diabetes25,26. However, these factors do not vary from the clinic to ambulatory settings, suggesting that there may be additional factors associated with the out-of-clinic setting that are associated with a selective increase in ambulatory BP over clinic BP.
PHYSICAL ACTIVITY
While patients are instructed to rest for several minutes prior to clinic BP measurements, ABPM takes readings while the patient is active throughout the day. Exaggerated BP responses to physical activity or exercise may increase the likelihood of masked hypertension among patients with non-elevated clinic BP. Sharman et al examined the prevalence of masked hypertension among 72 untreated participants with a hypertensive response to exercise (defined as clinic BP <140/90 mmHg and exercise SBP ≥ 210 mmHg in men or ≥ 190 mmHg in women, or DBP ≥ 105 mmHg)27. In this group, masked hypertension was highly prevalent (42 of 72 participants, 58%) and was associated with increased left ventricular mass index compared to participants with clinic normotension without a hypertensive response to exercise. In a group of 85 sedentary patients with diabetes and clinic normotension, Akilli et al demonstrated that masked hypertension was associated with decreased exercise capacity and exaggerated BP response on exercise treadmill testing25. In a case-control study of patients with diabetes, Kramer et al showed that in 37 patients with sustained normotension and 24 patients with masked hypertension who all underwent exercise treadmill testing, the proportion of patients who reached a systolic peak value of > 180 mmHg was higher in patients with masked hypertension (70.8%) than in those with sustained normotension (21.1%) and a peak SBP of >170 mmHg had 70% sensitivity and 73% specificity for identifying patients with masked hypertension28. It is possible that ABPM is detecting abnormally elevated BP in response to physical activity in the naturalistic setting that is not detected with clinic BP alone.
MENTAL STRESS
In addition to physical stress, out-of-clinic BP measurements may also be influenced by psychological stress. To determine if psychosocial stress at work affects the prevalence of masked hypertension, Trudel et al obtained BP measurements from 2,357 workers (mean age 44 years, 61% women)29. In men, having both high psychological demands and high decision latitude was associated with an odds ratio of 2.07 (95% CI 1.30 − 3.31) for masked hypertension compared to the passive group. Interestingly, among women, there was no significant association between high psychological demands/high decision latitude and masked hypertension. In a study looking at working conditions and masked hypertension by Landsbergis et al., 45 men and 119 women hospital and home care employees/volunteers were studied. Masked hypertension was observed in 24% of men and 17.6% of women with non-elevated clinic BPs, and was associated with evening, night and rotating shiftwork (odds ratio of 8.2, 95% CI 2.1 to 40.3). A combination of job strain and effort-reward imbalance had an odds ratio of 3.0 (95% CI 1.0 to 8.6) for masked hypertension after adjusting for age30. In a meta-analysis of 22 cross-sectional studies looking at job strain and ambulatory BP, Landsbergis et al.31 found that job strain was associated with a 3.4 mmHg (95% CI 2.0 to 4.8) increase in work systolic BP. These studies demonstrate an association between out-of-clinic job related stress and BP which may increase the likelihood of masked hypertension.
SMOKING AND ALCOHOL
In the meta-analysis by Verberk et al.26, the investigators found that participants with masked hypertension were more often smokers than were participants with either sustained normotension, white coat hypertension or sustained hypertension. Ishikawa et al.32 demonstrated that among 405 treated hypertensive patients with well-controlled clinic BPs, 246 patients (60.7%) had masked hypertension in the morning by HBPM. Patients who self-reported having at least one alcohol beverage every day, had an increased odds ratio (1.76, 95% CI 0.99 to 3.12, p=0.05) for morning-masked hypertension versus those who did not drink every day.
These out-of-clinic correlates of masked hypertension support the hypothesis that environmental factors are associated with BP in ways that are not detectable by clinic BP alone. Further research is required to determine if these associations are causal.
