Abstract
Since antizyme (AZ) is known to inhibit cell proliferation and to increase apoptosis, the question arises as to whether these effects occur independently of polyamines. Intestinal epithelial cells (IEC-6) were grown in control medium and medium containing 5mM difluoromethylornithine (DFMO) to inhibit ODC, DFMO + 5μM spermidine (SPD), DFMO+ 5μM spermine (SPM), or DFMO+ 10 μM putrescine (PUT) for 4 days and various parameters of growth were measured along with AZ levels. Cell counts were significantly decreased and mean doubling times were significantly increased by DFMO. Putrescine restored growth in the presence of DFMO. However, both SPD and SPM when added with DFMO caused a much greater inhibition of growth than did DFMO alone, and both of these polyamines caused a dramatic increase in AZ. The addition of SPD or SPM to media containing DFMO + PUT significantly inhibited growth and caused a significant increase in AZ. IEC-6 cells transfected with AZ-siRNA grew more than twice as rapidly as either control cells or those incubated with DFMO, indicating that removal of AZ increases growth in cells in which polyamine synthesis is inhibited as well as in control cells. In a separate experiment the addition of SPD increased AZ levels and inhibited growth of cells incubated with DFMO by 50%. The addition of 10 mM asparagine (ASN) prevented the increase in AZ and restored growth to control levels. These results show that cell growth in the presence or absence of ODC activity and in the presence or absence of polyamines depends only on the levels of AZ. Therefore, the effects of AZ on cell growth are independent of polyamines.
Keywords: Ornithine decarboxylase, α-difluoromethylornithine, spermidine, antizyme-siRNA, Cell number, doubling time
Introduction
The importance of polyamines to both normal and abnormal function has been recognized for many years. Spermidine, spermine and their diamine precursor, putrescine, are essential for eukaryotic cell proliferation and the growth of normal tissues (Tabor and Tabor 1984). Additional cellular functions including trans-membrane ion flux (Lopatin et al. 1994), apoptosis (Ray et al. 2000) and migration (McCormack et al. 1993) either require polyamines or are influenced by them. Numerous studies have demonstrated the importance of polyamines to the growth of cancer cells and the effects of polyamine analogs on inhibiting abnormal cell growth (Pegg 1988).
The intracellular levels of polyamines are tightly regulated through synthesis and degradation and uptake and release. The major factor in determining tissue polyamine levels is the activity of ornithine decarboxylase (ODC, EC 4.1.17), which catalyzes the conversion of ornithine to the diamine putrescine, the first rate-limiting step in polyamine synthesis. ODC activity itself is highly controlled, increasing dramatically from basal levels in response to tissue damage, growth factors, trophic hormones and other proliferative stimuli (Fausto 1971; Hovis et al. 1986). Increases in ODC activity are relatively transient, for it has one of the shortest half-lives of any mammalian enzyme (Canellakis et al. 1979; Chen et al. 1977). The activity of the enzyme is primarily regulated by a negative feedback mechanism involving ODC antizyme (AZ) (Coffino 2001). AZ levels increase via a frameshifting mechanism that is triggered by increasing tissue polyamine levels (Matsufuji et al. 1995). Active ODC consists of a dimer made up of identical monomers. AZ has a higher affinity for ODC monomers than they do for each other, and through the formation of ODC-AZ heterodimers prevents the formation of the active enzyme. AZ binding also mediated the degradation of ODC and other proteins involved in cell proliferation and apoptosis via the 26S proteosome (Pegg 2006). Cyclin D1, aurora-A and smad1 are among these proteins targeted for degradation by AZ (Newman et al. 2003; Lim and Gopalan 2007).
While it had been known for some time that amino acids could stimulate ODC activity both in vivo (Fausto 1971) and in cultured cells (Kay et al. 1972). Chen and Canellakis (1977) determined that asparagine (ASN) and glutamine (GLN) were the most effective. Using glucose salt solution (EBSS), they showed that several amino acids related to ASN and GLN including non-metabolizable α-amino-isobutyric acid (AIB) could stimulate ODC activity in the absence of media and serum. Some time later Rinehart and Canellakis (1985) found that the presence of ASN, GLN or a similar amino acid was essential before growth factors or hormones were able to induce OSC activity.
Our laboratory has recently shown that ASN and GLN stimulate ODC activity by inhibiting the synthesis of AZ. Their inhibition of AZ synthesis and its subsequent decrease then permit additional stimulation of ODC activity by other proliferating agents. In the absence of amino acids, for example, AZ synthesis increases rapidly for up to 6h, and this increase is essentially prevented by ASN (Ray et al. 2012).
