SUMMARY
D-type cyclins (D1, D2 and D3) are components of the mammalian core cell cycle machinery and function to drive cell proliferation. Here we report that D-cyclins perform a rate-limiting anti-apoptotic function in vivo. We found that acute shutdown of all three D-cyclins in bone marrow of adult mice resulted in massive apoptosis of all hematopoietic cell types. We demonstrate that adult hematopoietic stem cells are particularly dependent on D-cyclins for survival, and they are especially sensitive to cyclin D loss. Surprisingly, we found that the anti-apoptotic function of D-cyclins also operates in quiescent hematopoietic stem and progenitor cells. Our analyses revealed that D-cyclins repress the expression of the death receptor Fas and its ligand, FasL. Acute ablation of D-cyclins upregulated these pro-apoptotic genes, and led to Fas- and caspase 8-dependent apoptosis. These results reveal an unexpected function of cell cycle proteins in controlling apoptosis in normal cell homeostasis.
INTRODUCTION
The mammalian core cell cycle machinery is composed of cyclins and their associated cyclin-dependent kinases (Cdks). Cyclin-Cdk complexes phosphorylate several cellular proteins, thereby driving cell cycle progression (Malumbres and Barbacid, 2009). Among the cell cycle regulators, D-type cyclins have received particular attention, due to their very frequent involvement in the pathogenesis of human cancers and their role as oncogenes (Deshpande et al., 2005; Fu et al., 2004).
The family of mammalian D-type cyclins is composed of three highly homologous proteins, cyclin D1, D2 and D3. These proteins are expressed in an overlapping fashion in all proliferating cell types (Musgrove et al. 2011). D-cyclins bind and activate Cdk4 and Cdk6, resulting in phosphorylation of the retinoblastoma protein pRB, as well as of the related p107 and p130 proteins. pRB phosphorylation causes de-repression and activation of pRB-bound E2F transcription factors, which promote cell cycle progression and DNA synthesis (S-phase) (Sherr and Roberts, 2004).
Consistent with their growth-promoting functions, overexpression of D-type cyclins plays a causative role in development of a large number of human cancers. In several instances cyclin D-overexpression is driven by gene amplifications or translocations. Indeed, the gene encoding cyclin D1 represents the second most frequently amplified locus in the human cancer genome (Beroukhim et al. 2010). Also, genes encoding cyclins D2 and D3 are frequently amplified or rearranged in human cancers (Deshpande et al., 2005). Moreover, several human cancers overexpress particular cyclin D proteins without detectable genetic alterations (Diehl, 2002).
Analyses of knockout mice lacking D-type cyclins and their catalytic partners, Cdk4 and Cdk6 provided extensive genetic evidence for the role of cyclin D-Cdk4/6 complexes in driving cell proliferation. Thus, mice lacking cyclins D1, D2, D3, Cdk4 or Cdk6 displayed proliferative deficiencies in specific cell compartments (reviewed in Sherr and Roberts, 2004). Combined ablation of all three D-type cyclins resulted in an embryonic lethality of cyclin D1−/−D2−/−D3−/− mice, due to a block in proliferation of fetal liver hematopoietic cells (Kozar et al., 2004). These results were recapitulated in mice lacking Cdk4 and Cdk6, which also displayed severe proliferative failure in this compartment (Malumbres et al., 2004). Hence, analyses of knockout animals firmly established the function for D-cyclins in driving cell proliferation in vivo.
The embryonic lethality of cyclin D1−/−D2−/−D3−/− mice restricted analyses of the function of all three D-cyclins in vivo to fetal development. In the current study, we set out to circumvent this lethality by generating conditional cyclin D triple-knockout mice. Using these mice, we studied the consequence of an acute ablation of all three D-cyclins in the bone marrow of adult mice. Unexpectedly, shut down of D-cyclins led to extensive apoptosis of all hematopoietic cell types. We found that adult hematopoietic stem cells are particularly dependent on D-cyclins for survival, and they are especially sensitive to cyclin D loss. Our mechanistic analyses revealed that D-cyclins repress the expression of the death receptor, Fas and its ligand, FasL. These findings provide a mechanism linking a cyclin protein to cell survival in normal homeostasis in vivo.
RESULTS
Generation of Conditional ‘Triple-Knockout’ Cyclin D1F/FD2−/−D3F/F Mice
In order to address the requirement for cyclin D function in adult mice, we interbred cyclin D1F/F and D3F/F conditional knockout mice (Choi et al., 2011) with cyclin D2−/− animals. The latter strain develops normally, except for gonadal hypoplasia (Sicinski et al., 1996). After a few rounds of crosses, we generated conditional ‘triple-knockout’ cyclin D1F/FD2−/−D3F/F (TKO) mice (Figure S1A). These animals are viable, consistent with the expectation that the ‘floxed’ cyclin D alleles are functionally wild-type.
Acute Ablation of D-cyclins in Adult Bone Marrow
We previously found that D-cyclins are essential for embryonic hematopoiesis. Specifically, ablation of all three D-cyclins blocked rapid expansion of embryonic hematopoietic stem/progenitor cells, which takes place in fetal livers during midgestation. Consequently, cyclin D1−/−D2−/−D3−/− mice died during embryogenesis due to a severe anemia (Kozar et al., 2004). Outside hematopoiesis, other cell types seemed to be unaffected by cyclin D-loss, indicating that most embryonic cell types can proliferate without D-cyclins (Kozar et al., 2004).
In the current study, we decided to test the requirement for D-cyclins in adult hematopoiesis, which mostly takes place in the bone marrow. The hematopoietic system is hierarchically organized with the hematopoietic stem cell (HSC) at the apex. This defined hierarchy of hematopoietic stem cells, progenitors and lineage-committed cell types, combined with our ability to isolate these cells based on surface markers, allowed us to study the requirement for D-cyclins in stem/progenitor cells as well as at later stages of hematopoiesis.
To ablate D-cyclins in adult bone marrow, we intercrossed TKO mice with Mx1-Cre animals. The latter strain expresses inducible Cre recombinase in hematopoietic cells. Administration of polyI-polyC (pI-pC) to Mx1-Cre animals activates Cre leading to deletion of the ‘floxed’ sequences in bone marrow cells, including very efficient deletion in hematopoietic stem cells (Kuhn et al., 1995). We verified that Cre recombinase was expressed at the same levels in bone marrows of control Mx-Cre+;D1F/+D2+/−D3F/+ (MxCntrl) and experimental Mx-Cre+;D1F/FD2−/−D3F/F (MxTKO) animals (Figure S1B), and that pI-pC administration led to an efficient deletion of the floxed cyclin genes in hematopoietic cells (Figure S1C).
