Abstract
Female spontaneously hypertensive rats (SHR) have more regulatory T cells (Tregs) in their kidneys than males. The goal of this study was to determine the impact of blood pressure (BP) on the renal immune profile. We hypothesize that increases in BP promote a pro-inflammatory renal T cell and cytokine profile in SHR, although females will have greater hormone-dependent increases in Tregs and males will have greater increases in Th17 cells. Renal T cell and cytokine profiles were assessed in male and female WKY and male and female SHR treated with vehicle or hydrochlorothiazide and reserpine from 6 to 12 (6-HCTZ) or 11 to 13 weeks of age (2-HCTZ). Regardless of sex, SHR had a more pro-inflammatory renal immune profile than WKY. 6-HCTZ attenuated age-related increases in BP and 2-HCTZ reversed hypertension compared to vehicle-treated SHR. Neither 6-HCTZ nor 2-HCTZ altered CD3+, CD4+, or CD8+ T cells in either sex. Both treatments decreased Tregs only in female SHR abolishing sex differences in Tregs. 6-HCTZ has no impact on Th17 cells in either sex and 2-HCTZ had a minimal impact on renal Th17 cells. To further assess mechanisms mediating sex differences in the renal immune profile, male and female SHR were gonadectomized to determine the impact of sex hormones. Gonadectomy increased pro-inflammatory markers in both sexes, suggesting that both male and female sex hormones are anti-inflammatory. In conclusion, BP contributes to sex differences in the renal T cell profile of SHR; female SHR increase renal Tregs in response to increases in BP.
Keywords: hypertension, gender, T lymphocytes, Th17 cells, kidney, cytokines
Introduction
T cells play a pathogenic role in the development and progression of numerous cardiovascular diseases, including hypertension. T cells contribute to the development of hypertension in genetic, angiotensin (Ang)-II and salt-sensitive male experimental animals 1-3. Furthermore, elevated blood pressure (BP) is associated with an increase in renal CD3+ T cells in male experimental models of hypertension 3, while decreases in BP correlate with decreased immune cell infiltration 3-5. Male spontaneously hypertensive rats (SHR) have greater renal T cell infiltration and pro-inflammatory cytokine expression compared to normotensive Wistar Kyoto rats (WKY) as early as 3 weeks of age 6-12, suggesting that SHR exhibit a pro-inflammatory immune profile even before BP increases. We recently demonstrated that there are sex differences in T cells in kidneys of SHR 13; males have more pro-inflammatory Th17 cells and females have more immune-suppressive regulatory T cells (Tregs). However, the mechanisms responsible for sex differences in the renal T cell profile of SHR and the impact of BP status on the T cell profile of females remain unknown.
Cytokines are key determinants in coordinating immune responses and have also been implicated in BP control in male experimental models of hypertension. In male mice, interleukin (IL)-6 contributes to the development of high-salt and Ang-II induced hypertension 14, 15. Similarly, IL-17 has been suggested to be critical in promoting Ang-II induced hypertension in male mice 16. In contrast, IL-10 protects against hypertension-induced vascular dysfunction and attenuates increases in BP 17, 18. These studies demonstrate that cytokines also play a role in BP control, yet it is unclear whether or not sex influences the cytokine profile in experimental models of hypertension.
Historically, sex differences in hypertension have been attributed to sex hormones and sex hormones are known to impact the immune system 19, 20. In particular, both estrogen and testosterone have been shown to stimulate Treg formation in vitro and in vivo19, 21, 22. Therefore, despite the fact that estrogens are thought to be cardio-protective 23, 24 and androgens are perceived to promote cardiovascular disease 25, 26, both male and female sex hormones have been demonstrated to promote an anti-inflammatory immune profile.
Due to the expanding literature linking T cell infiltration and cytokines with hypertension in male hypertensive experimental models, the overall goal of this study was to determine the impact of sex, sex hormones, and BP status on the T cell and cytokine profile of the kidney. We hypothesize that increases in BP promote a pro-inflammatory renal T cell profile in both sexes of SHR, although females will have greater sex hormone-dependent increases in Tregs and males will have greater increases in Th17 cells.
Materials and Methods (Expanded methods are available in the online Supplement)
Animals
12-13 week old male and female SHR and WKY were used in this study (Harlan Laboratories, Indianapolis, IN). A subset of male and female SHR were gonadectomized at 10 weeks of age and studied at 13 weeks of age as previously described 26, 27.
Hydrochlorothiazide and Reserpine
2 week study (2-HCTZ): 9 week old male and female SHR were implanted with telemetry devices (Data Sciences International, St. Paul, MN) to record BP 13. Rats were allowed 1 week recovery and 1 week of stable baseline recording. At 11 weeks of age, rats were randomized to receive vehicle or hydrochlorothiazide (55 mg/kg/day) and reserpine (4.5 mg/kg/day) in drinking water from 11 to 13 weeks of age.
6 week study (6-HCTZ): 6 week old male and female SHR were randomized to receive vehicle or hydrochlorothiazide (10-55 mg/kg/day) and reserpine (0.6-4.5 mg/kg/day) in drinking water until 12 weeks of age. BP was measured weekly via tail-cuff plethsomyography as previously described 28.
