Abstract
Objective
To evaluate the feasibility and hearing outcome of a biocompatible degradable dexamethasone releasing implant for continuous drug delivery to the round window membrane in patients with idiopathic sudden sensorineural hearing loss (ISSHL) and insufficient recovery after systemic high dose glucocorticoid therapy.
Patients
Five patients with profound or moderate to severe hearing loss after systemic high dose prednisolone for ISSHL received local salvage therapy with a controlled release dexamethasone implant in the middle ear.
Intervention
Pieces of a sterile rod shaped poly(D,L-lactide-co-glycolide) PLGA polymer matrix containing a total of 0.7 mg dexamethasone which is approved for intravitreal use were implanted into the round window niche.
Main Outcome Measure(s)
Intraoperative handling and feasibility and hearing recovery as measured by change in pure tone threshold, final word recognition score and categories of improvement were evaluated.
Results
The implants were surgically placed without major difficulties. The mean hearing threshold significantly improved at follow up by 31±31 dB HL (from 94±27 to 63±36 dB HL; p<0.05). Two of five patients recovered completely. One patient showed partial hearing recovery with serviceable hearing.
Conclusions
Although no drugs are currently approved for local therapy of inner ear disorders, there is increasing evidence that intratympanic glucocorticoids are effective as salvage therapy in ISSHL. The present study has shown encouraging results with a biodegradable polymer delivery system, demonstrating the translation of preclinical studies with controlled drug delivery into clinical practice.
Keywords: controlled release, glucocorticosteroids, steroids, dexamethasone, sudden hearing loss, local drug delivery, round window, cochlea, rescue, salvage treatment, human
Introduction
Intratympanic glucocorticoid application appears to be beneficial as salvage (rescue) therapy in idiopathic sudden sensorineual hearing loss (ISSHL). Several application strategies have been suggested. Due to their feasibility, the most commonly used are single or repeated injections through the tympanic membrane (1,2). The round window membrane (RWM), however, shows various degrees of obstruction in 1/5th to 1/3rd of the cases (3,4). “Blind” intratympanic injections thus involve the risk of the drug not reaching the RWM. Therefore, standard or endoscopic tympanoscopy have been suggested for middle ear exploration and assessing the necessity for removing RMW obstructions (5-9). The width and shape of the ear canal and the limitations of the mini-endoscopes, however, do not in all cases allow a sufficient visualization of the RWM. Standard middle ear exploration with raising a tympanomeatal flap may cause bleeding (especially in patients on aspirin or coumarin derivates) and subsequent blockage of the RWM.
Since the time the drug is in contact with the RWM essentially determines the drug concentration in the inner ear (10,11), repeated single shot applications or continuous drug delivery have been suggested (12,13). Continuous drug delivery can be realized through implantation of a catheter into the middle ear connected to an external pump (14-16) or by biopolymers with controlled drug release properties of which a variety is currently been investigated for inner ear drug applications (17-20). Currently, however, no controlled release biopolymer is approved for local drug delivery to the inner ear. Therefore, in a limited number of patients with ISSHL and insufficient recovery after systemic high dose glucocorticoid therapy, we adopted an approved intravitreal implant for continuous dexamethasone application to the inner by implantation into the RW niche.
Methods
Between September 2011 and December 2012 five patients were treated with controlled release dexamethasone (DEX) implants in the RW niche as salvage therapy of ISSHL. All patients fulfilled the diagnostic criteria for ISSHL (2). In one patient the sudden hearing loss showed a possible association with an upper respiratory infection (patient 5, table 1). All patients were initially treated with high dose intravenous prednisolone (250 mg/d [200 mg/d for <70kg body weight]) over three days with subsequent dose reduction (2d 150mg, 2d 100mg, 2d 50mg, 2d 25 mg, 2d 12.5mg). Key demographic figures and patient characteristics are described in detail in tables 1 and 2.