BIOLOGIC CORRELATES
Arterial stiffness, inflammation and endothelial dysfunction are associated with masked hypertension. Veerabhadrappa et al.33 studied endothelial function and inflammation in 50 African Americans with clinic prehypertension and found that the 58% of patients with masked hypertension had impaired endothelial function and elevated levels of high sensitivity C-reactive protein compared to patients with true prehypertension (i.e., without elevated ambulatory BP). It is unclear whether these factors are selectively associated with masked hypertension or are also associated with sustained hypertension. It is possible that these biological correlates affect ambulatory BP first then clinic BP later, suggesting that masked hypertension is a precursor of sustained hypertension, which is a natural history we previously proposed8. Another possibility is that these biological factors interact with clinical and environmental factors to elevate BP outside the clinic setting in patients with masked hypertension. Because these associations are cross-sectional, causal direction cannot be ascertained. It is certainly possible that masked hypertension induces inflammation and endothelial function, rather than the other way around.
ISSUES RELATED TO PROPER DIAGNOSIS
In addition to the variation in the cutpoint used to define elevated ABP across studies, another source of variability in the diagnosis of masked hypertension is the number of valid ABPM readings required during either the awake or sleep period or 24-hour period4,7–9.
An additional issue is whether HBPM is an acceptable alternative to ABPM for measuring out-of-clinic BPs. As noted above, the original definition of masked hypertension by Pickering et al5 indicated ABPM for the out-of-clinic BP assessment; however, some studies have used HBPM instead of ABPM34–37. Viera et al10 evaluated the congruence of the diagnosis of masked hypertension comparing ambulatory and home BP monitoring in 50 participants with normal or elevated clinic BP (110–159/70–99 mmHg). Among the entire sample, ABPM and HBPM had only a 46.7% agreement in diagnosing masked hypertension when paired with their corresponding clinic BP measurement. In the PAMELA study4, home BP was elevated (defined as ≥135/83mmHg) in only half of the patients diagnosed with masked hypertension by mean 24-hour ambulatory BP (defined as ≥125/79 mmHg), again suggesting limited agreement between ambulatory and home BP readings in diagnosing masked hypertension. Finally, given that some studies have incorporated sleep BP in the definition of masked hypertension12,13, HBPM may not be an ideal methodology for identifying masked hypertension due to the inability of most devices to measure BP during sleep.
Despite the poor agreement between ABPM and HBPM in diagnosing masked hypertension, the diagnosis of masked hypertension by ABPM versus HBPM may have similar prognostic value. Angeli et al performed a meta-analysis on the risk of major cardiovascular disease in participants with masked hypertension stratified by out-of-clinic BP measurements with either ABPM or HBPM38. They found the hazard ratio of major cardiovascular disease to be 2.00 (95% CI 1.54 to 2.60, P < 0.001) for masked hypertension diagnosed by ABPM and 2.13 (95% CI 1.35 to 3.35, P = 0.001) for masked hypertension diagnosed by HBPM suggesting the risk associated with masked hypertension may not be affected by how out-of-clinic BP is measured. In the PAMELA study4, even though there was limited agreement between home and ambulatory BP readings in diagnosing masked hypertension, left ventricular mass index was similar between the participants who were diagnosed with masked hypertension by either 24-hour ABPM or HBPM.
The poor agreement between ABPM and HBPM in diagnosing masked hypertension while having similar relations with end-organ damage and cardiovascular outcomes is interesting. It could imply that both forms of out-of-clinic BP measurements are identifying non-overlapping populations of people with masked hypertension and similar cardiovascular risk. Alternatively and probably less likely, it could reflect the inability of either measurement modality to pick up all cases of masked hypertension with a single set of measurements. As these studies did not examine the contribution of masked nocturnal hypertension, which cannot be determined by HBPM, further research is needed to better elucidate these issues.