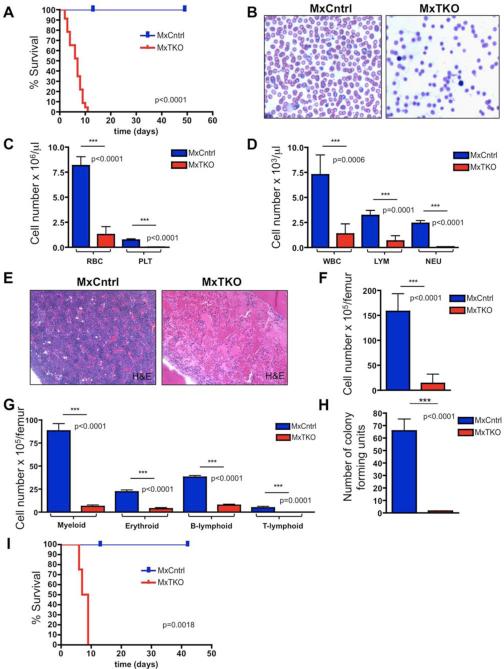
Acute ablation of all three D-cyclins in bone marrows of MxTKO mice resulted in 100% lethality within 11 days following pI-pC administration (Figure 1A). Moribund MxTKO mice developed severe pancytopenia, as evidenced by a 7-fold reduction in red blood cell counts and a 6-fold decrease in the number of white blood cells (Figures 1B-1D).
Figure 1. Consequences of Cyclin D Ablation in Hematopoietic Cells.
(A) Survival of Mx-Cre+;D1F/FD2−/−D3F/F (MxTKO) and control Mx-Cre+;D1F/+D2+/−D3F/+(MxCntrl) animals injected 1-3 times with pI-pC (to delete D-cyclins in MxTKO mice) (n = 23 per group). Day 0 corresponds to the first injection.
(B) Representative images of Wright-Giemsa stained peripheral blood smears at day 8 post-injection.
(C-D) Mean numbers of red blood cells (RBC), platelets (PLT), white blood cells (WBC), lymphocytes (LYM) and neutrophils (NEU) in the peripheral blood of MxTKO versus MxCntrl mice (n=5 per genotype).
(E) Images of hematoxylin and eosin stained bone marrow sections. Note that nucleated bone marrow cells are virtually absent in an MxTKO animal.
(F) Mean numbers of nucleated bone marrow cells per femur (n=5 per genotype).
(G) Mean numbers of Gr1+ CD11b+ (myeloid), Ter119+ (erythroid), B220+ (B-lymphoid), and CD3+ (T-lymphoid) cells in bone marrow (n=5 per genotype).
(H) Bone marrow cells from MxTKO and MxCntrl mice were collected 5 days after ablation of D-cyclins and plated in methylcellulose. Shown are the mean numbers of resulting colonies, scored after 12 days, from n=3 biological replicates.
(I) Results of non-competitive bone marrow reconstitution. Wild-type lethally irradiated mice received either undeleted MxTKO or MxCntrl bone marrow. Shown is the survival curve following pI-pC treatment (n=4 per group). Note that deletion of D-cyclins following bone marrow reconstitution recapitulated the phenotype seen in intact MxTKO mice (panel).
Error bars in (C), (D), (F), (G) and (H) denote SD.
See also Figure S1.
Histopathological analyses of the bone marrows revealed that nearly all nucleated cells were lost in MxTKO animals (Figures 1E-1G and S1D). This loss encompassed all lineages including myeloid (Gr1+,CD11b+), erythroid (Ter119+), lymphoid B-cells (B220+) and T-cells (CD3+) (Figure 1G). Consistent with these findings, MxTKO bone marrow was devoid of any colony forming units, as judged by methylcellulose assays (Figure 1H). The disappearance of hematopoietic cells following an acute shutdown of D-cyclins suggested that ablation of D-cyclins might have triggered cell death of all bone marrow cells.
To determine whether the loss of bone marrow cells was caused by an intrinsic defect in hematopoietic cells, we performed bone marrow transplantation experiments (Fleming et al., 2008). Wild-type recipient mice received a lethal dose of irradiation, which ablated their own bone marrow, and then were reconstituted with undeleted MxTKO bone marrow cells. Following reconstitution, wild-type recipients were treated with pI-pC, thereby deleting D-cyclins exclusively in the engrafted hematopoietic cells. This led to a disappearance of bone marrow cells and to death of the animals (Figure 1I), recapitulating the effect seen in \intact MxTKO mice (Figure 1A). Collectively, these analyses revealed that the D-cyclins play a cell autonomous, rate-limiting function in bone marrow hematopoietic cells.
Rapid Apoptosis of Hematopoietic Stem Cells Following Ablation of D-cyclins
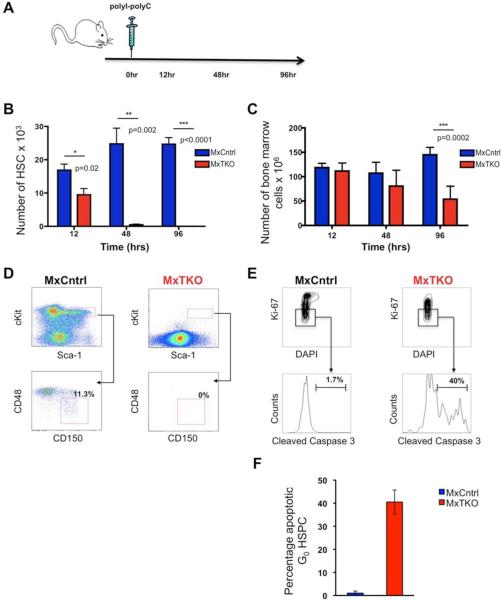
To gauge the response of hematopoietic stem cells to cyclin D shutdown, we investigated early time-points following ablation of D-cyclins, when the total numbers of bone marrow cells have not yet been impacted. We treated animals with a single dose of pI-pC, collected bone marrow cells after 12, 48 and 96 hours, and stained them with surface markers for hematopoietic stem cells (Linlow c-kit+ Sca+ CD48− CD150+, further called HSC). We then used flow cytometry to quantify HSC numbers at different time-points after cyclin D shutdown (Figure 2A). Strikingly, we observed that approximately 50% of stem cells disappeared as early as 12 hours after ablation of D-cyclins (Figure 2B), while the number of more mature bone marrow cells remained unchanged at this time-point (Figure 2C). Essentially 100% of stem cells disappeared within 48 hours (Figure 2B), a time-point when more mature bone marrow cells displayed a slight, but statistically non-significant drop in cell numbers (Figure 2C). At 96 hours, the HSC were essentially undetectable (Figures 2B and 2D), while the numbers of more mature bone marrow cells were reduced by 50% (Figure 2C). These observations suggested that HSC are particularly dependent on D-cyclins for their survival, as they are especially sensitive to cyclin D ablation.