A variety of reports indicate that proliferative agents such as cyclin D1 (Newman et al. 2004), aurora-A (Lim and Gopalan 2007), and smad1 (Lin et al. 2002) are targeted for destruction by AZ. In addition, Dulloo et al (2010) have reported that the antiapoptotic delta Np73 (DNp73) is degraded through the AZ-mediated pathway. Since both the removal of proliferative stimuli and an increase in the rate of apoptosis result in decreased growth, these findings suggest that AZ may regulate growth directly and independently of its inhibition of ODC and the subsequent depletion of tissue polyamines. In this study, we have measured proliferation and AZ levels while manipulating polyamine levels by inhibiting ODC with DFMO and by the addition of exogenous polyamines. The results show that AZ inhibits proliferation directly, independently of the levels of polyamines.
Material and Methods
Cell culture
Cell culture medium and fetal bovine serum (FBS) were obtained from Mediatech (Herndon, VA). Dialyzed FBS (dFBS), Putrescine, Spermidine, Spermine and L-Asparagine and mouse monoclonal anti-actin antibody were purchased from Sigma (St. Louis, MO), and trypsin-EDTA, antibiotics, and insulin were from GIBCO-BRL (Grand Island, NY). Rabbit polyclonal anti-AZ-1 antibody was a gift from Dr. Senya Matsufuji. CellTiter-blue cell viability assay reagent was obtained from Promega (Madison, WI). Protease inhibitors, phosphatase inhibitors, phosphate buffer saline (PBS), Dulbecco’s PBS (DPBS), bicinchoninic acid (BCA), and mammalian protein extraction reagent were purchased from Thermo Fisher Scientific (Rockford, IL). The enhanced chemiluminescence substrate Western Lightning TM was purchased from PerkinElmer Life and Analytical Sciences (Shelton, CT). DFMO was a gift from ILEX Oncology (San Antonio, TX). Disposable culture ware was purchased from Corning Glass. The IEC-6 cell line was obtained from the American Type Culture Collection (Manassas, VA) at passage 13. The stock was maintained in T-150 flasks in a humidified, 37°C incubator in an atmosphere of 90% air-10% CO2. The medium consisted of Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL) with 5% heat-inactivated FBS, 10μg/ml insulin, and 50-μg/ml gentamicin sulfate. The stock was passaged weekly and fed three times per week. Passages 15-20 were used in the experiments. All other chemicals were of the highest purity commercially available.
Experimental protocol
For most experiments, the cells were taken up with 0.05% trypsin plus 0.53 mM EDTA in Hanks’ balanced salt solution without Ca2+ and Mg2+. They were counted and plated (day 0) at 500,000 cells/well in 6-well plate or 20,000 cells/well in 24-well plate in DMEM plus 5% dialyzed FBS, 10 μg insulin, and 50 μg gentamicin sulfate/ml. without (control) or with DFMO (5mM), or DFMO + indicated concentrations of polyamines. The cells were fed each day with respective medium. On day 4, the medium was removed and the cells were subjected to cell counts or proliferation assay.
AZ siRNA transfection
Three AZ1 SiRNA oligonucleotide sequences were designed and cloned into a plasmid vector (pcDNA6.2-GW/EmGFP-MiR) and confirmed by sequencing using appropriate primer pairs. Selected clones for the vector (MiR-LacZ-EGFP) and AZ1 (MiR-LacZ-AZ1-EGFP) were used to prepare plasmid DNA for the transfection of IEC-6 cells using EndoFree Plasmid Maxi kit from QIAGEN. Vector (MiR-LacZ-EGFP) or AZ1 (MiR-AZ1-EGFP) plasmid DNA (10 μg) was transfected as described earlier (2). The success of transfection was ascertained by monitoring GFP expression, and stable clones were selected using blasticidin. Isolated clones were characterized by determining the expression of AZ1 and ODC activity as described earlier (2) and used for the proliferation experiments.
Proliferation assay
The cells were harvested with 0.05% trypsin plus 0.53 mM EDTA in Hanks’ balanced salt solution without Ca2+ and Mg2+. They were counted using a Beckman Coulter counter and plated 1 × 106/60mm plates containing either DMEM plus 5% dialyzed FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate with or without DFMO (5mM), DFMO + put, DFMO + spd or DFMO + spm in a humidified, 37°C incubator in an atmosphere of 90% air-10% CO2. The cells were fed everyday with respective culture media. On each day, one group of plates was washed once with HBSS and cells were counted using Beckman Coulter counter.