An equally important, but perhaps underappreciated issue is how clinic BP is ascertained. Myers et al compared conventional (manual) versus automated measurement of BP in 555 patients with systolic hypertension and no serious comorbidities in 67 primary care practices and found that the average manual clinic BP (149.5/81.4mmHg) was significantly higher than automated measurements (135.6/77.7 mmHg)39. These findings could reflect attenuation of the white coat response with the automated device. When compared to 24-hour ambulatory monitoring as a gold standard, the accuracy of automated clinic BP measurements was significantly better than manual BP measurements. In a separate study, Myers et al.40 demonstrated that the presence of masked hypertension was lower using automated clinic BP measurements. Across three clinic visits, masked hypertension was identified in a total of 42.1% single patient visits for automated clinic BP, and 60.7%single patient visits for manual clinic BP. It is plausible that the lower prevalence of masked hypertension associated with automated clinic BP reflects the greater correlation of ambulatory BP with automated versus manual clinic BP.
PRACTICAL APPROACH TO THE DIAGNOSIS OF MASKED HYPERTENSION
There is limited evidence on the best approach for detecting masked hypertension in individuals not taking antihypertensive medications. One approach would be to perform ABPM on everyone with non-elevated clinic BP. In 2011, based on a cost-effectiveness analysis, the National Institute for Health and Care Excellence (NICE) recommended that all patients with elevated clinic BP have ABPM to exclude white coat hypertension41. No study has been conducted on whether a parallel approach (i.e. performing ABPM in individuals with clinic normotension) would also be cost effective.
Given the added cost of ABPM, another approach would be to target screening to individuals whose risk for having masked hypertension exceeds some pre-specified threshold. Our group has previously examined the diagnostic overlap between masked hypertension and prehypertension (SBP 120–139mmHg or DBP 80–89mmHg)8. In a worksite-based study of 813 participants not on antihypertensive medications and free of cardiovascular disease, 769 participants had non-elevated clinic BP. The overall prevalence of masked hypertension in the participants with non-elevated clinic BP was 15.2% using a cutoff of 135/85 mmHg for mean awake ambulatory BP. In the subgroup of participants with prehypertension, the prevalence of masked hypertension increased to 34% and reached 52% in the higher prehypertensive range (SBP 130–139 mmHg or DBP 80–89 mmHg), whereas the prevalence of masked hypertension was only 3.9% in participants with normal clinic BP (SBP < 120 mmHg and DBP < 80 mmHg). Other studies have similarly shown that clinic BPs in the upper prehypertensive range predict masked hypertension42.
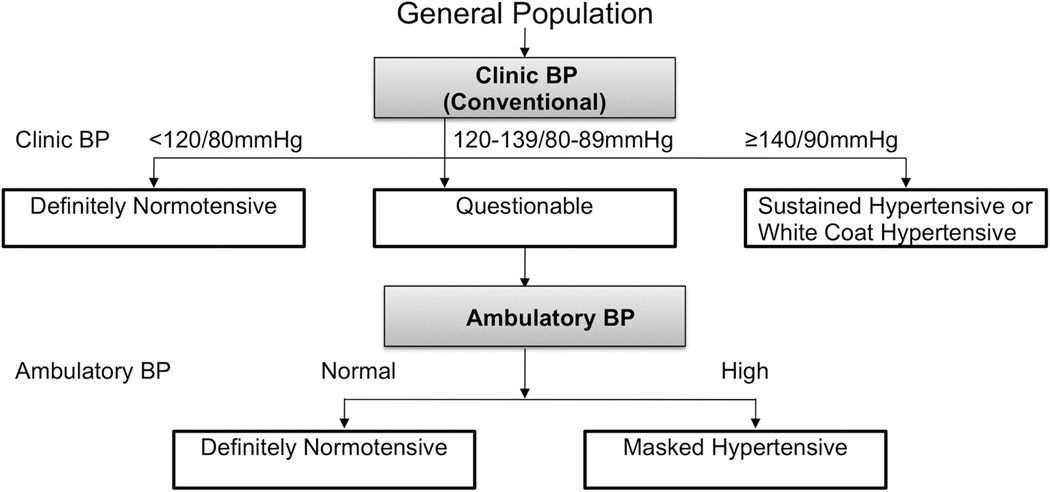
Therefore, a diagnostic approach may be to test individuals for masked hypertension if their clinic BP is in the prehypertensive range (Figure 2). If clinic BP is <120/80 mmHg there may be no need to do ABPM since the prevalence of masked hypertension in this group is low. An additional approach would be to perform targeted testing among untreated individuals who have a high prevalence of masked hypertension, including those with diabetes, obstructive sleep apnea, and possibly CKD.