For both studies, rats were individually housed throughout the study. Water intake and body weights were measured every three days and the doses of drugs titrated as needed to maintain consistent BP lowering (in Online Supplement Tables S1 and S2).
Statistical Analysis
All data are presented as mean ± SE. Flow cytometry data were compared using two-way ANOVA followed by a Bonferroni post-hoc test. Telemetry, tail cuff, body weight, and water intake data within each sex were analyzed using repeated-measures ANOVA. Telemetry and tail cuff data between sexes and between vehicle and treated rats were compared using T-test. Analyses were performed using GraphPad Prism Version 5.0 software (GraphPad Software Inc, La Jolla, CA) and for all comparisons, differences were considered statistically significant with P<0.05.
Results
SHR have greater renal T cell and cytokine expression than WKY, regardless of sex
Male SHR have a higher systolic BP than age-matched female SHR (mmHg: males 203±8, n=6; females 170±1, n=6; effect of sex: P=0.005). Male and female WKY had lower BP than SHR (mmHg: males 135±3, n=4; females 127±2 n=6; effect of strain: P<0.0001), although BP was comparable between male and female WKY (interaction: P=0.02).
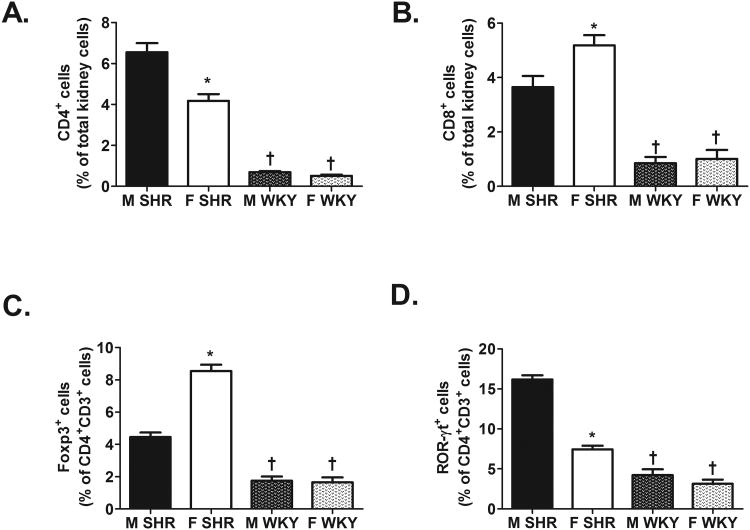
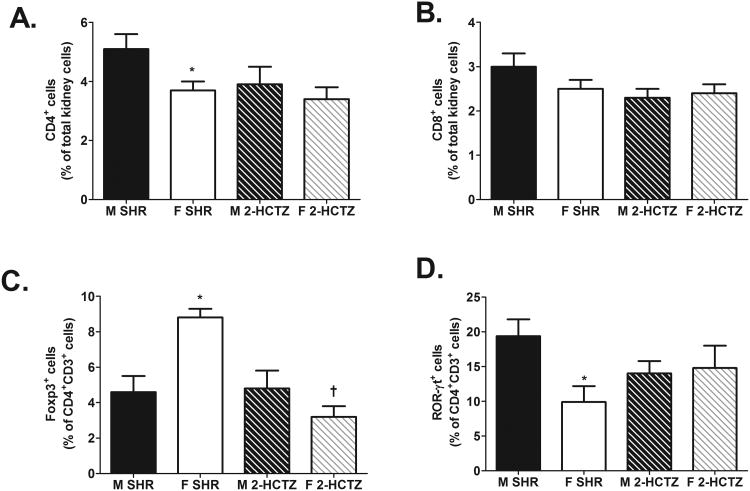
Regardless of sex, WKY had significantly fewer renal CD3+ T cells (expressed as % of total kidney cells: male SHR: 9±0.6%, female SHR: 7±0.5%; male WKY: 2±0.3%, and female WKY: 2±0.3%; effect of strain: P<0.0001; n=11-13), CD4+ T cells, CD8+ T cells, Tregs, and Th17 cells compared to same-sex SHR (Fig 1; for all effect of strain: P<0.0001). Sex differences in the renal T cell profile of male and female SHR from our previous study were confirmed in the present study 13, but there were no sex differences in any of the T cell subtypes in kidneys of WKY (Fig. 1; CD4+ effect of sex and interaction: P=0.0001; CD8+ effect of sex: P=0.01, interaction: P=0.04; Treg and Th17 effect of sex and interaction: P<0.0001).
Figure 1.
T cell profile in kidneys of 12-13 week old male and female SHR (n=11) and WKY (n=13). Shown are the percent of CD4+ T cells (panel A), CD8+ T cells (panel B), Foxp3+ Tregs (panel C), and ROR-γt+ Th17 cells (panel D). * indicates p<0.05 vs. same strain male; † indicates p<0.05 vs. sex matched SHR.