Figure 2. Apoptosis of Hematopoietic Stem/Progenitor Cells Following Shutdown of D-cyclins.
(A) Outline of the experimental design. Mx-Cre+;D1F/FD2−/−D3F/F (MxTKO) and control Mx-Cre+;D1F/+D2+/−D3F/+(MxCntrl) animals received a single injection of pI-pC (to delete D-cyclins in MxTKO mice). Animals were sacrificed after 12, 48 and 96 hours, and hematopoietic stem cells (HSC) were stained and quantified by FACS.
(B and C) Mean number of HSC (B), and mean number of total bone marrow cells (C), per animal (including femurs, tibias, Iliac crests and humerus) at the indicated time-points after ablation of D-cyclins.
(D) Representative FACS analysis of HSC (Linlow, Kit+, Sca1+, CD48−, CD150+) from bone marrows collected at 96 hours after deletion of D-cyclins. Note that essentially no HSC were detected in MxTKO animals.
(E) 24-48 hours after ablation of D-cyclins, hematopoietic stem/progenitor cells (HSPC) were stained with DAPI (4’,6-diamidino-2-phenylindole), Ki67 and cleaved caspase 3 (to mark apoptotic cells). HSPC then were gated on G0 cells (Wilson et al., 2008) and analyzed for cleaved caspase 3 staining.
(F) The mean percentage of caspase 3-positive cells among quiescent HSPC cells, gated as above, in MxCntrl and MxTKO animals.
In (B), (C) and (F), error bars denote SD.
See also Figure S2.
Ablation of D-cyclins Leads to Apoptosis of Quiescent Stem/Progenitor Cells
Unlike embryonic hematopoietic stem cells, which are rapidly proliferating, adult bone marrow hematopoietic stem cells represent a largely non-dividing, quiescent cell population (Wilson et al., 2008). On the other hand, D-cyclins are believed to be expressed in proliferating cells where they govern G1 phase progression (Sherr and Roberts, 2004). Intrigued by the loss of HSC following shutdown of D-cyclins, we flow sorted HSC from intact wild-type animals and determined that these cells express all three D-type cyclins (Figure S2A). We next injected MxTKO and MxCntrl mice with a single pI-pC dose and evaluated whether quiescent hematopoietic stem/progenitor cells (lineage-negative, c-Kit+ and Sca+, further called HSPC) undergo apoptosis upon acute ablation of D-cyclins. Quiescent (G0) HSPC cells were identified by means of Ki67 and DAPI staining, the standard procedure used to analyze quiescent stem cells (Gurumurthy et al., 2010; Wilson et al., 2008; Rossi et al., 2007). Staining of these cells for cleaved caspase 3 revealed wide-spread apoptosis of quiescent HSPC following ablation of D-cyclins (Figures 2E and 2F). In contrast, essentially no apoptosis was detected among HSPC of control animals, which were treated in the same way (Figures 2E and 2F). These observations indicated that D-cyclins play a rate-limiting, pro-survival function in quiescent hematopoietic stem/progenitor cells.
Apoptosis of All Bone Marrow Cells After Shutdown of D-cyclins
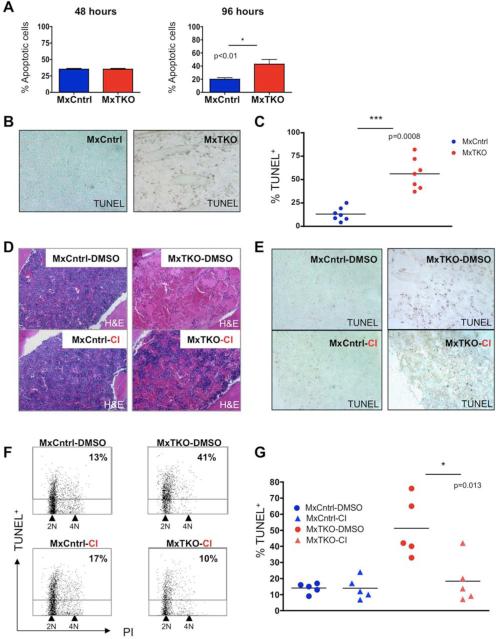
Analyses of bone marrow cells at later time-points indicated that eventually all hematopoietic cells underwent apoptosis following shutdown of D-cyclins, explaining the lethality seen in triple knockout animals. Within the whole bone marrow cell population, an increased apoptotic rate was first detected at 96 hours (Figure 3A), and by 6 days the majority of bone marrow cells were TUNEL positive (Figures 3B and 3C). These results indicate that all adult hematopoietic cells depend on D-cyclins for survival.
Figure 3. Apoptosis of Bone Marrow Cells After Ablation of D-cyclins.
(A and B) Mean percentage of Annexin V+ (apoptotic) bone marrow cells in MxTKO and MxCntrl mice (n=5 per genotype), at 48 and 96 hours after ablation of D-cyclins. Error bars, SD.
(B) Staining of femur sections for apoptotic cells (TUNEL). Cells were counterstained with methyl green. Note strongly increased number of apoptotic cells (TUNEL+) and paucity of viable nucleated cells (TUNEL− /methyl green+) in MxTKO bone marrow.
(C) Mean percentages of TUNEL-positive bone marrow cells analyzed by FACS 4-6 days after ablation of D-cyclins (MxTKO). Each dot corresponds to a different animal (n=7 mice per group), horizontal lines denote mean values.
(D-G) MxTKO and MxCntrl mice were injected with caspase inhibitor QVD-OPh (Cl), with vehicle only (DMSO), concomitantly with the deletion of D-cyclins, and bone marrows were collected 6 days later.
(D) Shown are representative images of hematoxylin and eosin stained sections of bone marrows. Note that ablation of D-cyclins resulted in loss of nucleated bone marrow cells (MxTKO-DMSO – upper right panel), and that treatment with caspase inhibitor partially rescued this effect (MxTKO-CI – lower right panel).
(E) TUNEL staining of femur sections to detect apoptotic cells. Cells were counterstained with methyl green. Note that treatment of animals with caspase inhibitor (CI) decreased the fraction of apoptotic cells in MxTKO mice (lower right panel) and increased the number of nucleated cells.