Table 1. Summary of demographic data and medical conditions.
| Nr. | M/F | Age | T | V | Db Pre |
Comments | ||
|---|---|---|---|---|---|---|---|---|
| Intraoperative findings | RWM obstruction | Secondary diagnoses | ||||||
| 1 | M | 76 | y | n | 5 | Intraoperative bleeding under aspirin and exostosis surgery | partial | Hypertension, hyperlipoproteinemia |
| 2 | M | 60 | y | n | 7 | Narrow ear canal, meatoplasty, mild bleeding under aspirin | none | Hypertension, dilated cardiomyopathia, IDDM, adipositas, hyperlipoproteinemia |
| 3 | M | 79 | y | n | 9 | Mild intraoperative bleeding under aspirin | subtotal | Coronary heart disease with St. p. CABG), bilateral ICA-stenosis (St. p. ICA surgery with patches), hypertension |
| 4 | M | 64 | y | n | 13 | Mild intraoperative bleeding in tympanic cavity after removal of adhesions and under aspirin | partial | Coronary heart disease with St. p. CABG), hypertension, IDDM, hyperlipoproteinemia |
| 5 | F | 59 | y | n | 7 | Mild intraoperative bleeding in tympanic cavity after removal of adhesions | partial | Hypertension, St. p. ISSHL contralateral; St. p. upper respiratory tract infection |
Nr.: Patient-Number; M: male; F: female; V/T: presence of vertigo/tinnitus at onset of ISSHL yes/no; DbPre: days before implantation of controlled release dexamethasone carrier; IDDM: insulin dependent diabetes mellitus; St. p.: status post; CABG: coronary artery bypass graft; ICA: internal carotid artery
Table 2. Summary of audiological baseline and outcome data.
| Nr. | 4PTA (dB HL) | WRS-65 (%) | SDmax (%) (at dB SPL) | DIFF 4PTA contr (dB) | Recovery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ini | pre | fin | f/u | ini | pre | fin | f/u | ini | pre | fin | f/u | ini | pre | fin | f/u | ||
| 1 | 116 | 116 | 120 | 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 80 | 80 | 84 | 84 | no |
| 2 | 84 | 116 | 83 | 74 | na | 0 | na | 0 | na | 0 | na | 5 (95) | 75 | 108 | 74 | 65 | no |
| 3 | 83 | 68 | 41 | 40 | na | n.a. | na | 65 | na | nd | 90 (70) | 100 (95) | 43 | 28 | 1 | 0 | complete |
| 4 | 64 | 61 | 63 | 48 | na | 0 | na | 40 | na | 95 (100) | 75 (95) | 100 (95) | 43 | 40 | 41 | 26 | partial/serviceable |
| 5 | 98 | 109 | 55 | 33 | 0 | 0 | 5 | 50 | 0 | 0 | 95 (95) | 100 (80) | 73 | 84 | 30 | 8 | complete |
| Mean | 89 | 94 | 72 | 63 | 31 | 61 | 63 | 68 | 46 | 37 | |||||||
| SD | 19 | 27 | 31 | 36 | 30 | 53 | 18 | 33 | 34 | 36 | |||||||
Nr.: Patient-Number; ini: initial presentation (before initial systemic therapy); pre: before reserve therapy with implantation of controlled release dexamethasone carrier; fin: final; f/u: long term follow-up. 4PTA: (pure tone average from 0.5-4kHz, WRS-65: % of monosyllables correctly understood at 65dB SPL in quiet, SDmax (%) at (dB): maximum number of monosyllables understood in quiet (in %) at XX dB SPL, DIFF 4PTA contr: Difference in pure tone average from affected to (not acutely affected) contralateral ear; na: not available; SD: standard deviation
The various therapeutic options for salvage therapy (see introduction) were discussed with the patient and informed consent was given for this individual treatment regimen involving a DEX releasing implant, approved for intravitreal use OZURDEX® (Allergan Inc., Irvine, CA, USA). The implant contains 0.7 mg dexamethasone in a poly(D,L-lactide-co-glycolide) PLGA polymer matrix containing a mixture of polymer chains with free and esterified carboxylic end groups without a preservative. The PLGA matrix slowly degrades to lactic acid and glycolic acid. The implant is approximately 0.46mm in diameter and 6mm in length. For intravitreal application, the rod-shaped implant is injected from a device consisting of a hollow stainless steel needle in disposable applicator. For the adopted local controlled release drug delivery to the inner ear the implant was removed from its applicator and cut into 3 to 5 pieces of approximately 0.8 to 1.5mm each, according to the size of the human RW niche (1.55 mm; range 0.8-1.6 mm by 1.2 mm; range 0.8-1.6 mm (21).