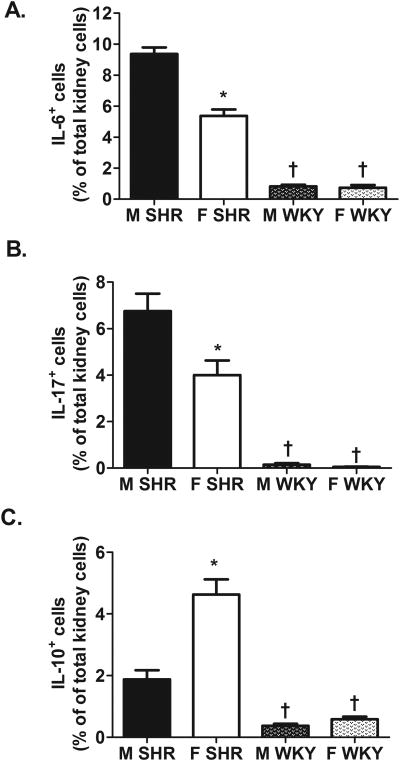
To further characterize the renal immune profile, renal cytokine levels were measured via flow cytometric analysis. Consistent with data in Figure 1, male SHR had significantly more renal cells expressing the pro-inflammatory cytokines IL-6 and IL-17 than female SHR (Fig. 2A and B; IL-6 effect of sex: P<0.0001 and IL-17 effect of sex: P=0.0006). In contrast, female SHR had more IL-10+ cells than male SHR (Fig. 2C; effect of sex: P<0.0001). Expression of IL-6, IL-17, and IL-10 were also greater in SHR compared to WKY, regardless of sex (Fig. 2; for all effect of strain: P<0.0001). Expression of all 3 cytokines were comparable in male and female WKY (IL-6 and IL-10 effect of sex and interaction: P<0.0001; IL-17 effect of sex: P=0.0006, interaction: P=0.0012).
Figure 2.
Cytokine profile in kidneys of 12-13 week old male and female SHR (n=8) and WKY (n=13). Shown are the percent of IL-6+ renal cells (panel A), IL-17+ renal cells (panel B), and IL-10+ renal cells (panel C). * indicates p<0.05 vs. same strain male; † indicates p<0.05 vs. sex matched SHR.
Attenuating age-related increases in BP prevents the development of a sex difference in renal Tregs
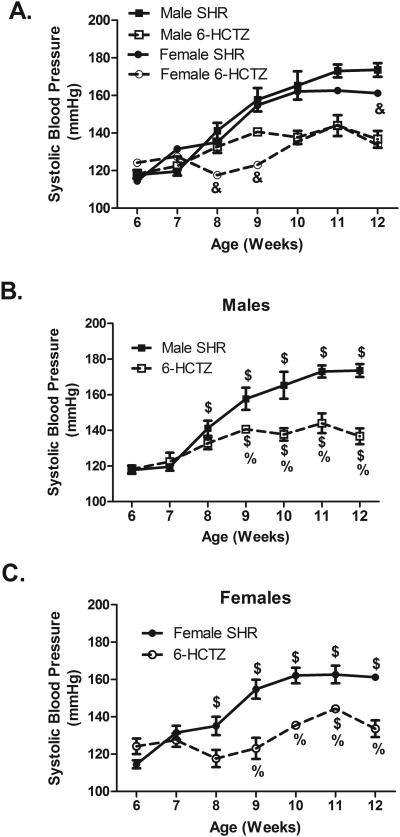
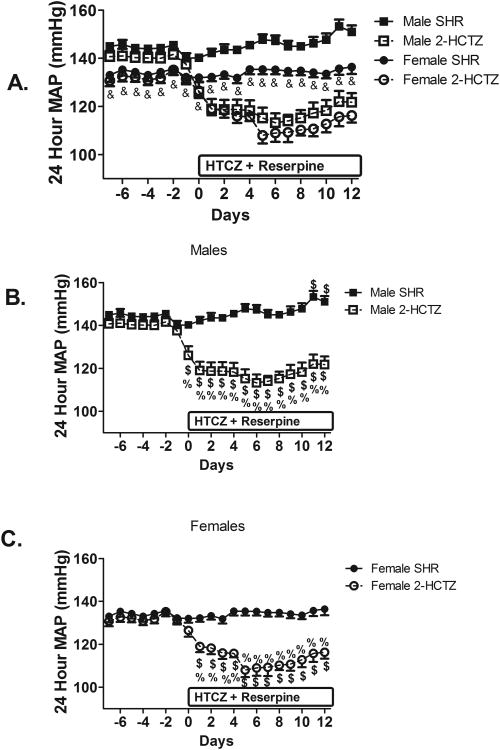
Male and female SHR were administered hydrochlorothiazide and reserpine (6-HCTZ) in the drinking water from 6 to 12 weeks of age. 6-HCTZ treatment attenuated age-related increases in BP in SHR and abolished the sex difference in BP observed in 12 week old vehicle-treated SHR (Fig. 3). 6-HCTZ treatment did not change weight gain or water intake compared to vehicle treated animals (Table S1).
Figure 3.
Systolic BP in male (panel B), female (panel C), and both sexes of SHR (panel A) treated from 6 to 12 weeks of age with hydrochlorothiazide and reserpine; n=5. & indicates p<0.05 vs. male SHR from same treatment conditions; $ indicates p<0.05 vs. same sex and treatment at 6 weeks of age; % indicates p<0.05 vs. same sex and age vehicle control SHR.