(F) Representative FACS profiles of bone marrow cells stained with TUNEL (to detect apoptotic cells) and with propidium iodide (PI, for cell cycle analysis) and analyzed by FACS. The percentages of apoptotic (TUNEL+) cells are marked. Note that treatment with Cl blocked apoptosis triggered by cyclin D ablation (MxTKO-DMSO vs. MxTKO-Cl). Also note that ablation of D-cyclins reduced the fraction of cells with >2N DNA content and increased the proportion of 2N cells, which is indicative of cell cycle arrest (please see also Figures S2C-S2F).
(G) Percentages of TUNEL+ cells in bone marrows of MxTKO and MxCntrl mice treated with DMSO or with caspase inhibitor QVD-OPh (Cl). TUNEL+ cells were quantified by FACS. Each dot corresponds to a different animal (n=5 for each group), horizontal lines represent mean values.
See also Figure S2.
Given this widespread apoptosis of bone marrow cells, we asked whether blocking the apoptotic process might at least partially recue the phenotype of cyclin D shutdown. Since activation of caspases represents a central step in the execution of apoptosis (Strasser et al., 2009), we attempted to block this step by administering an irreversible broad-spectrum caspase inhibitor, QVD-OPh (Caserta et al., 2003) to MxTKO animals concomitantly with ablation of D-cyclins. Inhibition of caspase activation significantly reduced the extent of bone marrow cell apoptosis following D-cyclin ablation (Figures 3D-3G) and partially protected against the loss of bone marrow cells (Figures 3D and S2B).
It should be noted that in addition to the apoptotic response, ablation of D-cyclins blocked proliferation of bone marrow cells (Figures S2C and S2D). For this reason, inhibition of the apoptotic process only partially rescued the phenotype of cyclin D-deficiency, as cell cycle arrest was still in place following QVD-OPh treatment (Figures S2E and S2F). Collectively, these analyses established caspase-dependent hematopoietic cell death as a mechanism of the loss of bone marrow cells following ablation of D-cyclins.
D-Cyclins Regulate Apoptosis through Fas and FasL
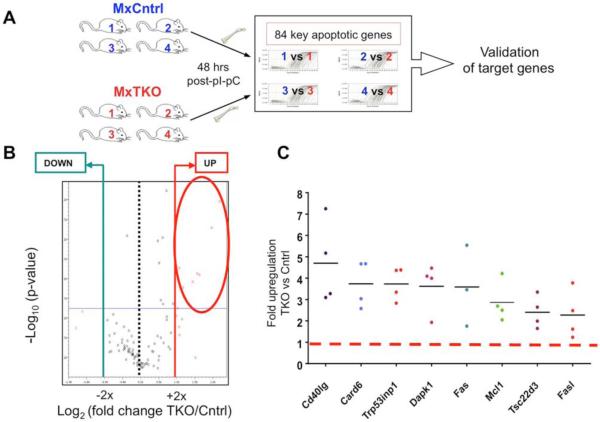
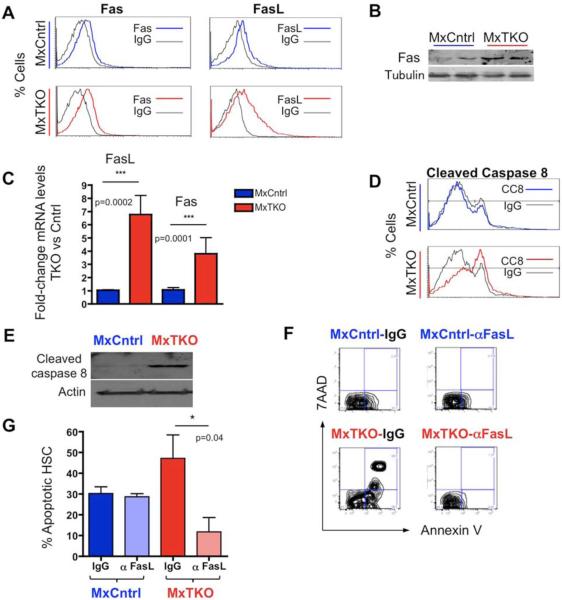
In order to gain mechanistic insights into the pro-survival function of D-cyclins in hematopoietic cells, we evaluated the transcriptional changes in bone marrow cells following an acute ablation of D-cyclins. We focused our analysis on 84 key apoptosis-relevant genes, using an RT-PCR based Apoptosis PCR Array (Figure 4A). Transcripts of eight genes were consistently upregulated upon cyclin D shutdown in bone marrow cells (Figures 4B, 4C and Table S1). Two of these genes encode pro-apoptotic proteins, which function within the same pathway to induce cell death: the death receptor Fas and its ligand, FasL (Strasser et al., 2009). We confirmed upregulation of the Fas protein as well as increased cell surface expression of both Fas and FasL in cyclin D-deleted hematopoietic cells (Figures 5A and 5B). We also confirmed upregulation of Fas and FasL transcripts in hematopoietic stem/progenitor cells in vivo following ablation of D-cyclins (Figure 5C).
Figure 4. Molecular Analyses of Hematopoietic Cells Following Cyclin D Shutdown.
(A) Strategy for the identification of genes with altered expression upon ablation of D-cyclins. Bone marrows from MxTKO and MxCntrl mice (n = 4 per group) were collected 48 hrs after one dose of pI-pC (to delete D-cyclins in MxTKO mice) and analyzed for the expression levels of apoptotic genes by RT-PCR-based array (See Supplemental Experimental Procedures).
(B) Volcano plot analysis using web-based software for Apoptosis RT2 Profiler™ Apoptosis PCR SArray PAMM-3012 (Qiagen) for 4 biological replicates from MxTKO and MxCntrl mice. The volcano plot displays significance of changes (-Log10 p-values) versus fold-change (Log2 fold change TKO/Cntrl) on the y- and x-axes, respectively. Eight genes encircled in red satisfied the following criteria: (1) genes that had >2-fold increased (red vertical line) or decreased (blue vertical line) expression in MxTKO, as compared to MxCntrl; (2) The significance of changes had p-value <0.05 (thin horizontal line).
(C) Results of RT-PCR analysis for eight genes which were identified by Apoptosis PCR Array as having altered expression (>2 fold change, p<0.05) in cyclin D-deleted bone marrows. Each dot shows the fold-change expression in MxTKO versus MxCntrl bone marrows, for each of the 4 independent biological replicates. Horizontal lines denote mean fold-change for the indicated genes, red dashed line = no change (ratio of 1).
See also Table S1.
Figure 5. Fas/FasL Signaling Is a Critical Mediator of Apoptosis Induced by Cyclin D Ablation.
(A) FACS analysis of Fas and FasL surface expression in MxTKO and MxCntrl bone marrow cells 2 days after ablation of D-cyclins. Note increased staining in MxTKO as compared to MxCntrl.