Cell Viability assay
The cells were harvested, counted and plated in 12-well plate containing DMEM plus 5% dialyzed FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate. Culture media was changed to either DMEM with or without DFMO (5mM), DFMO + put, DFMO + spd or DFMO + spm along with Insulin and gentamicin sulfate after 24 hrs. Culture media was changed everyday. On each day, one plate was washed once with HBSS and CellTiter-blue Cell viability assay reagent was added with DMEM, 5% dialyzed without phenol. CellTiter-Blue Reagent undergo a “blue shift” upon reduction of resazurin to resorufin by metabolically active cells. The pink resorufin fluorescence was read at an excitation wavelength of 560nm and an emission wavelength of 590 nm after 3 hours of incubation.
Western blot analysis
After experimental treatments, IEC-6 cells were washed twice with DPBS. The DPBS was removed, 500 μl of MPER containing protease and phosphatase inhibitors was added, and the plates were frozen overnight and scraped. The cell lysate was centrifuged at 10,000 rpm for 10 min. The supernatant was used to determine the protein concentration by the BCA method. Total cell protein (30 μg) was separated on 10% SDS-PAGE and transferred to a PVDF membrane for Western blotting with a specific primary (AZ1, 1μg/ml, Actin 0.1 μg/ml) antibodies and appropriate secondary antibodies labeled with horseradish peroxidase (HRP) (1:10,000 dilution). Immune complexes were detected by chemiluminescence system.
Statistics
All experiments were repeated three times (n = 3). Data were expressed as means ± SE. Experiments involving Western blots were performed three times with similar results, and a representative blot is shown. ANOVA with appropriate post hoc testing was used to determine the significance, and the Student’s t-test was performed to determine the significance of the differences between means. p < 0.05 was regarded as statistically significant.
Results
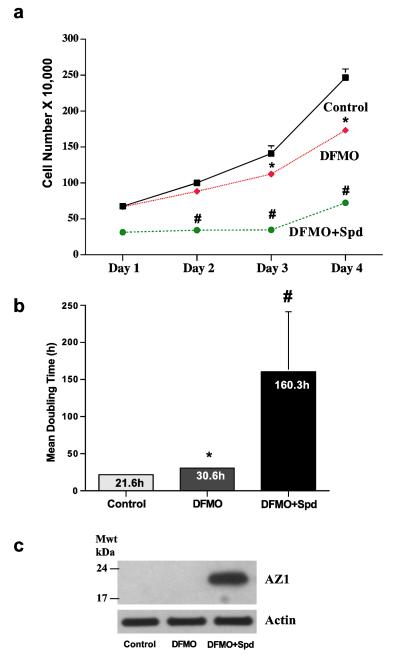
As shown in Fig.1a, incubation of IEC-6 cells, a nontransformed line originally developed from rat crypt cells by Quaroni et al (1988), for 4 days in the presence of 5μM DFMO significantly decreased the rate of growth compared to controls. The presence of 5.0 μM spermidine not only failed to prevent the effects of polyamine depletion by DFMO but also caused an additional significant inhibition of cell growth. These data are reflected in the mean cell doubling times (Fig. 1b), which were increased from 30.6 h in DFMO alone to 160.3 h when spermidine was added to the medium containing DFMO. In Fig.1c, it can be seen that spermidine caused a large increase in the synthesis of AZ.
Fig. 1.
(A) IEC-6 cells (500,000/well) were plated in control, DFMO, or DFMO + 5 μM Spd containing medium and incubated at 37°C. Total cell counts were determined using a coulter counter. (B) Growth rate are shown by calculating mean doubling times. (C) Cells grown as described above for 4 days were used to determine antizyme-1 and actin by western blot analysis. Values are means ± SE of triplicates. *, p < 0.05 compared with Control. #, p < 0.05 compared with Control and DFMO. Blots shown are representative of 3 observations.
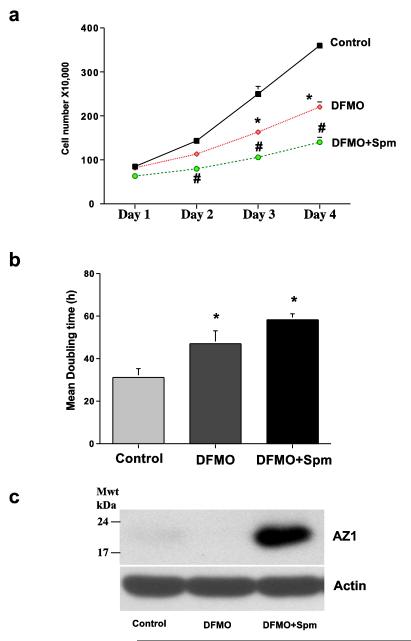
An identical experiment was done substituting spermine for spermidine (Fig. 2). DFMO again caused a significant decrease in cell number by the third and fourth days of incubation, and like spermidine, spermine inhibited growth further instead of restoring it to normal levels (Fig. 2a). This was also reflected in the doubling times for the different groups (Fig. 2b), although, it was apparent that the inhibitory effect of spermine was less than that of spermidine in the preceding experiment. As seen in Fig. 2c spermine also caused a large increase in AZ protein. Thus, the data in Figs. 1 and 2 are qualitatively similar. Both spermidine and spermine at 5.0 μM caused an additional inhibition of cell growth produced by DFMO, and both polyamines significantly increased the levels of AZ protein present in the cultured cells.