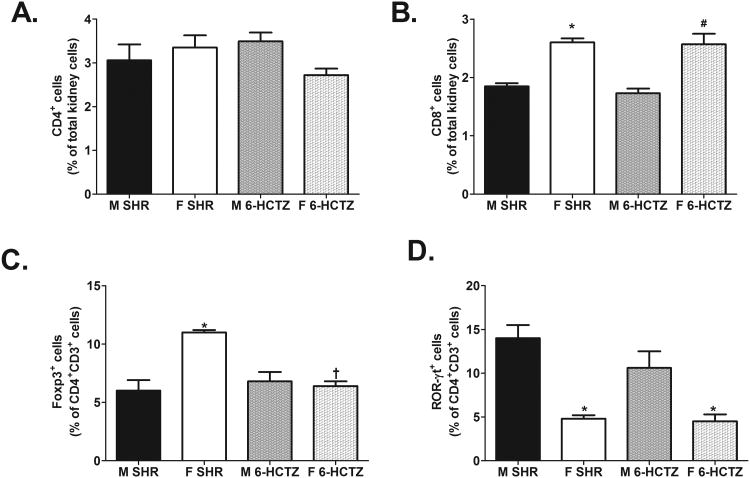
6-HCTZ treatment did not significantly alter renal CD3+ (expressed as % of total kidney cells: M SHR: 4±0.2%; M 6-HCTZ: 4±0.3%; F SHR: 5±0.5%; F 6-HCTZ: 5±0.3%; effect of treatment: P=0.8; n=5-6), CD4+, or CD8+ T cells compared to same sex vehicle controls (Fig. 4A and 4B: CD4+ effect of treatment: P=0.7; CD8+ effect of treatment: P=0.5). However, attenuating age-related increases in BP significantly decreased the percent of renal Tregs only in female SHR, thereby abolishing the sex difference in renal Tregs (Fig. 4C; effect of sex: P=0.0007; effect of treatment: P=0.004; interaction: P=0.0005). 6-HCTZ treatment did not significantly alter Th17 cell counts in either sex, and males treated with 6-HCTZ maintained more renal Th17 cells than treated females (Fig. 4D; effect of sex: P<0.0001; effect of treatment: P=0.2; interaction: P=0.2).
Figure 4.
T cell profile in kidneys of male and female SHR treated from 6 to 12 weeks of age with hydrochlorothiazide and reserpine; n=5-6. Shown are the percent of CD4+ T cells (panel A), CD8+ T cells (panel B), Foxp3+ Tregs (panel C) and ROR-γt+ Th17 cells (panel D). * indicates p<0.05 vs. same strain male; † indicates p<0.05 vs. sex matched SHR; # indicates p<0.05 vs. male 6-HCTZ.
Reversing hypertension in adult SHR abolishes the sex difference in Tregs and minimizes the sex difference in Th17 cells
To determine if sex differences in renal Tregs could also be abolished by reversing hypertension, additional male and female SHR were administered hydrochlorothiazide and reserpine from 11 to 13 weeks of age (2-HCTZ). Baseline BP was greater in male SHR than female SHR (Figure 5; P<0.05). 2-HCTZ treatment lowered BP relative to same-sex vehicle controls in both males and females and abolished the sex difference in BP. Body weight and daily water intake increased in both sexes from 11 to 13 weeks of age, but there were not differences between vehicle and treated groups of the same sex (Table S2).
Figure 5.
Mean arterial pressure (MAP) in male and female SHR treated from 11 to 13 weeks of age with hydrochlorothiazide and reserpine; n=4-7. & indicates p<0.05 vs. male SHR from same treatment conditions; $ indicates p<0.05 vs. same sex and treatment at Day 0; % indicates p<0.05 vs. same sex and age vehicle control SHR.
2-HCTZ treatment did not affect renal CD3+ T cells (expressed as % of total kidney cells: M SHR: 5±0.8%; M 2-HCTZ: 5±0.7%; F SHR: 5±0.3%; F 2-HCTZ: 5±0.4%; effect of treatment: P=0.5; n=4-7), CD4+, or CD8+ T cells (Fig 6A and 6B; CD4+ effect of treatment: P=0.1; CD8+ effect of treatment: P=0.1). However, decreasing BP significantly lowered Tregs in female SHR relative to vehicle-treated female SHR with no effect in males and the sex differences in Tregs observed in control SHR was abolished (Fig. 6C; effect of sex: P=0.2; effect of treatment: P=0.003; interaction, P=0.002). In contrast to the 6-HCTZ treatment, 2-HCTZ treatment also minimized the sex difference in Th17 cell counts in SHR (Fig. 6D; effect of treatment: P=0.9; effect of sex: P=0.09; interaction: P=0.06).
Figure 6.
T cell profile in kidneys of male and female SHR treated from 11 to 13 weeks of age with hydrochlorothiazide and reserpine; n=5-8. Shown are the percent of CD4+ T cells (panel A), CD8+ T cells (panel B), Foxp3+ Tregs (panel C) and ROR-γt+ Th17 cells (panel D). * indicates p<0.05 vs. same strain male; † indicates p<0.05 vs. sex matched SHR.