Figure 2.
A possible diagnostic approach for the identification of masked hypertension in the general population. BP: blood pressure
Important questions remain including whether non-research clinic BPs obtained in routine practice can be used, whether this approach is valid across a broad range of age, gender, and racial/ethnic groups, and whether HBPM is useful as an intermediary assessment tool for deciding on whom to perform ABPM.
TREATMENT
One approach to the treatment of masked hypertension would be to reduce ambulatory BP in masked hypertensives, using antihypertensive medications, despite the absence of elevated clinic BP and then perform periodic ABPM to determine on-treatment ambulatory BP. While it is assumed that lowering ambulatory BP will have similar clinical benefits to lowering clinic BP43, it is still unclear if the treatment of masked hypertension is associated with a reduction in clinical adverse events. In a randomized, double-blind, placebo-controlled study, Hare et al44 examined the impact of fixed dose spironolactone (25mg daily) in 115 untreated participants without a history of hypertension, but with a hypertensive response to exercise (exercise SBP ≥ 210 mmHg in men or ≥ 190 mmHg in women, or DBP ≥ 105 mmHg). In a subgroup analysis of the 40% of participants with masked hypertension based on daytime ABPM using a cutoff of 135/85 mmHg, the spironolactone group showed significantly greater reductions in exercise systolic BP (−10.0±12.9 mmHg vs. 0.3±8.7 mmHg, P < 0.01) and 24-hour ambulatory pulse pressure (−2.4 ± 4.7 mmHg vs. 2.1 ± 8.4 mmHg, P < 0.05); effects on 24-hour systolic and diastolic ambulatory BP were not reported. However, no difference in left ventricular mass index reduction was observed between the spironolactone and placebo groups after three months.
Another treatment paradigm is to consider masked hypertension a prognostic marker of increased CVD and mortality, and rather than treating ambulatory BP, the focus would be on aggressively treating the modifiable risk factors associated with masked hypertension including obesity, diabetes, sleep apnea, and avoidance of smoking and alcohol intake. Masked hypertension may identify a “high risk” group that requires aggressive CVD risk factor control.
Given that there is some evidence that masked hypertension predicts subsequent sustained hypertension45, a third paradigm is to wait for individuals with masked hypertension to manifest sustained hypertension. This approach would include performing clinic BP measurements and ABPM at frequent intervals to diagnose sustained hypertension or exclude other phenotypes such as sustained normotension, and less commonly white coat hypertension, which may also follow an initial diagnosis of masked hypertension45.
FUTURE RESEARCH
Many important questions regarding masked hypertension remain unanswered. Table 2 summarizes relevant questions. In order to further unmask the extent of masked hypertension’s public health impact and establish effective clinical screening and treatment guidelines, these questions should be addressed through focused research.
Table 2.