After raising a tympanomeatal flap, the middle ear and especially the oval and RW niches were inspected with the operation microscope and an endoscope (1.7mm, 30°). After no signs of an oval or RW fistula were found, the DEX-PLGA controlled release implant pieces were placed in the RW niche through transportation with a 0.7 mm suction tip. The implants were held in place with drops of fibrin glue. The tympanomeatal flap was then replaced and a standard ear canal dressing was inserted. Dressing and sutures were removed 8-10 days after the procedure. The surgery can be performed with local anesthesia. In the presented cases, however, the patients requested general anesthesia. Pure-tone and speech audiometry (German Freiburger monosyllables) were performed with an Auritec AT900 clinical audiometer (Hamburg, Germany) and Bayer Dynamic DT48A calibrated headphones (Berlin, Germany) in a sound-attenuated booth (Industrial Acoustics Company, Niederkrüchten, Germany). Thresholds at frequencies of 0.5, 1, 2, and 4 kHz (4PTA) were averaged.
Results
The implants were surgically placed without major difficulties (figure 1). Special care, however, has to be taken when placing the implants onto the RWM. The pieces of the rigid implants may show sharp edges at their breaking points. No complications like infection, worsening of hearing or tympanic membrane perforation were observed. In the eye, the slow disintegration of the implants can be evaluated by funduscopy initially observing an opaque, round cylinder, which becomes smaller, translucent, and fragmented from day 60 onward (22). In the ear, the process of disintegration could not be evaluated, since for ethical reasons the middle ear was not re-opened.
Fig. 1.
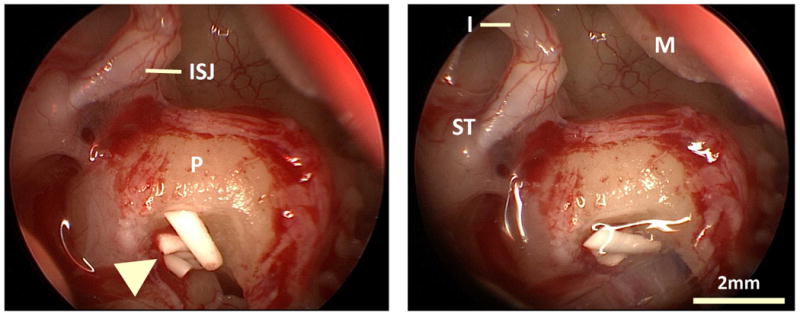
Intraoperative endoscopic of view showing pieces of the solid polymer drug delivery system (arrow head) in the RW niche. ISJ: Incudostapedial joint; P: promontory; I: incus (long process); ST: stapedial tendon; M: malleus handle. Right: implant position fixed with fibrin glue.
The mean hearing threshold (4PTA) significantly improved at follow up (217±62 days after implantation) by 31±31 dB HL (from 94±27 to 63±36 dB HL; p<0.05, paired student t-test, MS EXCEL 2011 for MAC version 14.3.9). Two of five patients recovered completely. One of these two patients (nr. 5 in table 1) had a recurrent episode of sudden hearing loss six month after the initial intratympanic treatment, which again was possibly associated with an upper respiratory tract infection. Hearing loss recovered completely within one week during systemic therapy with steroids. One patient showed partial hearing recovery with serviceable hearing, i.e. a maximum speech discrimination of 100% for monosyllables in quiet under amplified conditions (95dB SPL). Two patients with anacusis (no measurable threshold) from 1 kHz onward showed no signs of recovery. Audiological results for each patient are provided in detail in table 2.
Discussion
The intravitreal controlled release dexamethasone drug delivery system which was adopted for the implantation in a selected group of patients with ISSHL is primarily indicated for the treatment of macular edema following branch or central retinal vein occlusion and for the treatment of patients with inflammation of the posterior segment of the eye presenting as non-infectious uveitis (23-25). Pharmacokinetic studies in the eye of monkeys showed that DEX concentration in the vitreous humor was characterized by two distinct phases: From days 7 to 60, high concentrations of DEX were detected. At days 90 to 180, DEX release was sustained at low concentrations, and from days 210 to 270, DEX was below the limit of quantification (22).
By the means of computer simulations it can be estimated that a solid controlled release drug delivery system will deliver more drug to the inner ear than multiple intratympanic injections of DEX solution, (see text and figure 2 in supplementary content 1, describing the method and results of the computer simulations). Based on experience from treatment of Menière's disease by intratympanic injections of gentamicin, there is evidence that continuous application protocols result in higher cochlear doses associated with higher risk to hearing but without increasing the success rate concerning the control of vertigo (26).