Sex hormones are anti-inflammatory in SHR regardless of sex
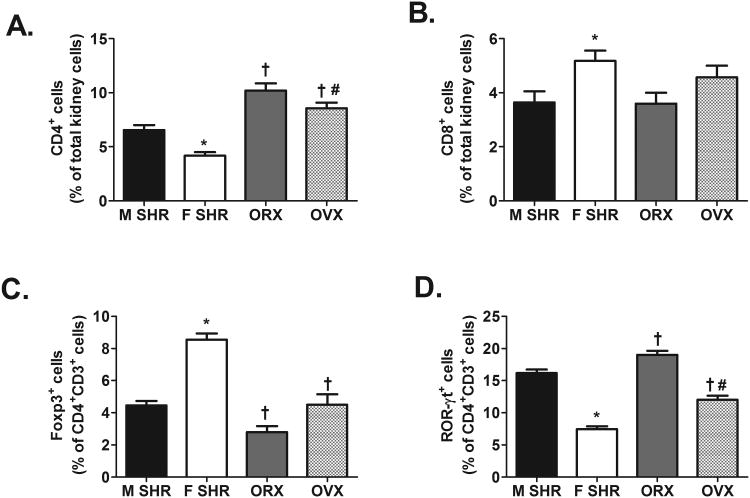
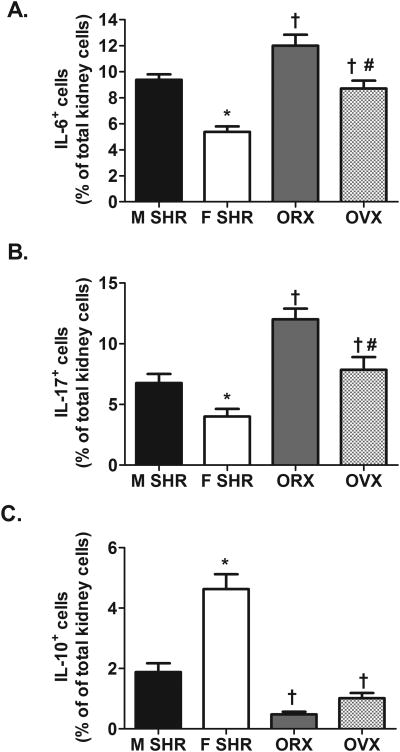
To gain additional mechanistic insight into sex differences in the immune profile of SHR, renal T cell and cytokine profiles were measured in gonad-intact and gonadectomized male (orchidectomized, ORX) and female (ovariectomized, OVX) SHR. Sex differences observed in the renal T cell profile of gonad-intact SHR were verified. Gonadectomy had no effect on renal CD3+ T cell counts in either sex (CD3+ expressed as % of total kidney cells: intact male: 9±1%; ORX: 8±1%; intact females: 7±1%; OVX: 9±1%; n=5-11; effect of sex hormones: P=0.4). Gonadectomy increased renal CD4+ T cells and Th17 cells relative to gonad-intact males and females; however the increase was comparable in both sexes such that ORX maintained more renal CD4+ T cells and Th17 cells than OVX (Fig. 7A and 7D; CD4+ effect of sex: P=0.0003; Th17 effect of sex: P<0.0001; for both effect of sex hormones: P<0.0001; CD4+ interaction: P=0.5; Th17 interaction: P=0.2). Gonadectomy did not change renal CD8+ T cells in either sex, therefore OVX maintained more CD8+ T cells than ORX (Fig. 7B; effect of sex: P=0.008; effect of sex hormones: P=0.5; interaction: P=0.5). Tregs were decreased by gonadectomy in both sexes, although, OVX resulted in a greater decrease in Tregs than ORX (Fig. 7C; effect of sex: P<0.001; effect of sex hormones: P<0.0001; interaction, P=0.01). Gonadectomy also increased the number of IL-6+ and IL-17+ cells to a similar degree in both sexes thereby maintaining the sex difference observed in gonad-intact SHR (Fig. 8; for both effect of sex: P<0.0001; for both effect of sex hormones: P<0.0001; IL-6 interaction: P=0.5; IL-17 interaction: P=0.4). In contrast, gonadectomy decreased IL-10+ renal cells in both sexes (Fig 8; effect of sex: P<0.0001; effect of sex hormones: P<0.0001), however, the decrease in renal IL-10 was greater in OVX than in ORX (interaction: P=0.004).
Figure 7.
T cell profile in kidney of 12-13 week old intact (n=11) and gonadectomized (n=5-7) male and female SHR. Shown are the percent of CD4+ T cells (panel A), CD8+ T cells (panel B), Foxp3+ Tregs (panel C) and ROR-γt+ Th17 cells (panel D). * indicates p<0.05 vs. gonad-intact male SHR; † indicates p<0.05 vs. gonad-intact, sex matched SHR; # indicates p<0.05 vs. ORX.
Figure 8.