Fig. 2.
(A) IEC-6 cells (500,000/well) were plated in control, DFMO, or DFMO + 5 μM Spm containing medium and incubated at 37°C. Total cell counts were determined using a coulter counter. (B) Growth rates were determined by calculating mean doubling times. (C) Cells grown as described above for 4 days were used to determine antizyme-1 and actin by western blot analysis. Values are means ± SE of triplicates. *, p < 0.05 compared with Control. #, p < 0.05 compared with Control and DFMO. Blots shown are representative of 3 observations.
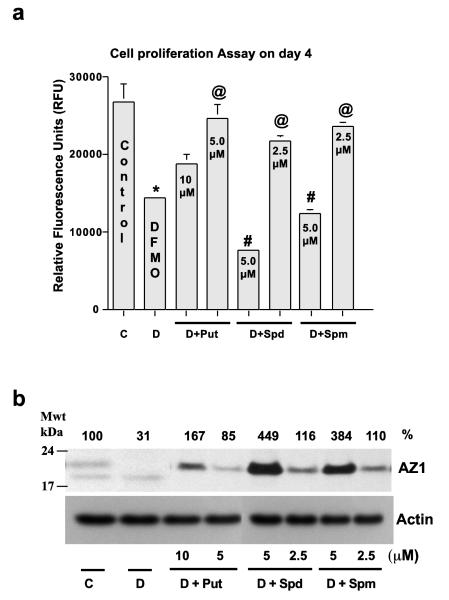
It has been shown many times that the effects of DFMO can be prevented by the addition of exogenous polyamines (Tabor and Tabor 1984; McCormack et al. 1993, Ray et al. 2000). Figure 3 shows the results of an experiment based along the same lines as those shown in Figs. 1 and 2 except that cell proliferation was measured using a fluorescent assay and different doses of polyamines were added to the DFMO containing medium. DFMO retarded cell proliferation by approximately 50 %, and both a low (5.0 μM) and a higher (10.0 μM) concentration of putrescine prevented some of the effects of DFMO. The low dose of putrescine nearly restored growth to control levels. In the presence of DFMO, low doses (2.5 μM) of both spermidine and spermine restored growth significantly to near normal levels. However, as in Figs. 1 and 2, 5.0 μM spermidine and spermine given in the presence of DFMO caused a further and significant inhibition of growth. Interestingly, as before, the inhibitory effect of spermidine was more pronounced than that of spermine.
Fig. 3.
(A) IEC-6 cells (20,000/well) were seeded in a 48-well plate in control, DFMO, or DFMO plus medium containing the indicated amounts of polyamines and incubated at 37°C. Total cell counts were determined on day 4 using cell Blue proliferation assay reagent. Cell proliferation was determined by measuring the fluorescence and expressed as Relative Fluorescence Units (RFU). (B) Cells grown as described above for 4 days were used to determine antizyme-1 and actin by western blot analysis. Values are means ± SE of triplicates. *, p < 0.05 compared with Control. @, p < 0.05 compared with DFMO. #, p < 0.05 compared with Control or DFMO. Blots shown are representative of 3 observations.
While the rates of proliferation in cultures incubated with DFMO and the different concentrations of polyamines (Fig. 3a) were apparently unrelated to the amount of polyamines present in the medium, in each case they were inversely correlated with the amount of AZ protein present (Fig. 3b). Both doses of all three amines increased AZ levels. The lower doses of all three amines partially restored growth in the presence of DFMO and only caused small increases in AZ. Even so, the increase in AZ appeared to correlate inversely with the degree of restoration, in that AZ levels for Put < Spm < Spd. The high dose of putrescine increased AZ significantly and had less effect than the low dose in restoring growth. Interestingly, two bands of AZ detected in control cells appear to be due to the post-translational modification of AZ protein. As in Figs. 1 and 2, spermidine and spermine at 5.0 μM caused large increases in AZ and further inhibited proliferation in the presence of DFMO. Spermidine increased AZ more than spermine and, as before, inhibited growth more.