(B) Western blot analysis of Fas protein levels in bone marrow 2 days after ablation of D-cyclins.
(C) Upregulation of Fas and FasL mRNA levels in hematopoietic stem/progenitor cells (HSPC) 24 hours after ablation of D-cyclins (MxTKO). N=4 mice for each genotype, error bars, SD.
(D) Intracellular staining for cleaved caspase 8 in bone marrow cells 2 days after cyclin D-deletion. Note increased staining in MxTKO as compared to MxCntrl.
(E) Western blot analysis of the levels of cleaved caspase 8 in bone marrow cells of MxCntrl and MxTKO mice, three days after pI-pC injection (to delete D-cyclins in MxTKO).
(F and G) MxTKO and MxCntrl mice were injected with neutralizing anti-FasL antibody (αFasL), or with isotype control (IgG) concomitantly with administration of pI-pC (to delete D-cyclins in MxTKO mice). Two days later, hematopoietic stem/progenitor cells, HSPC (F), or hematopoietic stem cells, HSC (G) were flow sorted and stained with Annexin V and 7AAD to mark apoptotic (Annexin V+/7AAD+) cells. Note that inhibition of FasL abrogated apoptosis of HSC and HSPC triggered by ablation of D-cyclins (compare MxTKO-IgG vs. MxTKO-αFasL). (F) Shows representative staining with Annexin V 7AAD. (G) Mean percentage of Annexin V+ HSC (n=3 mice for MxCntrl and n=5 MxTKO). Error bars, SD.
See also Figure S3.
Interaction of the death receptor Fas with its ligand, FasL triggers cell death via an extrinsic apoptotic process that involves activation of caspase 8 (Strasser et al., 2009). Indeed, we observed activation of caspase 8 following cyclin D deletion in hematopoietic cells (Figures 5D and 5E). At these early time-points we detected no changes in mitochondrial membrane potential (Figure S3A). However, at later times, changes in mitochondrial membrane potential were observed (Figure S3A), consistent with the fact that the extrinsic pathway may lead to mitochondrial activation (Strasser et al., 2009). Collectively, these observations indicate that ablation of D-cyclins leads to upregulation of Fas and FasL and triggers the Fas/FasL → caspase 8 apoptotic pathway in hematopoietic cells.
To further substantiate upregulation of Fas and FasL as the cause of apoptosis of hematopoietic cells, we asked whether inhibition of Fas activation could block cell death following cyclin D shutdown. To address this question, we injected MxTKO mice with anti-FasL neutralizing antibody (or with isotype-matched IgG, as a control) concomitantly with deletion of D-cyclins. Subsequently, the in vivo apoptotic rate of hematopoietic stem cells and hematopoietic stem/progenitor cells was examined by Annexin V staining. Strikingly, inhibition of Fas signaling blocked apoptosis of HSC and HSPC triggered by cyclin D shutdown (MxTKO-αFasL, Figures 5F and 5G). We also confirmed that anti-FasL antibody blocked death of unsorted bone marrow cells (data not shown). These analyses point to upregulation of Fas signaling as a cause of death of all hematopoietic cells, including stem cells, upon ablation of D-cyclins. On the other hand, we did not detect upregulation of p53 transcriptional targets Bax, Noxa and Puma, nor activation of TNFα (Figure S3B and data not shown), suggesting that these pathways do not play a major role in hematopoietic cell death following shutdown of D-cyclins.
Collectively, our findings suggest a model that D-cyclins repress the expression of Fas and FasL in hematopoietic cells. Ablation of D-cyclins leads to upregulation of these proteins, and triggers caspase 8-initiated apoptosis.
D-cyclins Repress Expression of Fas and FasL via E2F1
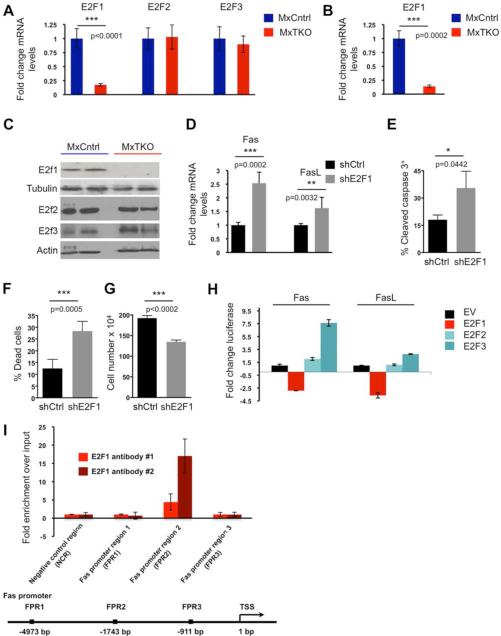
In order to understand how D-cyclins regulate the expression of Fas and FasL in bone marrow cells, we focused on the pRB→E2F pathway. It is well established that D-cyclins control the activity of E2F transcription factors by phosphorylating pRB; pRB phosphorylation results in release or de-repression of E2F transcriptional activity (Trimarchi and Lees, 2002; Sherr and Roberts, 2004). Consistent with this model, we observed that ablation of D-type cyclins in bone marrow cells strongly decreased pRB phosphorylation (Figures S4A and S4B), leading to reduced levels of E2F transcriptional targets (Figure S4C). The E2F1 gene represents an established E2F target (Neuman et al., 1995), and its expression was strongly reduced in total bone marrow, in hematopoietic stem/progenitor cells and in hematopoietic stem cells upon cyclin D shutdown (Figures 6A, 6B, and data not shown). Moreover, the E2F1 protein was virtually undetectable in bone marrow cells following acute cyclin D shutdown (Figure 6C). In contrast, the levels of E2F2 and E2F3 were not affected (Figures 7A and 7C).
Figure 6. D-cyclins Repress Expression of Fas and FasL via E2F1.
(A) Relative expression of E2F1, E2F2 and E2F3 transcripts in bone marrow cells 48 hours following an acute ablation of D-cyclins (MxTKO).
(B) Relative expression of E2F1 transcripts in hematopoietic stem cells (HSC) 24 hours after ablation of D-cyclins (MxTKO). N=3 mice for each genotype.
(C) Protein levels of E2F1, E2F2 and E2F3 in bone marrow cells 48 hours following acute ablation of D-cyclins (MxTKO).
(D-G) Bone marrow cells were infected with viruses encoding anti-E2F1 shRNA (shE2F1), or with control, scrambled shRNA (shCtrl). Please note that bone marrow cells did not undergo any selection after viral transduction. Therefore, E2F1 knockdown took place only in a fraction of cells and hence the observed changes likely underestimate the effects of E2F1 depletion. See also Figure S5A.