Important questions that remain unanswered in the area of masked hypertension
| What ambulatory blood pressure cutpoint should be used in the definition of masked hypertension? |
| How many clinic (and over how many visits) and ambulatory readings should be obtained for the diagnosis of masked hypertension? |
| Which ambulatory blood pressure monitoring time frame (daytime, nighttime, or 24 hours) should be used in the definition of masked hypertension? |
| Should manual or automated devices be used for clinic blood pressure assessment for the diagnosis of masked hypertension? |
| What diagnostic strategy is most accurate and cost-effective for the detection of masked hypertension? |
| Should home blood pressure monitoring be used to diagnose masked hypertension? |
| Does treatment of masked hypertension improve clinical outcomes? |
| Do patients with masked (uncontrolled) hypertension taking antihypertensive medications have the same prognosis as patients with masked hypertension on no antihypertensive medications? |
| Does treatment of these groups have similar benefits in reducing end-organ damage and adverse clinical outcomes? |
| What are the mechanisms that underlie masked hypertension? |
CONCLUSIONS
Masked hypertension is associated with an increased risk of cardiovascular morbidity and mortality compared to sustained normotension. If we assume that the prevalence of masked hypertension ranges from 15 to 30%in the U.S. adult population of approximately 160 million with non-elevated clinic BP, then currently it is estimated that 24 to 48 million American adults may be at increased risk for heart disease and stroke due to masked hypertension. There are fundamental issues about masked hypertension that need to be addressed. These include standardizing the definition for diagnosis, comparing the cost-effectiveness of strategies for screening and detection, gaining further insight into the mechanisms that underlie masked hypertension, and determining the optimal treatment strategy for treating masked hypertension. Until these issues are addressed, masked hypertension will likely remain outside mainstream clinical care, and this public health concern will be largely unaddressed.
Acknowledgements
None.
Funding sources
This work was supported by P01-HL047540 (PI: JE Schwartz) and R01 HL098604 (PI: AJ Viera) from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH). The research was also supported by T32-HL007854-15, General Clinical Research Center grant MO1-RR10710 (to Stony Brook University), an NIH Diversity Supplement P01-HL047540-19S1 (KM Diaz), and the National Center for Advancing Translational Sciences, NIH, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156. The contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Conflicts of interest.
The authors have no conflicts of interest.
References
- 1.Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009 Feb;27(2):280–286. doi: 10.1097/HJH.0b013e32831b9e6b. [DOI] [PubMed] [Google Scholar]
- 2.Pickering TG. How Common Is White Coat Hypertension? JAMA. American Medical Association. 1988 Jan 8;259(2):225–228. [PubMed] [Google Scholar]
- 3.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens [Internet] 2007 Nov;25(11):2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 5.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. Am Heart Assoc. 2002;40(6):795–796. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005 Apr 1;23(4):697. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 7.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006 Mar;19(3):243–250. doi: 10.1016/j.amjhyper.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Shimbo D, Newman JD, Schwartz JE. Masked hypertension and prehypertension: diagnostic overlap and interrelationships with left ventricular mass: the Masked Hypertension Study. Am J Hypertens [Internet] 2012 Jun;25(6):664–671. doi: 10.1038/ajh.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, et al. Prognosis of "masked" hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. Journal of the American College of Cardiology [Internet] 2005 Aug 2;46(3):508–515. doi: 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 10.Viera AJ, Hinderliter AL, Kshirsagar AV, Fine J, Dominik R. Reproducibility of masked hypertension in adults with untreated borderline office blood pressure: comparison of ambulatory and home monitoring. Am J Hypertens [Internet] 2010 Nov;23(11):1190–1197. doi: 10.1038/ajh.2010.158. [DOI] [PubMed] [Google Scholar]