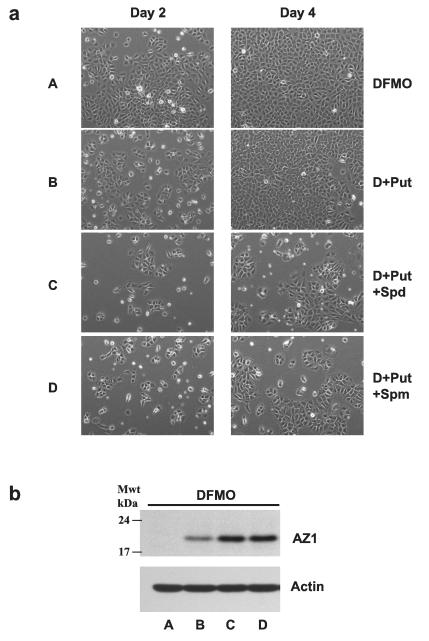
We next reasoned that since spermidine and spermine stimulated the synthesis of AZ, they should be able to inhibit the growth of cells incubated in the presence of both DFMO and putrescine. Fig. 4a consists of phase contrast microscopic images of representative cultures from these four groups after 2 and 4 days incubations. On day 2 there were fewer cells present in DFMO cultures treated with putrescine than in those grown in the presence of DFMO alone, but by day 4 there was no discernible difference between the two groups. However, both spermidine and spermine significantly inhibited cell growth as seen on both days 2 and 4. In the presence of DFMO, all three amines increased AZ levels, although the effect of putrescine was much less than the increases caused by spermidine and spermine (Fig. 4b).
Fig. 4.
(A) IEC-6 cells (500,000/well) were plated in DFMO, or DFMO + Put (10 μM), DFMO + Put + Spd (5 μM) or DFMO + Put + Spm (5 μM) containing medium and incubated at 37°C. Cells were photographed on day 2 and day 4 using a phase contrast microscope. 20X images are shown for each group. Representative images from three experiments carried out in triplicate are shown. (B) Cells grown as described above for 4 days were used to determine antizyme-1 and actin by western blot analysis. Blots shown are representative of 3 observations.
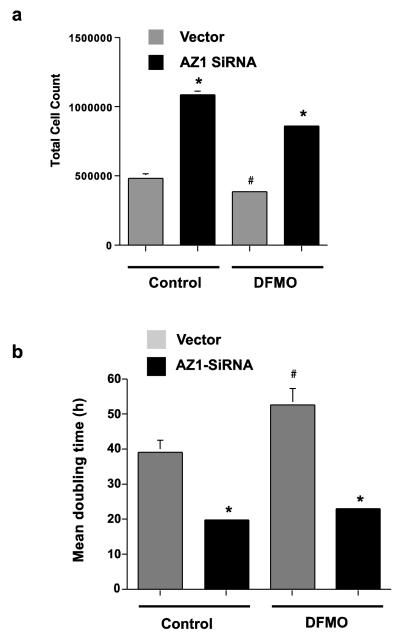
In the next experiment cells transfected with either vector or AZ-siRNA were grown in control or DFMO containing medium for 4 days. Cells in which AZ had been knocked down with siRNA grew more than twice as rapidly as did the vector-transfected cells in both the control and DFMO cultures. This was apparent in both increased cell counts (Fig. 5a) and reduced doubling times (Fig. 5b). Thus, the effects of inhibiting AZ were identical in control cells and those in which ODC was inhibited.
Fig. 5.
IEC-6 cells transfected with vector (Vector) or AZ1 SiRNA (AZ1 siRNA) were grown in control or DFMO containing medium for 4 days. Cell counts were determined at intervals of 24h. (A) Total cell count on day 4. (B) Growth rates are shown as mean doubling times. Values are means ± SE of triplicates. *, p < 0.05 compared with vector-transfected control and DFMO cells. #, p < 0.05 compared with vector-transfected control cells.