Cytokine profile in kidneys12-13 week old intact (n=11) and gonadectomized (n=5-7) male and female SHR. Shown are the percent of IL-6+ renal cells (panel A), IL-17+ renal cells (panel B), and IL-10+ renal cells (panel C). * indicates p<0.05 vs. gonad-intact male SHR; † indicates p<0.05 vs. gonad-intact, sex matched SHR; # indicates p<0.05 vs. ORX.
Discussion
The majority of studies showing increased immune cell infiltration in experimental models of hypertension have been conducted in males, despite the fact that females account for ∼50% of all hypertensive cases in the United States. We recently published that there are sex differences in the immune profile of SHR; female SHR have more renal Tregs while males have greater Th17 cells counts 13. The major novel finding of the current study is that the sex differences in renal Tregs in young, adult SHR is BP dependent. There are no apparent sex differences in the renal immune profile of normotensive WKY, and lowering BP in SHR abolished the sex difference in Tregs by decreasing counts in females. In addition, both male and female sex hormones are anti-inflammatory. Therefore while female sex hormones likely contribute to the higher incidence of Tregs in females relative to males, sex hormones alone cannot account for sex differences in the immune profile of SHR. Taken together, the current studies suggest that Tregs serve as an important feedback mechanism which may account for the consistently lower BP in female SHR relative to males.
It is well established that hypertension is associated with an increase in renal T cells in male experimental models of hypertension 3-5. The current study expands our understanding of the immune system in hypertension by demonstrating that hypertensive females also exhibit greater renal T cell counts than normotensive females which is consistent with our previous publication demonstrating that lymphocyte suppression decreases BP in female SHR 13. Consistent with reports showing greater CD3+ renal T cell infiltration in male SHR relative to male WKY 29, SHR of both sexes also have more renal CD4+ and CD8+ T cells, Tregs, and Th17 cells than same sex WKY. Expanding on our previous results and consistent with a sex difference in the T cell profile of male and female SHR 13, the current study also demonstrates that male SHR also have higher levels of the pro-inflammatory cytokines, IL-6 and IL-17, whereas females had greater numbers of kidney cells expressing the anti-inflammatory cytokine IL-10. In contrast to our findings, male SHR have been reported to have lower levels of circulating IL-6 than female SHR 30, 31, however, the authors did not report tissue levels and the local cytokine environment of the kidney may be quite different from the circulation. Indeed, we published that male SHR have fewer circulating Th17 cells and more Tregs compared to female SHR, underscoring the importance of measuring local levels of cytokines and immune cells at the tissue level. It should be noted that cytokines in the current study were assessed by flow cytometry and reflect the percent of total kidney cells that are positive for the individual cytokines. This is a potential limitation of the current study, however, multiple commercially available ELISA's were tested and levels in the rat were below the sensitivity threshold of the individual assays. Based on the key these cytokines have been shown to play in BP control in other studies, future studies will determine the impact of these cytokines on BP and renal function in male and female SHR.
Although the current study clearly demonstrates greater numbers of renal T cells in SHR compared to WKY, they do not conclusively link greater T cells in SHR to higher BP. Indeed, strain differences in renal T cells between male SHR and WKY have been reported to be evident as early as 3 weeks of age, well before BP increases in SHR 6, 32. These data suggest that male SHR inherently have more renal T cells than male WKY. Therefore, additional experiments were designed to directly address the impact of elevated BP on the renal T cell profile in SHR. In addition, since male SHR exhibit a more rapid age-related increase in BP compared to females, sex differences in the renal T cell profile in young, adult SHR may simply reflect the higher BP in the male. Attenuating the degree of hypertension and abolishing the sex difference in BP in SHR did not alter total T cell counts in the kidney of either male or female SHR, although there were sex-specific effects on T cell subtypes following BP lowering. Female SHR exhibited a decrease in renal Tregs following an attenuation of the increase in BP or a decrease in BP that was not apparent in males. Therefore, the renal T cell profile is impacted by BP in a sex-dependent manner in SHR and the sex difference in renal Tregs in adult SHR can be explained by a BP-mediated increase in Tregs exclusively in the female.
Our results are consistent with previous reports in the literature showing that inclusion of the BP-lowering drug hydralazine does not alter Ang-II-induced increases in renal CD4+ or CD8+ T cell counts 33. In contrast, we previously published that triple antihypertensive therapy including hydralazine, hydrochlorothiazide and reserpine attenuated L-NAME-mediated increases in renal T cell counts in both sexes of SHR 34. However, hydralazine has been shown to reduce the number of adherent and migrating leukocytes in the vasculature of male SHR independent on an effect on BP 35, therefore the current study did not include hydralazine to lower BP. Hydrochlorothiazide and reserpine were used in the current study to lower BP in order to minimize non-specific drug effects on immune cells. To our knowledge, there are no studies that have reported an effect of hydrochlorothiazide or reserpine on T cell differentiation or tissue infiltration, although, reserpine has been shown to block T cell activation and proliferation in vitro36. While we cannot rule out an effect of HCTZ treatments on the immune profile independent of blood pressure, it seems unlikely that this would impact only one T cell subtype in one sex. However, this is a potential limitation of the current study. Since Ang-II has been demonstrated to impact immune cells 4, 37, pharmacological drugs that alter the renin angiotensin system were not used.