(D) Results of RT-PCR analysis for the levels of the endogenous Fas and FasL transcripts.
(E to G) Mean percentages of cleaved caspase-3 positive cells (E), trypan blue-positive dead cells (F), and mean total number of remaining bone marrow cells (G), following knock-down of E2F1.
(H) Fas and FasL promoter-luciferase constructs were co-transfected with vectors encoding E2F1, E2F2, E2F3, or with an empty vector (EV). Note that E2F1 expression significantly reduced transcription from both Fas and FasL promoters (see also Figures S5E and S5G).
(I) Chromatin immunoprecipitation (ChIP) studies of E2F1 binding to the Fas promoter in HSPC. Binding of E2F1 to a region of the Fas promoter was observed with both anti-E2F1 antibodies used (Santa Cruz, bright red; Millipore, dark red). Data represent mean ± SEM of three replicates. Negative control region (NCR) is a control region away from the Fas promoter. Below, a schematic representation of the Fas promoter.
In (A), (B), (D-I), error bars denote SD.
See also Figures S4 and S5.
Figure 7.
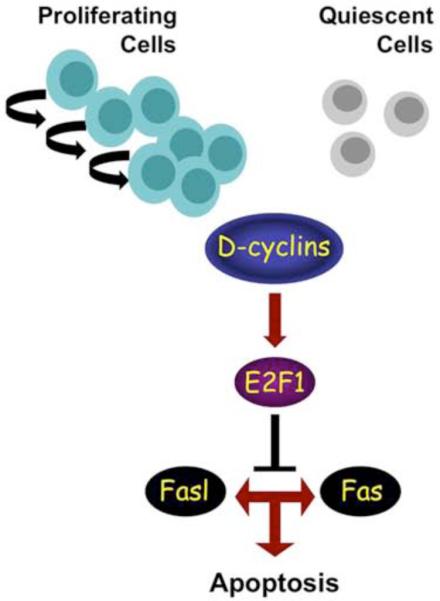
A Proposed Model Explaining a Pro-Survival Function of D-cyclins in Hematopoietic Cells Via a Cyclin D → E2F → Fas/FasL Pathway
Genetic ablation of E2Fs1-3 was shown to result in apoptosis of several compartments, including bone marrow cells, revealing that E2Fs play pro-survival roles in vivo (Chen et al., 2009; Chong et al., 2009; Trikha et al., 2011). Therefore, we asked whether an acute loss of E2F1 upon cyclin D ablation might be responsible for upregulation of the Fas and FasL transcripts. To address this, we first knocked down E2F1 in hematopoietic cells (Figures S5A-S5C). We observed that this led to increased expression of the endogenous Fas and FasL transcripts (Figure 6D) and was sufficient to trigger apoptosis of hematopoietic cells (Figures 6E-6G). Importantly, treatment of hematopoietic cells with an anti-FasL neutralizing antibody inhibited apoptosis triggered by E2F1 knockdown (Figure S5D).
To establish a direct link between E2F1 and Fas/FasL expression, we utilized promoters of these two genes in promoter-reporter assays, and gauged their activity in the presence of ectopically expressed E2F1, E2F2 or E2F3. We observed that ectopic expression of E2F1 repressed activity of Fas and FasL promoters, whereas E2F2 or E2F3 did not have a repressive effect (Figures 6H, and S5E). We next flow sorted hematopoietic stem/progenitor cells from wild type mice and examined the association of the endogenous E2F1 with the Fas gene promoter using chromatin immunoprecipitation (ChIP). We observed that E2F1 physically associates with the Fas promoter in HSPC (Figures 6I, and S5F). Moreover, deletion of the E2F1-binding region (defined by the ChIP analysis) from the Fas promoter abrogated the repressive effect of E2F1 in promoter-reporter assays (Figures S5G and S5H). Furthermore, the effect of E2F1 on the wild-type Fas promoter was essentially lost when E2F1 DNA non-binding and transactivation-deficient mutant (Hsieh et al., 1997) was used in these assays (Figure S5G).
Collectively, our findings suggest that the cyclin D → E2F1 → Fas signaling axis represents an important regulatory pathway of the apoptotic machinery in vivo (Figure 7). In hematopoietic cells E2F1 physically binds the promoter of Fas (and likely FasL) genes and represses their expression. Ablation of D-cyclins causes strong reduction of E2F1 levels and results in de-repression of the Fas and FasL genes. This leads to upregulation of Fas and FasL proteins and triggers ‘extrinsic’, caspase 8-dependent apoptosis. Consistent with this model, inhibition of Fas/FasL signaling protects hematopoietic cells from apoptosis triggered by cyclin D-ablation, while knockdown of E2F1 causes upregulation of Fas/FasL and induces apoptosis despite the continued expression of D-cyclins.
DISCUSSION
D-type cyclins represent components of the ancestral core cell cycle machinery, that has been conserved from yeast to humans. It is well established that the major function of D-cyclins is to drive cell proliferation. This notion is supported by overwhelming evidence revealing that germline ablation of D-cyclins, or knock-down of D-cyclins in cancer cells, ectopic expression of cyclin D-Cdk4/6 inhibitor p16INK4a, or treatment of cells with a chemical inhibitor of cyclin D-associated kinase, all resulted in cell cycle arrest (Fry et al., 2004; Lukas et al., 1995; Sherr and Roberts, 2004).
It has recently become increasingly clear, however, that the function of cyclin D-Cdk4/6 complexes is not restricted to regulation of the cell cycle, and that the consequences of inhibition of these complexes extend beyond a transient cell cycle arrest (Choi and Sicinski, 2013). For instance, D-cyclins, together with their catalytic partner proteins, Cdk4 and Cdk6, are powerful suppressors of cellular senescence (Zou et al., 2002; Ruas et al., 2007; Puyol et al., 2010; Anders et al., 2011).
In order to delineate the in vivo functions of D-cyclins in an adult organism, in this study we examined the consequences of an acute shutdown of all three D-cyclins in bone marrow cells of adult mice. We focused on this compartment, because our previous work revealed that during embryogenesis D-cyclins are essential for proliferation of hematopoietic stem/progenitor cells (Kozar et al., 2004). Thus, constitutive, germline ablation of all three D-type cyclins resulted in a proliferative block in embryonic liver hematopoietic stem/progenitor cells, consistent with the well-established function for D-cyclins in driving cell proliferation (Kozar et al., 2004). Unexpectedly, we now observed that ablation of D-cyclins in hematopoietic cells of adult mice triggered apoptosis. All hematopoietic cells died after shutdown of D-cyclins, indicating that D-cyclins are required for survival of all bone marrow cell types. Importantly, we found that adult hematopoietic stem cells are particularly sensitive to cyclin D loss, as apoptosis occurred first in stem cell compartments. These findings reveal that D-cyclins play a previously unanticipated major pro-survival function in the cells of adult organisms.