- 11.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013 Sep;:1. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 13.Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, et al. Hypertension. 1. Vol. 53. Lippincott Williams & Wilkins; 2009. Jan, Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease; pp. 20–27. [DOI] [PubMed] [Google Scholar]
- 14.Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Inoue R, et al. Prevalence of masked uncontrolled and treated white-coat hypertension defined according to the average of morning and evening home blood pressure value: from the Japan Home versus Office Measurement Evaluation Study. Blood Press Monit. 2005;10:311–316. doi: 10.1097/00126097-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Bjorklund K, Lind L, Zethelius B, Andren B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation [Internet] 2003 Mar 11;107(9):1297–1302. doi: 10.1161/01.cir.0000054622.45012.12. [DOI] [PubMed] [Google Scholar]
- 16.Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007 Aug;25(8):1554–1564. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 17.Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013 May;61(5):964–971. doi: 10.1161/HYPERTENSIONAHA.111.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Dov IZ, Kark JD, Mekler J, Shaked E, Bursztyn M. The white coat phenomenon is benign in referred treated patients: a 14-year ambulatory blood pressure mortality study. J Hypertens. 2008;26:699–705. doi: 10.1097/HJH.0b013e3282f4b3bf. [DOI] [PubMed] [Google Scholar]
- 19.Leitao CB, Canani LH, Silveiro SP, Gross JL. Ambulatory blood pressure monitoring and type 2 diabetes mellitus. Arquivos brasileiros de cardiologia. 2007;89:315–321. 347–354. doi: 10.1590/s0066-782x2007001700012. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Dov IZ, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Increased prevalence of masked blood pressure elevations in treated diabetic subjects. Arch Intern Med. 2007;167:2139–2142. doi: 10.1001/archinte.167.19.2139. [DOI] [PubMed] [Google Scholar]
- 21.Gorostidi M, Sarafidis PA, la Sierra de A, Segura J, la Cruz de JJ, Banegas JR, et al. YAJKD. 2. Vol. 62. Elsevier Inc; 2013. Aug 1, 1-s2.0-S0272638613007026-main; pp. 285–294. [Google Scholar]
- 22.Baguet JP, Levy P, Barone-Rochette G, Tamisier R, Pierre H, Peeters M, et al. Masked hypertension in obstructive sleep apnea syndrome. J Hypertens. 2008 May;26(5):885–892. doi: 10.1097/HJH.0b013e3282f55049. [DOI] [PubMed] [Google Scholar]
- 23.Drager LF, Diegues-Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010 Mar;23(3):249–254. doi: 10.1038/ajh.2009.246. [DOI] [PubMed] [Google Scholar]
- 24.Drager LF, Pedrosa RP, Diniz PM, Diegues-Silva L, Marcondes B, Couto RB, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–555. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 25.Akilli H, Kayrak M, Arıbas A, Tekinalp M, Ayhan SS, Gündüz M, et al. The relationship between exercise capacity and masked hypertension in sedentary patients with diabetes mellitus. Clin Exp Hypertens. 2013 Jun 4; doi: 10.3109/10641963.2013.783047. [DOI] [PubMed] [Google Scholar]
- 26.Verberk WJ, Kessels AGH, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008 Sep;21(9):969–975. doi: 10.1038/ajh.2008.221. [DOI] [PubMed] [Google Scholar]
- 27.Sharman JE, Hare JL, Thomas S, Davies JE, Leano R, Jenkins C, et al. Association of masked hypertension and left ventricular remodeling with the hypertensive response to exercise. Am J Hypertens. 2011 Aug;24(8):898–903. doi: 10.1038/ajh.2011.75. [DOI] [PubMed] [Google Scholar]
- 28.Kramer CK, Leitão CB, Canani LH, Ricardo ED, Pinto LC, Gross JL. Blood pressure responses to exercise in type II diabetes mellitus patients with masked hypertension. J Hum Hypertens. 2009;23:620–622. doi: 10.1038/jhh.2009.24. [DOI] [PubMed] [Google Scholar]
- 29.Trudel X, Brisson C, Milot A. Job strain and masked hypertension. Psychosom Med. 2010;72:786–793. doi: 10.1097/PSY.0b013e3181eaf327. [DOI] [PubMed] [Google Scholar]
- 30.Landsbergis PA, Travis A, Schnall PL. Working conditions and masked hypertension. High Blood Press Cardiovasc Prev. 2013 Jun;20(2):69–76. doi: 10.1007/s40292-013-0015-2. [DOI] [PubMed] [Google Scholar]
