Abstract
Chronic inflammation in physically ill patients is often associated with the development of symptoms of depression. The mechanisms that are responsible for inflammation-associated depression have been elucidated over the last few years. Kynurenine produced from tryptophan in a reaction catabolized by indoleamine 2,3 dioxygenase is transported into the brain where it is metabolized by microglial enzymes into a number of neurotropic compounds including quinolinic acid, an agonist of N-methyl-D-aspartate receptors. Quinolinic acid can synergize with glutamate released by activated microglia. This chain of events opens the possibility to treat inflammation-induced depression using therapies that target the transport of kynurenine through the blood-brain barrier, the production of quinolinic acid and glutamate by activated microglia, or the efflux of glutamate from the brain to the blood.
Inflammation is associated with an increased prevalence of major depressive disorders
Major depressive disorders are characterized by a combination of symptoms that interfere with the patient’s inability to function normally. These symptoms as defined by DSM-IV include persistent depressed mood or irritability, decreased interest or pleasure in most activities, changes in appetite, sleep and activity, fatigue or loss of energy, feelings of guilt and worthlessness, diminished ability to think and concentrate, and suicidality. According the World Health Organization, major depressive disorders are the second largest burden of disease in the developed world and a leading cause of disability (http://www.who.int/mediacentre/factsheets/fs369/en/index.html). The limited knowledge on the pathophysiology of major depressive disorders remains an important obstacle for the development of effective preventive and curative therapies.
Several converging sources of evidence point to a role for inflammation in the development of symptoms of depression. This is particularly the case in physically ill patients with chronic inflammation as the prevalence of depression in this population can be up to three times higher than in the general population.1–3 In addition, depression in physically ill patients is associated with increased rates of morbidity and mortality. Several lines of evidence confirm the biological nature of the association between inflammation and depression. At the clinical level, symptoms of depression are associated with elevated circulating levels of biomarkers of inflammation such as C-reactive protein and interleukin-6 (IL-6).4,5 The strongest evidence in favor of a causal role of inflammation in the development of depression comes from the observation that cytokine immunotherapy based on chronic administration of interferon-alpha (IFNα) and/or interleukin-2 (IL-2) to patients with kidney cancer or melanoma with metastasis induces an episode of depression in a significant proportion of patients.6,7 The development of depression is gradual, starting with neurovegetative symptoms akin to sickness (fatigue, loss of appetite, sleep disorders) and culminating with psychological and cognitive symptoms of depression.8 Further evidence for a role of inflammation in depression comes from the observation that administration of an antagonist of the proinflammatory cytokine tumor necrosis factor-alpha (TNFα) was found to attenuate depressed mood in patients with psoriasis9 and in patients with major depressive disorders and elevated C-reactive protein.10
Inflammation induces depression by activating the tryptophan degrading enzyme indoleamine 2,3 dioxygenase (IDO)
The mechanisms by which inflammation induces depression have been studied at the preclinical level using acute and chronic activation of the immune system in murine models of depression-like behavior.11 Acute administration of the cytokine inducer lipopolysaccharide (LPS) via the intraperitoneal route increases the duration of immobility in the forced swim test and the tail suspension test; two behavioral tests that are commonly used to detect the anti-depressant properties of drugs.11 LPS also decreases preference for sucrose when rodents are presented with two bottles, one that contains tap water and the other a solution of sucrose at a concentration close to the detection threshold.11 This decrease in sucrose preference is interpreted as evidence for anhedonia; the inability to experience reward, which is a key feature of depression. The same effects are observed in animals of which the immune system is chronically stimulated such as during infection with the attenuated form of Mycobacterium bovis, Bacillus Calmette-Guerin.12 As activation of the peripheral immune system is typically associated with behavioral signs of sickness including decreased motor activity and reduced food and water intake, it was important to show that depression-like behavior is not just another expression of sickness. In patients treated chronically with cytokines, there is a temporal dissociation between sickness and depression, with symptoms of