Attenuating age-related increases in BP had no effect on the renal T cell profile in male SHR, although male SHR maintained more Th17 cells than even though BP was comparable in both sexes. This result suggests that the greater number of Th17 cells in the male SHR does not simply reflect them having a higher BP. In contrast, reversing established hypertension narrowed the sex difference in Th17 cells (p=0.06), due to a non-significant decrease in Th17 cell counts in male SHR with treatment. It is of interest that Th17 cells were potentially differentially altered in SHR depending on whether their BP was allowed to reach hypertensive levels. These data may suggest that although the development of hypertension is not dependent on Th17 cells, the maintenance of hypertension in male SHR may be. There is controversy in the literature regarding the role of the Th17 cell pathway in hypertension. It was initially reported that male IL-17 knockout mice fail to develop Ang-II hypertension 16, although a more recent study demonstrated that knockout of key Th17 cell effector cytokines, IL-17 and IL-23, does not alter increases in BP to DOCA-salt+Ang-II administration in male mice despite a decrease in Th17 cells 38. A separate study also showed that although triple therapy lowers BP in male uninephrectomized Sprague-Dawley rats on a DOCA-salt diet, renal levels of Th17 cells were comparable to control 39. When taken together, these studies call into question the role of Th17 cells in hypertension in either sex. Future studies are needed to directly assess the contribution of the different T cell subtypes to BP control in both sexes.
In addition to increases in BP, aging of SHR from 6 to 12 weeks of age is also associated with sexual maturation and increases in sex hormones. To gain additional mechanistic insight potentially driving sex differences in the renal immune profile and to determine the contribution of sex hormones to the immune profile, we assessed the impact of gonadectomy on the renal T cell and cytokine expression in male and female SHR. Although male sex hormones have primarily been associated with pro-hypertensive pathways in SHR 25, 26 and female sex hormones are thought to be cardio-protective 23, 24, sex hormones in both sexes were anti-inflammatory. However, this result may not be surprising based on available data in the literature. Castration of healthy men reduces circulating Tregs 21 and testosterone supplementation to male experimental autoimmune orchitis rats increases Treg expression in the testis 22. Similarly, estrogen stimulates Treg production in vitro and in vivo in CB57BL/6 mice and estrogen stimulated-conversion of CD4+ T cells into Tregs can be blocked by an estrogen receptor antagonist 19. Regardless, sex hormones cannot account for all of the observed sex differences in the renal T cell profile in SHR. However, gonadectomy of female SHR resulted in a greater decrease in Tregs than in males suggesting that female sex hormones contribute to greater Tregs in female SHR. Future studies will continue to pursue additional mechanisms driving sex differences in the immune profile of SHR.
Perspectives
Despite all of the current therapeutics available for the treatment of hypertension, there is a critical need for new treatment options to increase the percentage of individuals with controlled BP. This is a challenge due to our lack of knowledge regarding the molecular mechanism(s) driving essential hypertension in either sex. In this study, we identify that female SHR have a compensatory increase in renal Tregs in response to elevated BP. We propose that this is one way in which female SHR maintain a lower BP than male SHR 26. Future studies are needed to determine the molecular mechanism(s) responsible for the compensatory increase in renal Tregs in female SHR because it might be a potential therapeutic target for the treatment of hypertension in both sexes.
Supplementary Material
Novelty and Significance.
1) What Is New
The current study supports that there is an increase in pro-inflammatory mediators in both hypertensive males and females. However, female SHR have a compensatory increase in renal Tregs following increases in elevated BP which is not observed in males. This is an important finding that advances the field because it suggests that Tregs, which have been shown to protect against hypertension in various experimental models, serve as an important feedback mechanism which may account for the consistently lower BP in female SHR relative to males.
2) What Is Relevant?
Understanding the factors that lead to differential regulation of the cardiovascular system between male and female is an important endeavor that will ultimately promote better and more individualized treatment strategies. Although it is now widely accepted that the immune system plays an important role in the pathogenesis of hypertension in males, very little is known with regards to how the immune system contributes to disparate cardiovascular responses between males and females.
3) Summary
This study provides an inclusive analysis of the renal immune cell profile in SHR by further categorizing T cells based on subtype and cellular cytokine expression. The major finding of the current study is that Tregs in the kidney are directly influenced by BP in hypertensive females.
Acknowledgments
The authors will like to thank the excellent technical assistance of Erica M. Ralph and Beverly Li.
Sources of Funding: This study was supported by HL093271 to JC Sullivan and a predoctoral fellowship from the American Heart Association Greater Southeast Affiliate to AJ Tipton.
Footnotes
Disclosures: None.