It remains to be seen why D-cyclins play a pro-survival role in adult bone marrow cells, but not in embryonic cells. One possibility is that in adult hematopoietic cells E2F1 transcription factor plays a rate-limiting role in repressing Fas and FasL expression, whereas in embryonic cells Fas/FasL genes are repressed by additional mechanism(s). Consequently, ablation of D-cyclins, and concomitant loss of E2F1 expression would cause apoptosis in adult hematopoietic cells but not in cells of a developing embryo.
It was originally reported, based largely on in vitro cell culture systems, that E2Fs1-3 act as activators of transcription, and can induce pro-apoptotic genes when overexpressed (reviewed in DeGregori and Johnson, 2006; Iaquinta and Lees, 2007). However, genetic experiments from the Leone and Bremner groups revealed that E2Fs1-3 can function in mouse development as transcriptional repressors. Thus, conditional ablation of E2Fs1-3 in retinas, intestine and in bone marrow resulted in an increased apoptotic rate, although not as wide-spread as the one described here upon cyclin D loss (Chen et al., 2009; Chong et al., 2009; Trikha et al., 2011). In the context of retinas, the apoptosis was caused by altered expression of an E2F target, Sirt1 (Chen et al., 2009). However, Sirt1 expression was not affected by ablation of D-cyclins in bone marrow cells of MxTKO mice (data not shown). Trikha et al. (2011) used MxCre mice to delete E2Fs1-3 in bone marrow cells, and observed increased apoptosis in the myeloid cells. The authors did not report the molecular basis of this phenomenon, but observed that E2Fs1-3 act in this lineage as transcriptional repressors. It should be noted that Trikha et al. (2011) used constitutive E2F1 knockout mice that could have masked the full effect of a sudden E2F1 loss due to possible compensatory mechanisms.
Our study suggests a model that in adult hematopoietic cells D-cyclins inhibit apoptosis by maintaining levels of E2F1, which represses the death receptor Fas and its ligand, FasL (Figure 8). Cellular apoptosis can be mediated by two distinct pathways: an ‘extrinsic’ pathway triggered by the engagement of the death receptor, which involves activation of caspase 8, and an ‘intrinsic’ pathway propelled by mitochondrial damage, which causes release of cytochrome c and activation of caspase 9 (Strasser et al., 2009). Our study revealed that D-cyclins control the extrinsic pathway, as evidenced by Fas-mediated activation of caspase 8 upon cyclin D ablation, the lack of mitochondrial involvement at early stages of apoptosis, and by the ability of anti-FasL antibodies to block apoptosis of hematopoietic cells. It will be interesting to determine whether breeding of cyclin D-triple knockout animals into Fas-null background (Adachi et al., 1995) would rescue at least some phenotypic manifestations of cyclin D-deficiency.
One of the most unexpected findings from our study is the observation that D-cyclins play a pro-survival function also in quiescent hematopoietic stem/progenitor cells. According to the current understanding, D-cyclins are expressed and function in proliferating cells. However, we demonstrated that quiescent hematopoietic stem cells express D-cyclins, and that D-cyclins protect these cells from apoptosis. At present, the extreme paucity of hematopoietic stem cells precludes detailed mechanistic analyses specifically in this cell type.
The work presented in this study revealed an unexpected anti-apoptotic function of critical cell cycle proteins. The functional significance of our findings is highlighted by the observation that widespread apoptosis represented a major consequence of cyclin D ablation in the bone marrow of adult animals. These analyses reveal that D-cyclins play a particularly important role in maintaining cellular mass, both by driving cell division and by acting as essential, rate-limiting regulators of cell survival.
EXPERIMENTAL PROCEDURES
Generation of Triple-Knockout Cyclin D1F/FD2−/−D3F/F Mice and Ablation of D-Cyclins in Bone Marrow
Genotyping of cyclin D triple-knockout mice is described in Supplemental Experimental Procedures. These animals were crossed with Mx1-Cre mice (from Jackson Laboratory). To delete D-cyclins in hematopoietic cells, 6-10 weeks-old Mx1-Cre+;D1F/FD2−/−D3F/F (MxTKO) and control Mx1-Cre+;D1F/+D2+/−D3F/+ animals were injected intraperitoneally, 1-5 times on alternate days, with 2.5 μg/g body weight of polyinosinic-polycytidylic acid (pI-pC, Amersham).
Histological Analyses and In Vivo BrdU Incorporation Assay
Paraffin-embedded 5 μm-thick sections were stained with Wright-Giemsa, hematoxylin and eosin, BrdU or TUNEL as described below. For BrdU incorporation, mice were injected intraperitoneally with 1mg of BrdU and sacrificed after 2 hours. Bone marrow cells were harvested, assayed with BrdU-Flow kit (BD Biosciences) and analyzed by FACS.
Apoptosis Assays
The apoptotic rate was quantified by TUNEL staining using in situ cell death kit (Roche, Germany) or by FACS analysis using ApoBrdU kit (Phoenix Flow Systems). Cleaved caspase 3 and cleaved caspase 8 were quantified by intracellular staining (CaspGlow 3 or CaspGlow 8 kits from Biovision). To gauge apoptosis of HSC or HSPC, cells were stained with Annexin V-FITC and 7AAD (BD Biosciences) and analyzed by FACS.
Flow Cytometry, Hematological Analyses and Bone Marrow Reconstitution
Description of hematological and flow cytometric analyses, including staining of HSC and HSPC (LKS), as well as non-competitive marrow transplantation assays are described in the Supplemental Experimental Procedures.
Knockdown of E2F1 in Bone Marrow Cells
Primary wild-type bone marrow cells were cultured in RPMI supplemented with 10% heat-inactivated FBS (Hyclone), Pen/Strep (100U/ml, Invitrogen), mSCF (1 or 10ng/ml), mIL-3 (10ng/ml; Peprotech) and mIL-6 (10ng/ml; Peprotech), and infected with shE2F1 lentivirus (sc-35247, Santa Cruz) or Scramble control lentivirus (sc-108080, Santa Cruz) and assayed for knockdown of E2F1 using RT-qPCR and nested PCR (sc-35247PR). Three anti-E2F1 shRNA sequences were used: (a) 5’-CGC TAT GAA ACC TCA CTA ATT CAA GAG ATT AGT GAG GTT TCA TAG CGT TTT T-3’; (b) 5’-CCA AGA AGT CCA AGA ATC ATT CAA GAG ATG ATT CTT GGA CTT CTT GGT TTT T-3’ and (c) 5’-GAA GGA TGT TGT ACA GTG TTT CAA GAG AAC ACT GTA CAA CAT CCT TCT TTT T-3’. In experiments shown in Figure S5D, 50 μg/ml of anti-FasL neutralizing antibody (clone MFL3, BioLegend) or isotype control Hamster IgG (clone HTK888, BioLegend) was added to the culture medium contemporaneously with E2F1 knockdown.