sickness appearing first in response to inflammation and symptoms of depression developing later.13 This temporal dissociation also occurs in animal models of inflammation-induced depression. Signs of sickness appear within 1 to 2 hours in lipopolysaccharide-treated mice. They last for several hours and are usually gone by 24 h. However, mice still show at this time depression-like behavior in the form of increased immobility in the forced swim test and in the tail suspension test as well as reduced preference for a sucrose solution when given the choice between water and a normally preferred sucrose solution.11 Given that sickness is temporally dissociated from depression-like behavior, it is possible to explore the molecular mechanisms that underpin the transition from sickness to depression-like behavior. Proinflammatory cytokines such as IFNγ and TNFα activate indoleamine 2,3 dioxygenase (IDO).14–16 This ubiquitous enzyme metabolizes tryptophan along the kynurenine pathway. Activation of IDO leads to a decrease in circulating levels of tryptophan and an increase in circulating levels of kynurenine.17 IDO activation is an important mechanism for the down-regulation of the immune response, as cytotoxic T cells need tryptophan to proliferate and exert their cytotoxic activity. Activation of IDO mediates the development of inflammation-induced depression-like behavior but not the occurrence of sickness. Inhibition of activation of IDO by administration of the competitive inhibitor of IDO, 1-methyl tryptophan, abrogated LPS-induced depressive-like behavior but not LPS-induced sickness responses nor LPS-induced cytokine expression.16 In the same manner, genetic deletion of ido1, the gene that codes for IDO, blocked the development of depressive-like behavior induced by inoculation of Bacillus Calmette-Guerin but had no influence on sickness.15
Clinical studies have also contributed greatly towards exposing IDO activation by cytokines as a primary mechanistic candidate. As mentioned above, there is temporal dissociation in the development of symptoms of sickness and depression in patients treated with IFNα. 8 Neurovegetative symptoms of sickness develop in response to the very first injections of IFNα whereas psychological symptoms of depression including anhedonia, feelings of worthlessness and suicidal ideation take longer to emerge. Secondly, patients treated with IFNα exhibit decreased circulating levels of tryptophan. This association between enhanced tryptophan degradation and depression has been confirmed in several clinical studies in psychiatric as well as in physically ill patients.18–24
Activation of IDO induces depression-like behavior by a glutamatergic-dependent mechanism
IDO-dependent degradation of tryptophan along the kynurenine pathway can lead to depression either via decreased bioavailability of the essential amino acid tryptophan for the synthesis of serotonin or increased formation of cytotoxic kynurenine metabolites. Tryptophan is an essential amino acid and its bioavailability is a limiting factor for the synthesis of serotonin. It is therefore tempting to speculate that the IDO-dependent decrease in circulating tryptophan leads to reduced brain serotoninergic neurotransmission. However, the decrease in blood tryptophan that takes place during inflammation does not translate into decreased brain tryptophan.24,25 An increase in brain tryptophan together with an enhanced turnover of serotonin is actually observed instead in rodents of which the peripheral immune system is activated. Another way serotoninergic neurotransmission can be detrimentally affected is via the up-regulation of the brain serotonin transporter SERT. Proinflammatory cytokines increase the expression of SERT via a mitogen-activated protein kinase p38 pathway. Genetic deletion of SERT abrogates the depression-like behavioral effects of LPS,26,27 although contradictory results concerning the ability of cytokines to stimulate SERT have been reported.28
An alternative hypothesis to that of the tryptophan starvation hypothesis is the formation of potentially neurotoxic kynurenine metabolites downstream of IDO.29 Kynurenine is further metabolized to 3-hydroxy kynurenine, a potent free radical donor, and quinolinic acid, a NMDA receptor agonist (Figure 1). Elevated levels of quinolinic acid have been associated with low grade systemic inflammation and suicide attempts in patients with major depression.30,31
Figure 1. The kynurenine pathway of tryptophan metabolism.
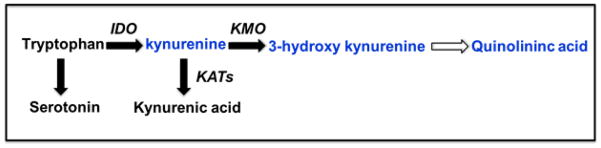
Tryptophan is the biochemical precursor for the production of serotonin. Activation of the enzyme indoleamine 2,3 dioxygenase (IDO) metabolizes tryptophan into kynurenine which can then be metabolized by kynurenine aminotransferases (KATs) into the neuroprotective kynurenic acid or by kynurenine mono-oxygenase (KMO) into the potentially neurotoxic 3-hydroxykynurenine and subsequent quinolinic acid. Under conditions of inflammation there is a bias for kynurenine metabolism by KMO.