- 31.Landsbergis PA, Dobson M, Koutsouras G, Schnall P. Am J Public Health. 3. Vol. 103. American Public Health Association; 2013. Mar, Job Strain and Ambulatory Blood Pressure: A Meta-Analysis and Systematic Review; pp. e61–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa J, Kario K, Eguchi K, Morinari M, Hoshide S, Ishikawa S, et al. Regular alcohol drinking is a determinant of masked morning hypertension detected by home blood pressure monitoring in medicated hypertensive patients with well-controlled clinic blood pressure: the Jichi Morning Hypertension Research (J-MORE) study. Hypertens Res. 2006 Sep;29(9):679–686. doi: 10.1291/hypres.29.679. [DOI] [PubMed] [Google Scholar]
- 33.Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, et al. Endothelial-dependent flow-mediated dilation in African Americans with masked-hypertension. Am J Hypertens. 2011 Oct;24(10):1102–1107. doi: 10.1038/ajh.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, et al. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA: the journal of the American Medical Association. 2004;291:1342–1349. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo S, Wen W, Li-Yuan M, Shu-Yu W, Yi-Xin W. Home blood pressure measurement in prehypertension and untreated hypertension: comparison with ambulatory blood pressure monitoring and office blood pressure. Blood Press Monit. 2009;14:245–250. doi: 10.1097/MBP.0b013e328332fd25. [DOI] [PubMed] [Google Scholar]
- 36.Stergiou GS, Argyraki KK, Moyssakis I, Mastorantonakis SE, Achimastos AD, Karamanos VG, et al. Home blood pressure is as reliable as ambulatory blood pressure in predicting target-organ damage in hypertension. Am J Hypertens. 2007;20:616–621. doi: 10.1016/j.amjhyper.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Matsui Y, Eguchi K, Ishikawa J, Hoshide S, Shimada K, Kario K. Subclinical arterial damage in untreated masked hypertensive subjects detected by home blood pressure measurement. Am J Hypertens. 2007 Apr;20(4):385–391. doi: 10.1016/j.amjhyper.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Angeli F, Reboldi G, Verdecchia P. Masked hypertension: evaluation, prognosis, and treatment. Am J Hypertens [Internet] 2010 Sep;23(9):941–948. doi: 10.1038/ajh.2010.112. [DOI] [PubMed] [Google Scholar]
- 39.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, et al. BMJ. feb07 1. BMJ Group; 2011. Feb 7, 342. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial; pp. d286–d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. The conventional versus automated measurement of blood pressure in the office (CAMBO) trial: masked hypertension sub-study. J Hypertens. 2012 Oct;30(10):1937–1941. doi: 10.1097/HJH.0b013e328356abd3. [DOI] [PubMed] [Google Scholar]
- 41.National Clinical Guideline Centre (UK) Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. London: Royal College of Physicians (UK); 2011. [PubMed] [Google Scholar]
- 42.Hanninen M-RA, Niiranen TJ, Puukka PJ, Mattila AK, Jula AM. Determinants of masked hypertension in the general population: the Finn-Home study. J Hypertens. 2011;29:1880–1888. doi: 10.1097/HJH.0b013e32834a98ba. [DOI] [PubMed] [Google Scholar]
- 43.Turnbull F, Neal B, Algert C, Chalmers J, Woodward M, Macmahon S, et al. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. The Lancet. 2000 Dec;356(9246):1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 44.Hare JL, Sharman JE, Leano R, Jenkins C, Wright L, Marwick TH. Impact of spironolactone on vascular, myocardial, and functional parameters in untreated patients with a hypertensive response to exercise. Am J Hypertens. 2013;26:691–699. doi: 10.1093/ajh/hpt008. [DOI] [PubMed] [Google Scholar]
- 45.Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Friz HP, et al. Long-Term Risk of Sustained Hypertension in White-Coat or Masked Hypertension. Hypertension. 2009 Jul 22;54(2):226–232. doi: 10.1161/HYPERTENSIONAHA.109.129882. [DOI] [PubMed] [Google Scholar]
- 46.Hanninen M-RA, Niiranen TJ, Puukka PJ, Johansson J, Jula AM. Prognostic significance of masked and white-coat hypertension in the general population: the Finn-Home. Study. J Hypertens. 2012;30:705–712. doi: 10.1097/HJH.0b013e328350a69b. [DOI] [PubMed] [Google Scholar]