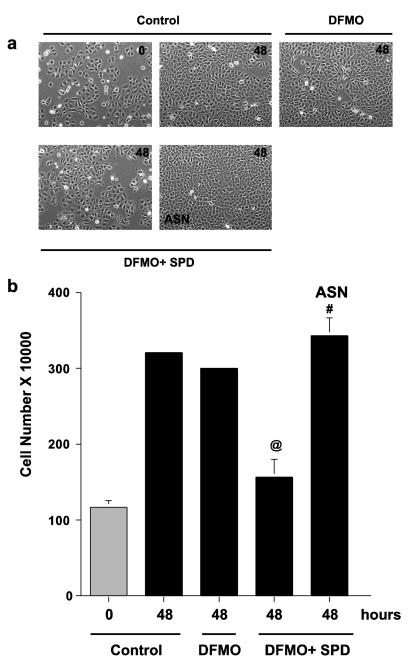
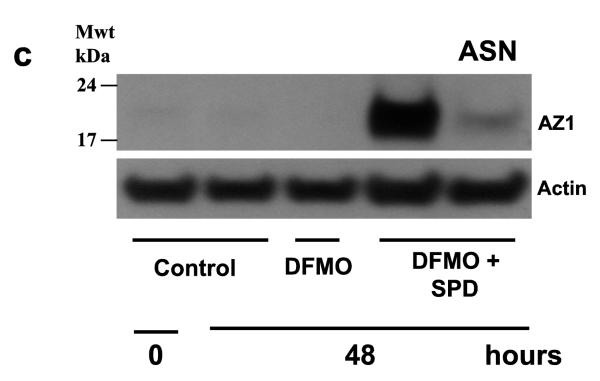
As shown in Figs. 1, 3 and 4, spermidine dramatically increased the synthesis of AZ and inhibited cell growth. Since ASN inhibits AZ synthesis (Ray et al. 2012), we reasoned that the amino acid might restore the growth of cell cultures incubated with spermidine. Phase contrast micrographs in Fig. 6a show that control cultures and those incubated with DFMO were nearly confluent by 48 h. Those incubated with spermidine in addition to DFMO were only about half (50%) confluent. The addition of ASN to DFMO plus spermidine cultures restored growth to near confluence. These results are duplicated by the cell counts shown in Fig. 6b. As can be seen in Fig. 6c, spermidine significantly increased AZ levels in the presence of DFMO, and the addition of ASN to those cultures almost totally blocked AZ synthesis. Thus, by virtue of its ability to inhibit AZ synthesis, ASN restored the growth of cells to normal levels in cultures in which ODC activity was inhibited by DFMO and which had an unchanging and significant supply of exogenous polyamines.
Fig. 6.
(A) IEC-6 cells (500,000/well) were plated in control, DFMO, DFMO + spermidine (Spd 5 μM) or DFMO + spd + Asparagine (ASN 10 mM) containing medium and incubated at 37°C. (A) Cells were photographed on day 0 and day 2 using a phase contrast microscope. 20X images are shown for each group. Representative images from three experiments carried out in triplicate are shown. (B) Total cell counts were determined using a coulter counter on day 2. (C) Cells grown as described above for 2 days were used to determine antizyme-1 and actin by western blot analysis. @, p < 0.05 compared with 48h control and DFMO. #, p < 0.05 compared with 48h DFMO+SPD. Blots shown are representative of 3 observations.
Discussion
The growth of a tissue in situ or a population of cultured cells depends on the balance between cell proliferation and cell death, usually related to apoptosis. In non-dividing cells, the levels of polyamines are low but rapidly increase following the activation of ODC by a mitogenic stimulus. ODC activity usually peaks within 2-3 h of a stimulus and then decreases rapidly due to its short half-life, which usually ranges between 15 and 60 min (Coffino 2001). The turnover of ODC depends on cellular polyamine levels, and the addition of exogenous polyamines increases the degradation of the enzyme. Discovered by Fong et al. (1976), AZ rapidly appeared in cell cultures following the addition of putrescine and was named “ ornithine decarboxylase inhibitory protein “, AZ rapidly inactivates ODC and targets it for degradation. Another protein, AZ inhibitor (AZin) binds to AZ monomers inactivating them and targeting them for degradation (Fujita et al. 1982). Overall ODC activity depends on the relative proportions of ODC monomers, AZ and AZin (Kahana 2009).
That AZ stimulates the degradation of ODC and inhibits the cellular uptake of polyamines, suggested that it also should inhibit proliferation. Murakami et al. (1994) forced the expression of AZ in HZ7 cells transfected with AZ cDNA driven by a glucocorticoid-inducible promoter and found that ODC was inhibited and levels of putrescine and spermidine decreased along with cell growth. Decreased cell growth was similar to that seen with DFMO and was restored by the addition of putrescine. Similar results were described in transgenic mice overexpressing AZ in the basal layer of the forestomach (Fong et al. 2003). In addition to decreased proliferation, the rate of apoptosis increased. The overexpression of AZ also inhibited the induction of tumors by N-nitrosomethylbenzylamine. This group also found that DFMO, and the resulting decrease in polyamines, had effects similar to the ability of AZ to deplete polyamine levels.