The possibility that NMDA receptor activation mediates major depressive disorders stems from the many clinical observations of an improvement in the symptoms of depression such as suicidal ideation and a decrease in the severity of scores on several validated depressive inventories in response to intravenous injection of the NMDA receptor antagonist ketamine. 32–34 Animal studies confirm that ketamine has anti-depressant properties, as demonstrated by the decrease in immobility in rats submitted to the forced swim test.35,36
To test the possibility that NMDA receptor activation also mediates the development of inflammation-induced depression, ketamine was administered at very low doses to mice treated with LPS.25 Ketamine was administered at the dose of 6 mg/kg either in a preventive manner, just before LPS, or in a curative manner, 10 h after the administration of LPS. In both cases ketamine abrogated LPS-induced depression-like behavior without altering the cytokine response to LPS. To confirm that the blockade of the NMDA receptor was responsible for the antidepressant-like effects of ketamine in LPS-treated mice, we conducted a subsequent experiment in which we pretreated mice with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione (NBQX). NBQX blocked the increase in AMPA receptor glutamatergic neurotransmission after NMDA receptor antagonism by ketamine and thus restored LPS-induced decreased sucrose preference. These findings indicate that the formation of brain NMDA receptor agonists compounds during systemic inflammation is responsible for the development of LPS-induced depression.25
The blood-brain barrier plays an active role in immune-to-brain communication
The ketamine experiments described in the previous paragraph demonstrate that LPS-induced depression like behavior is mediated by NMDA receptor activation but they do not inform on the nature and origin of the compounds that activate NMDA receptors. During systemic immune activation IDO is activated both at the periphery and in the brain. In studies that were carried out in the late 1980s and early 1990s, Heyes and colleagues already noted that the decrease in blood tryptophan in response to systemic LPS is associated with an increase in brain tryptophan and brain serotonin turnover.37 The decrease in blood tryptophan was caused by IDO and not by hepatic tryptophan 2,3 dioxygenase as the activity of this last enzyme was actually decreased in response to LPS. Increased brain kynurenine was proposed to derive from peripheral kynurenine.38 This hypothesis was confirmed by experiments carried out in gerbils using tritiated kynurenine. In the normal brain up to 78% of kynurenine derives from the blood. During systemic inflammation nearly all of brain kynurenine derives from circulating kynurenine.39
The blood-brain barrier strictly regulates the transport of blood-borne substances into the brain. This is particularly the case for tryptophan and kynurenine whereas kynurenine metabolites are almost exclusively formed in situ.29,40 Tryptophan and kynurenine enter the brain via a sodium-independent large neutral amino-acid transporter known as System L (see Figure 2).41,42 This transporter is composed of two subunits, a heavy glycoprotein chain, CD98, and one catalytic chain known as large neutral amino acid transporter, LAT-1.41,42 Although it should be theoretically possible to block the entry of kynurenine into the brain by targeting LAT-1 during systemic inflammation to prevent inflammation-induced depression, this has not yet been tested.
Figure 2. Transportation of tryptophan and kynurenine across the blood brain barrier by the large neutral amino acid transporter (LAT-1).
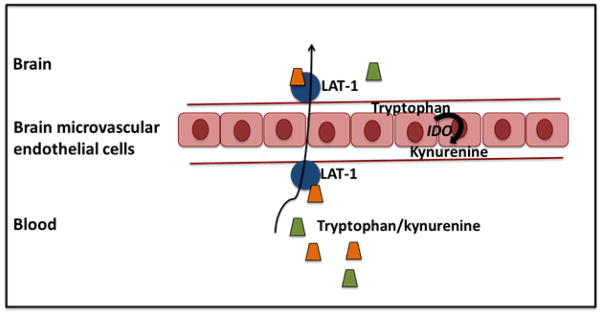
Tryptophan and kynurenine enter the brain via a sodium-independent large neutral amino-acid transporter known as System L which sits on either side of the blood brain barrier (BBB). This allows peripheral tryptophan and kynurenine to be transported through the microvascular endothelial cells of the BBB as well as the transportation of kynurenine endogenously produced in these endothelial cells of the brain when indoleamine 2,3 dioxygenase (IDO) is activated.