For the treatment of inner ear disorders, controlled release biodegradable polymers (gels or solid implants) have been suggested promising drug delivery strategies (13, 17, 18,20,27) and continuous intratympanic application of glucocorticoids has been favored by several clinical studies (14, 15,28,29). Intratympanic injections show a fast loss of drug from the middle and inner ear (10,30). Even repeated applications show a poor level of control, and implanted catheters with external pump systems are limited in their feasibility (4, 13).
Although the amount of secondary recovery in this small group of patients appears promising, due to the small number of patients, the experience to date is limited. The 4PTA threshold values in the three patients with profound hearing loss are at least partially based on non-measurable (“out of range”) frequencies, which have been dummy-coded with a level of 120 dB HL as suggested previously (31-33). Nevertheless, since no drug is approved so far for local therapy of inner ear disorders accompanied by increasing evidence for the effectiveness of intratympanic glucocorticoids as salvage therapy in ISSHL and the rapidly growing number of preclinical data on controlled drug delivery to the inner ear using biodegradable polymers, the above observations encourage following this approach further. For application into the RW niche, development of more suitably shaped controlled release devices (e.g. a disc with smooth edges fitting onto the RWM) would be beneficial. Additional studies, such as extended case series compared to historical controls, the results of which could form the basis of randomized controlled trials, are necessary. Due to the greater invasiveness as compared to intratympanic injections, these studies will most likely be first designed as secondary or tertiary strategies in patients with higher degrees of ISSHL.
Supplementary Material
Acknowledgments
We would like to thank Ruth Gill (Dept. of Otolaryngology, Washington University School of Medicine, St. Louis, USA) for her contribution to the computer simulations. Part of this research was supported by a grant from NIH/NIDCD (R01 DC01368) to Alec N. Salt.
Footnotes
Disclosures: Stefan K. Plontke has been a consultant for Otonomy, Inc., San Diego, USA and is member of the scientific advisory board of AudioCure Pharma, Berlin, Germany
Alec N. Salt is a member of the scientific advisory board of Otonomy, Inc. This work was not sponsored by any of the above mentioned companies not any third party outside the university.
References
- 1.Garavello W, Galluzzi F, Gaini RM, et al. Intratympanic steroid treatment for sudden deafness: a meta-analysis of randomized controlled trials. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:724–9. doi: 10.1097/MAO.0b013e318254ee04. [DOI] [PubMed] [Google Scholar]
- 2.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;146:S1–35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 3.Alzamil KS, Linthicum FH., Jr Extraneous round window membranes and plugs: possible effect on intratympanic therapy. Ann Otol Rhinol Laryngol. 2000;109:30–2. doi: 10.1177/000348940010900105. [DOI] [PubMed] [Google Scholar]
- 4.Plontke SK, Zimmermann R, Zenner HP, et al. Technical note on microcatheter implantation for local inner ear drug delivery: surgical technique and safety aspects. Otol Neurotol. 2006;27:912–7. doi: 10.1097/01.mao.0000235310.72442.4e. [DOI] [PubMed] [Google Scholar]
- 5.Crane BT, Minor LB, Della Santina CC, et al. Middle ear exploration in patients with Meniere's disease who have failed outpatient intratympanic gentamicin therapy. Otol Neurotol. 2009;30:619–24. doi: 10.1097/MAO.0b013e3181a66d2b. [DOI] [PubMed] [Google Scholar]
- 6.Plontke SK. Evaluation of the round window niche before local drug delivery to the inner ear using a new mini-otoscope. Otol Neurotol. 2011;32:183–5. doi: 10.1097/mao.0b013e3181f6cb25. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein H, Choo D, Rosenberg SI, et al. Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report) Ear Nose Throat J. 1996;75:468–71. 74, 76. [PubMed] [Google Scholar]
- 8.Kanzaki S, Saito H, Inoue Y, et al. A new device for delivering drugs into the inner ear: otoendoscope with microcatheter. Auris, nasus, larynx. 2012;39:208–11. doi: 10.1016/j.anl.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Hiraumi H, Nakagawa T, Ito J. Efficiency of a transtympanic approach to the round window membrane using a microendoscope. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2009;266:367–71. doi: 10.1007/s00405-008-0760-3. [DOI] [PubMed] [Google Scholar]