References
- 1.Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011;187:1778–1787. doi: 10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco M, Bautista R, Perez-Mendez O, Gonzalez L, Pacheco U, Sanchez-Lozada LG, Santamaria J, Tapia E, Monreal R, Martinez F. Renal interstitial adenosine is increased in angiotensin II-induced hypertensive rats. American J Physiol Renal Physiol. 2008;294:F84–92. doi: 10.1152/ajprenal.00123.2007. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. American J Physiol Renal Physiol. 2002;282:F191–201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 4.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. American J Physiol Regul Integr Comp Physiol. 2010;298:R1089–1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57:269–274. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Iturbe B, Quiroz Y, Ferrebuz A, Parra G, Vaziri ND. Evolution of renal interstitial inflammation and NF-kappab activation in spontaneously hypertensive rats. Am J Nephrol. 2004;24:587–594. doi: 10.1159/000082313. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. 2011;58:489–496. doi: 10.1161/HYPERTENSIONAHA.111.172114. [DOI] [PubMed] [Google Scholar]
- 8.Andrzejczak D, Gorska D, Czarnecka E. Influence of amlodipine and atenolol on lipopolysaccharide (LPS)-induced serum concentrations of TNF-alpha, IL-1, IL-6 in spontaneously hypertensive rats (SHR) Pharmacol Reports. 2006;58:711–719. [PubMed] [Google Scholar]
- 9.Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. Hdac inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu XP, Pang YJ, Zhu WW, Zhao TT, Zheng M, Wang YB, Sun ZJ, Sun SJ. Benazepril, an angiotensin-converting enzyme inhibitor, alleviates renal injury in spontaneously hypertensive rats by inhibiting advanced glycation end-product-mediated pathways. Clinical Exp Pharmacol Physiol. 2009;36:287–296. doi: 10.1111/j.1440-1681.2008.05078.x. [DOI] [PubMed] [Google Scholar]
- 11.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in deoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension. 2010;55:172–179. doi: 10.1161/HYPERTENSIONAHA.109.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol. 2012;303:R359–367. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magen E, Feldman A, Cohen Z, Alon DB, Minz E, Chernyavsky A, Linov L, Mishal J, Schlezinger M, Sthoeger Z. Circulating endothelial progenitor cells, Th1/Th2/Th17-related cytokines, and endothelial dysfunction in resistant hypertension. Am J Med Sci. 2010;339:117–122. doi: 10.1097/MAJ.0b013e3181c6a968. [DOI] [PubMed] [Google Scholar]
- 15.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 16.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler ThrombVasc Biol. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viel EC, Lemarie CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H938–944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 19.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 20.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- 21.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, Amory JK, Nelson PS, Wu JD. Effect of medical castration on CD4+CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: A physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 22.Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: Evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- 23.Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, Hamar P, Amann K, Ritz E. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRSP rats. J Am Soc Nephrol. 2004;15:348–358. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- 24.Sandberg K, Ji H. Sex and the renin angiotensin system: Implications for gender differences in the progression of kidney disease. Advances Renal Replacement Ther. 2003;10:15–23. doi: 10.1053/jarr.2003.50006. [DOI] [PubMed] [Google Scholar]
- 25.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1573–1579. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 27.Case J, Davison CA. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exper Ther. 1999;291:524–530. [PubMed] [Google Scholar]
- 28.Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in sprague-dawley rats from two suppliers. Am J PhysiolRegul Integr Comp Physiol. 1998;275:R1719–1723. doi: 10.1152/ajpregu.1998.275.5.R1719. [DOI] [PubMed] [Google Scholar]
- 29.Abumiya T, Masuda J, Kawai J, Suzuki T, Ogata J. Heterogeneity in the appearance and distribution of macrophage subsets and their possible involvement in hypertensive vascular lesions in rats. Laboratory Invest J Tech Methods Pathol. 1996;75:125–136. [PubMed] [Google Scholar]
- 30.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R713–719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 31.Dalpiaz PL, Lamas AZ, Caliman IF, Medeiros AR, Abreu GR, Moyses MR, Andrade TU, Alves MF, Carmona AK, Bissoli NS. The chronic blockade of angiotensin I-converting enzyme eliminates the sex differences of serum cytokine levels of spontaneously hypertensive rats. Brazilian J Med Biological Res. 2013;46:171–177. doi: 10.1590/1414-431X20122472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswas SK, de Faria JB. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic Res. 2007;41:216–224. doi: 10.1080/10715760601059672. [DOI] [PubMed] [Google Scholar]
- 33.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 2003;42:31–38. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 34.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol. 2013;305:R701–710. doi: 10.1152/ajpregu.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues SF, de Oliveira MA, dos Santos RA, Soares AG, de Cassia Tostes R, Carvalho MH, Fortes ZB. Hydralazine reduces leukocyte migration through different mechanisms in spontaneously hypertensive and normotensive rats. Eur J Pharmacol. 2008;589:206–214. doi: 10.1016/j.ejphar.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Mekori YA, Blickstein D, Baram D, Alter A, Radnay J, Rozenszajn LA, Ravid M. Characterization of the interference of T cell activation by reserpine. Cellular Immunol. 1989;124:308–319. doi: 10.1016/0008-8749(89)90133-0. [DOI] [PubMed] [Google Scholar]
- 37.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, Stahl RA, Benndorf RA, Velden J, Paust HJ, Panzer U, Ehmke H, Wenzel UO. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin II-induced hypertension. Hypertension. 2014;63:565–571. doi: 10.1161/HYPERTENSIONAHA.113.02620. [DOI] [PubMed] [Google Scholar]
- 39.Amador CA, Barrientos V, Pena J, Herrada AA, Gonzalez M, Valdes S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L. Spironolactone decreases doca-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63:797–803. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.