QVD-OPh Caspase Inhibitor Rescue In Vivo
MxTKO and MxCntrl mice were injected once with pI-pC and twice with QVD-OPh (irreversible broad-spectrum caspase inhibitor, 25 mg/kg body weight, from Biovision) on days 0 and 3. On day 6, mice were sacrificed and bone marrow sections were stained with hematoxylin and eosin or TUNEL. In addition, TUNEL staining was quantified by FACS.
Anti-FasL Neutralization in Vivo
MxTKO and MxCntrl mice were injected intraperitoneally once with pI-pC plus one injection of either anti-FasL neutralizing antibody (clone MFL3, BioLegend), or isotype control Hamster IgG (clone HTK888, BioLegend). Mice were sacrificed 48 hours after treatment, and HSC and HSPC were analyzed by Annexin V/7AAD staining.
Western Blotting and Fas/FasL Surface Staining
Whole cell extracts were prepared from bone marrows and proteins solubilized as described before (Anders et al., 2006), immunoblotted and probed with antibodies against cyclin D1 (Neomarker), cyclin D3 (C-16, Santa Cruz), E2F1 (Santa Cruz), E2F2 (Santa Cruz), E2F3 (Santa Cruz), Fas (Millipore), Cre (Novagen), cleaved caspase 8 (8592, Cell Signaling), tubulin (DM1A, Sigma) and hemagglutinin (HA.11, Covance). For pRB phosphorylation analysis, cells were fixed, permeabilized, and stained with phycoerythrin (PE)-mouse antiphospho-pRbS780 antibody (BD Biosciences) or isotype IgG1 control and analyzed by FACS. For surface staining of bone marrow cells, anti-Fas-PE (BD Biosciences) and anti-FasL-FITC or -PE (BioLegend) antibodies were used, followed by FACS analysis.
Apoptosis Array and Gene Expression Analysis
Expression analyses of apoptotic target genes were conducted as described in Supplemental Experimental Procedures. The levels of Fas, FasL, E2F1, E2F2 E2F3, Bax, Noxa, Puma, cyclin D1, D2, D3, E1, E2 and A2 transcripts were quantified in total bone marrow, HSPC, and HSC by reverse-transcription – quantitative PCR as described in Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
D-cyclins perform a rate-limiting anti-apoptotic function in vivo
D-cyclins control the levels of Fas and Fas ligand via E2F1 and repress cell death
Adult hematopoietic stem cells are particularly dependent on D-cyclins for survival
D-cyclins play an anti-apoptotic function also in quiescent stem/progenitor cells
ACKNOWLEDGEMENTS
We thank Drs. R. Bremner, T. Yu, G. Leone, T. Prashant , N. Dyson, K. Tschoep, P. Farnham, M. Gaddis, J. Nevins and L. Jakoi, X. Lu, L. Fajas, A. Jean-Sebastien, K. Helin and W. Kaelin for various E2F reagents, W. Sellers for the FasL promoter construct, R. Shivdasani, N. Kodandaramireddy, J. Quackenbush and Rene Rubio for help and advice. This work was supported by R01 CA108420 grant from NIH (to PS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five Supplemental Figures and one Supplemental Table.
REFERENCES
- Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P, Sicinski P. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21:469–476. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature. 2009;462:925–929. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1809. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Hydbring P, Sanda T, Stefano L, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer H, Sicinski P. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Sicinski P. Unexpected outcomes of CDK4/6 inhibition. Oncotarget. 2013;4:176–7. doi: 10.18632/oncotarget.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AM, Myant K, Reed KR, Ridgway RA, Athineos D, et al. Cyclin D2-cyclin-dependent kinase 4/6 is requried for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 2010;70:8149–58. doi: 10.1158/0008-5472.CAN-10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739–748. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- Ethell DW, Buhler LA. Fas ligand-mediated apoptosis in degenerative disorders of the brain. J Clin Immunol. 2003;23:439–446. doi: 10.1023/b:joci.0000010420.96419.a8. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J-K, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- Iaquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. Curr Opin Cell Biol. 2007;19:649–657. doi: 10.1016/j.ceb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Ronai Z, Hei TK. Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem. 2006;281:1840–1852. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases CDK4 and CDK6. Cell. 2004;118 doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman E, Flemington EK, Sellers WR, Kaelin WG., Jr. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol Cell Biol. 1994;14:6607–6615. doi: 10.1128/mcb.14.10.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Gandara R, Mahida YR, Loeffler M, Wright MA. The stem cells of small intestinal crypts; where are they? Cell Prolif. 2009;42:732–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, Guerra C, Santamaria D, Barbacid M. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Seita J, Czechowicz A, Bhattacharya D, Bryder D, Weissman IL. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6:2371–2376. doi: 10.4161/cc.6.19.4759. [DOI] [PubMed] [Google Scholar]
- Ruas M, Gregory F, Jones R, Poolman R, Starborg M, Rowe J, Brookes S, Peters G. CDK4 and CDK6 Delay Senescence by Kinase-Dependent and p16INK4a-Independent Mechanisms. Mol. Cell. Biol. 2007;27:4273–4282. doi: 10.1128/MCB.02286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai CM, Freund J, Oh P, Ndiaye-Lobry D, Bretz JC, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell. 2012;22:452–465. doi: 10.1016/j.ccr.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474.psok. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha P, Sharma N, Opavsky R, Reyes A, Pena C, Ostrowski MC, Roussel MF, Leone G. E2f1-3 are critical for myeloid development. J Biol Chem. 2011;286:4783–4795. doi: 10.1074/jbc.M110.182733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nature Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Yang R, Bie W, Haegebarth A, Tyner AL. Differential regulation of D-type cyclins in the mouse intestine. Cell Cycle. 2006;5(2):180–3psok. doi: 10.4161/cc.5.2.2306. [DOI] [PubMed] [Google Scholar]
- Zou X, Ray D, Aziyu A, Christov K, Boiko AD, Gudkov AV, Kiyokawa H. Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev. 2002;16:2923–2934. doi: 10.1101/gad.1033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.