- 10.Plontke SK, Mikulec AA, Salt AN. Rapid clearance of methylprednisolone after intratympanic application in humans. Comment on: Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007;28:1124-30. Otol Neurotol. 2008;29:732–3. doi: 10.1097/MAO.0b013e318173fcea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn H, Kammerer B, DiMauro A, et al. Cochlear microdialysis for quantification of dexamethasone and fluorescein entry into scala tympani during round window administration. Hear Res. 2006;212:236–44. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plontke SK, Salt AN. Simulation of application strategies for local drug delivery to the inner ear. ORL J Otorhinolaryngol Relat Spec. 2006;68:386–92. doi: 10.1159/000095284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14:350–60. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopke RD, Hoffer ME, Wester D, et al. Targeted topical steroid therapy in sudden sensorineural hearing loss. Otol Neurotol. 2001;22:475–9. doi: 10.1097/00129492-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Plontke SK, Lowenheim H, Mertens J, et al. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2009;119:359–69. doi: 10.1002/lary.20074. [DOI] [PubMed] [Google Scholar]
- 16.Schwab B, Lenarz T, Heermann R. Use of the round window micro cath for inner ear therapy - results of a placebo-controlled, prospective study on chronic tinnitus. Laryngorhinootologie. 2004;83:164–72. doi: 10.1055/s-2004-814278. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Ito J. Local drug delivery to the inner ear using biodegradable materials. Therapeutic delivery. 2011;2:807–14. doi: 10.4155/tde.11.43. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Glueckert R, Johnston H, et al. Strategies for drug delivery to the human inner ear by multifunctional nanoparticles. Nanomedicine (London, England) 2012;7:55–63. doi: 10.2217/nnm.11.84. [DOI] [PubMed] [Google Scholar]
- 19.Swan EE, Mescher MJ, Sewell W, et al. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60:1583–99. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert PR, Nguyen S, Maxwell KS, et al. A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral Meniere's disease. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:1257–65. doi: 10.1097/MAO.0b013e318263d35d. [DOI] [PubMed] [Google Scholar]
- 21.Lang J, Kothe W. Measurements of the tympanic cavity. Gegenbaurs morphologisches Jahrbuch. 1987;133:469–505. [PubMed] [Google Scholar]
- 22.Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investigative ophthalmology & visual science. 2011;52:80–6. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 23.Haller JA, Bandello F, Belfort R, Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–46 e3. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Archives of ophthalmology. 2007;125:309–17. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 25.Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. American journal of ophthalmology. 2009;147:1048–54. 54 e1–2. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Salt AN, Gill RM, Plontke SK. Dependence of hearing changes on the dose of intratympanically applied gentamicin: a meta-analysis using mathematical simulations of clinical drug delivery protocols. Laryngoscope. 2008;118:1793–800. doi: 10.1097/MLG.0b013e31817d01cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salt AN, Hartsock J, Plontke S, et al. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurootol. 2011;16:323–35. doi: 10.1159/000322504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou YF, Chen PR, Kuo IJ, et al. Comparison of intermittent intratympanic steroid injection and near-continual transtympanic steroid perfusion as salvage treatments for sudden sensorineural hearing loss. Laryngoscope. 2013;123:2264–9. doi: 10.1002/lary.23909. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Ren J, Yin T, et al. Intratympanic dexamethasone perfusion versus injection for treatment of refractory sudden sensorineural hearing loss. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2013;270:861–7. doi: 10.1007/s00405-012-2061-0. [DOI] [PubMed] [Google Scholar]
- 30.Salt AN, Hartsock JJ, Gill RM, et al. Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. Journal of the Association for Research in Otolaryngology : JARO. 2012;13:771–83. doi: 10.1007/s10162-012-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plontke S, Lowenheim H, Preyer S, et al. Outcomes research analysis of continuous intratympanic glucocorticoid delivery in patients with acute severe to profound hearing loss: basis for planning randomized controlled trials. Acta Otolaryngol. 2005;125:830–9. doi: 10.1080/00016480510037898. [DOI] [PubMed] [Google Scholar]
- 32.Chen CY, Halpin C, Rauch SD. Oral steroid treatment of sudden sensorineural hearing loss: a ten year retrospective analysis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003;24:728–33. doi: 10.1097/00129492-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Plontke SK, Bauer M, Meisner C. Comparison of pure-tone audiometry analysis in sudden hearing loss studies: lack of agreement for different outcome measures. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2007;28:753–63. doi: 10.1097/mao.0b013e31811515ae. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