Throughout the current experiments we have examined cell proliferation relating it to AZ levels while controlling the polyamine levels. In all the studies, DFMO was used to inhibit ODC and prevent the synthesis of polyamines. DFMO is a specific and irreversible suicide inhibitor of ODC, having no effects except those caused by ODC inhibition and the subsequent depletion of polyamines (Russell, 1985; Pegg et al. 1987). Following the addition of DFMO, putrescine is depleted by 6 h, spermidine by 24 h, and by 4 days only approximately 40 % of spermine remain which is probably bound to negatively charged macromolecules. We have also used exogenous putrescine, spermidine and spermine to manipulate polyamine levels. In none of our experiments could the rates of cell proliferation be positively correlated with polyamine levels. In each instance, however, proliferation was inversely correlated with AZ levels. This striking correlation is readily apparent if one relates the effects of different polyamines and doses on proliferation in Fig. 3a to AZ levels from the same cultures shown in Fig. 3b. Moreover, the restoration of proliferation by putrescine in cultures inhibited by DFMO could be prevented by spermidine or spermine, which increased AZ levels substantially (Fig. 4).
The data in Figs. 5 and 6 are of particular significance. Knocking down AZ with its siRNA stimulated growth compared to that seen in vector-transfected control cells, which could be attributed to increased ODC activity and polyamine levels. However, growth was also more than doubled in cells incubated with DFMO and, therefore, having no ODC activity. The experiment described in Fig. 6 shows that the addition of ASN to cultures in which ODC is inhibited by DFMO fully restored proliferation that had been significantly inhibited by spermidine. It is obvious from Fig. 6c that ASN exerted its effect by blocking the synthesis of AZ. These data also suggest that some amino acids may exert their well-known effects on growth by regulating AZ levels.
Throughout our experiments the greater decreases in cell proliferation and increase in AZ levels were caused by spermidine. We have recently shown that spermidine is the only polyamine that directly stimulates AZ synthesis (Ray et al. 2014). In that study blocking the conversion of putrescine to spermidine by inhibiting S-adenosylmethionine decarboxylase (SAMDC), eliminated the increase in AZ levels caused by the diamine. In addition, the stimulation of AZ synthesis by spermine required its back conversion to spermidine by spermidine/spermine acetyl transferase (SSAT).
The major conclusion from the current study is that AZ can regulate growth directly, independent of its effects on polyamine levels. Both AZ and AZin have been shown to function outside of the polyamine pathway (for review see Olsen and Zetter 2011). The realization that AZ might control cell growth without affecting polyamine levels followed observations that AZ could degrade proteins other than ODC. These proteins were involved in growth regulation, and the major ones were discussed in the “introduction”. Our study is the first to actually correlate cell proliferation and AZ levels while manipulating and controlling polyamines. It is obvious from our data that the direct effects of AZ on growth are significant. How they compare to its effects as the result of changes in polyamines remain to be determined.
ACKNOWLEDGEMENTS
This publication was made possible by grants (Number DK -16505) from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and by support from the Thomas A. Gerwin endowment. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health.
Footnotes
CONFLICT OF INTEREST The authors declare that they have no conflict of interest.
References
- Canellakis ES, Viceps-Madore D, Kariakidis DA, Heller JS. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Chen KY, Canellakis ES. Enzyme regulation in neuroblastoma cells in a salts-glucose medium: induction of ornithine decarboxylase by asparagine and glutamine. Proc Natl Acad Sci USA. 1977;74:3791–3795. doi: 10.1073/pnas.74.9.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- Dulloo I, Gopalan G, Melino G, Sabapathy K. The antiapoptotic deltaNp73 is degraded in a c-jun-dependent manner upon genotoxic stress through the antizyme-mediated pathway. Proc Natl Acad Sci USA. 2010;107:4902–4907. doi: 10.1073/pnas.0906782107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. The control of ornithine decarboxylase activity during liver regeneration. Biochim. Biophys. Acta. 1971;238:116–128. doi: 10.1016/0005-2787(71)90015-3. [DOI] [PubMed] [Google Scholar]
- Fong WF, Heller JS, Canellakis ES. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochem Biophys Acta. 1976;428:456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Fong LY, Feith DJ, Pegg AE. Antizyme over expression in transgenic mice reduces cell proliferation, increases apoptosis, and reduces N-nitrosomethylbenzylamide-induced forestomach carcinogenesis. Cancer Res. 2003;63:3945–3954. [PubMed] [Google Scholar]
- Fujita K, Murakami Y, Hayashi S. A macromolecular inhibitor of the antizyme to ornithine decarboxylase. Biochem J. 1982;204:647–652. doi: 10.1042/bj2040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovis JG, Stumpo DJ, Halsey DL, Blackshear PJ. Effects of mitogens on ornithine decarboxylase activity and messenger RNA lels in normal and protein kinase C-deficient NIH3T3 fibroblasts. J Biol Chem. 1986;261:10380–10386. [PubMed] [Google Scholar]
- Kahana C. Regulation of cellular polyamine levels and cellular proliferation by antizyme and antizyme inhibitor. Essays in Biochem. 2009;46:47–61. doi: 10.1042/bse0460004. [DOI] [PubMed] [Google Scholar]
- Kay JE, Lindsay VJ, Cooke A. Ornithine decarboxylase in phytohaemagglutinin stimulated lymphocytes: Control of degradation by amino acids. FEBS Lett. 1972;21:123–126. doi: 10.1016/0014-5793(72)80118-2. [DOI] [PubMed] [Google Scholar]
- Lim SK, Gopalan G. Antizyme1 mediates AURKAIP1-dependent degradation of aurora-A. Oncogene. 2007;26:6593–603. doi: 10.1038/sj.onc.1210482. [DOI] [PubMed] [Google Scholar]
- Lin Y, Martin J, Gruendler C, Farley J, Meng X, Li BY, Lechleider R, Huff C, Kim RH, Grasser WA, Paralkar V, Wang T. A novel link between the proteasome pathway and the signal transduction pathway of the bone morphogenetic proteins (BMPs) BMC Cell Biol. 2002;3:15. doi: 10.1186/1471-2121-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- McCormack SA, Viar MJ, Johnson LR. Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 1993;264:G367–G374. doi: 10.1152/ajpgi.1993.264.2.G367. [DOI] [PubMed] [Google Scholar]
- Matsufuji S, Matsufuji T, Miyazaki Y, Murakami Y, Atkins JF, Gesteland RF, Hayashi S. Auto-regulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell. 1995;80:51–60. doi: 10.1016/0092-8674(95)90450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Matsufuji S, Miyazaki Y, Hayashi S. Forced expression of antizyme abolishes ornithine decarboxylase activity, suppresses cellular levels of polyamines and inhibits cell growth. Biochem J. 1994;46:47–61. doi: 10.1042/bj3040183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RM, Mosbascher A, Mangold U, Koike C, Diah S, Schmidt M, Finley D, Zetter BR. Antizyme targets cyclin D1 for degradation. A novel mechanism for cell growth repression. J Biol Chem. 2004;279:41504–41511. doi: 10.1074/jbc.M407349200. [DOI] [PubMed] [Google Scholar]
- Olsen RR, Zetter BR. Evidence of a role for antizyme and antizyme inhibitor as regulators of human cancer. Mol Cancer Res. 2011;9:1285–1293. doi: 10.1158/1541-7786.MCR-11-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- Pegg AE, McGovern KA, Weist L. Decarboxylation of alpha-difluoromethylornithine by ornithine decarboxylase. Biochem J. 1987;241:305–307. doi: 10.1042/bj2410305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelial cell culture from rat small intestine. J Cell Biol. 1988;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RM, Viar MJ, Yuan Q, Johnson LR. Polyamine depletion delays apoptosis of rat intestinal epithelial cells. Am J Physiol. 2000;278:C400–C489. doi: 10.1152/ajpcell.2000.278.3.C480. [DOI] [PubMed] [Google Scholar]
- Ray RM, Viar MJ, Johnson LR. Amino acids regulate the expression of antizyme-1 to modulate ornithine decarboxylase activity. J Biol Chem. 2012;287:3674–3690. doi: 10.1074/jbc.M111.232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RM, Bhattacharya S, Bavaria MN, Viar MJ, Johnson LR. Spermidine, a sensor for antizyme 1 expression regulates intracellular polyamine homeostasis. Amino Acids. 2014 doi: 10.1007/s00726-014-1757-4. (DOI 10.1007/s00726-014-1757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart CA, Jr., Viceps-Madore D, Fong WF, Ortiz JG, Canellakis ES. The effect of transport system A and N amino acids and nerve and epidermal growth factors on the induction of ornithine decarboxylase activity. J Cell Physiol. 1985;123:435–441. doi: 10.1002/jcp.1041230321. [DOI] [PubMed] [Google Scholar]
- Russell DH. Ornithine decarboxylase. A key regulatory enzyme in normal and neoplastic growth. Drug Met Rev. 1985;16:1–88. doi: 10.3109/03602538508991430. [DOI] [PubMed] [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]