The blood-brain barrier can be circumvented when inflammation develops locally in the brain. This can be mimicked by direct administration of LPS in the brain. LPS administered into the lateral ventricle of the brain was found to activate brain but not peripheral IDO and this was associated with the development of depression-like behavior.43 IDO activation was responsible for the development of LPS-induced depression-like behavior as depression-like behavior was abrogated in mice in which ido1 had been genetically deleted as well as in mice treated with the competitive antagonist of IDO, 1-methyl tryptophan.44
Quinolinic acid might not act alone but require glutamate as a co-factor
Based on the data presented above inflammation-induced depression should be amenable to treatment by targeting the development of inflammation (e.g., cytokine or cytokine signaling pathways antagonists), the formation of peripheral kynurenine (e.g., IDO antagonist) or the transport of kynurenine into the brain (e.g., competing amino acids). This strategy would certainly work at its best when it is deployed in a preventive manner before depression occurs. Once depression has developed, another strategy aiming at repairing the inflamed brain needs to be put in place.
Quinolinic acid should a priori be a druggable target, by blocking its formation or antagonizing its effect on NMDA glutamatergic receptors. The neurotoxic activity of quinolinic acid has been known for more than 30 years and its role in the pathophysiology of Huntington’s disease has been explored intensively over the last two decades.29 However, it is not clear that inflammation-induced quinolinic acid is the sole culprit in the development of LPS-induced depression as endogenous quinolinic acid is elevated but does not reach neurotoxic concentrations during inflammation. According to microdialysis studies, neurotoxicity can be achieved only when quinolinic acid is infused in the brain at concentrations that are 10 to 100 fold the concentrations that are reached during neuroinflammation.45
If quinolinic acid alone is not sufficient to induce excitotoxicity, its effect could be potentiated by other endogenous neurotoxic factors. An obvious candidate is the potent radical oxygen species donor 3-hydroxy kynurenine as the brain levels of this metabolite increase considerably in response to LPS.25 However, the fact that targeting NMDA receptor activation with ketamine fully abrogates LPS-induced depression does not support a possible role of 3-hydroxy kynurenine in inflammation-induced depression.
Another potential candidate is glutamate. Activated microglia are able to release large quantities of glutamate as demonstrated by Adriano Fontana in the early 1990s.46 Activated microglial cells and macrophages uptake extracellular glutamine that is metabolized to glutamate via glutaminase.47,48 Microglial glutamate is then released via the connexin hemichannels of gap junctions that form in vivo in response to brain injury and in vitro in response to inflammatory mediators (Figure 3).49
Figure 3. Glutamate release by activated microglia.
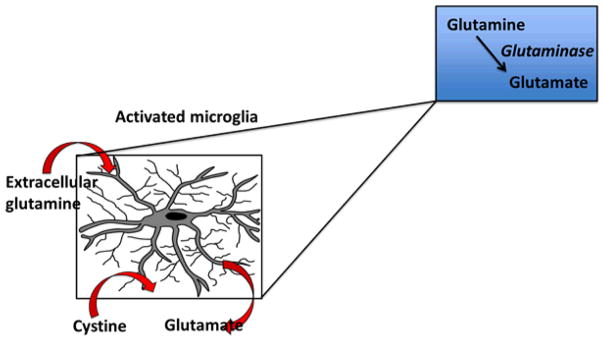
Activated microglia and macrophages take in extracellular glutamine that is metabolized to glutamate via the enzyme glutaminase, which is then released into the extracellular space either via gap junctions induced by injury or a transporter known as system xc− that transports cystine into the cell in exchange for glutamate.
In the absence of cellular aggregates allowing the formation of gap junctions between microglial cells, the release of microglial glutamate occurs via a transporter known as system xc− that transports cystine into the cell in exchange for glutamate.50,51 System xc− consists of two protein components, a 4F2 heavy chain and the xCT protein responsible for transport activity. The entry of cystine into the cell is necessary for the intracellular synthesis of glutathione and the preservation of the redox status of the cell. Activation of system xc− during neuroinflammation functions as an endogenous antioxidant response as the cystine influx helps maintain intracellular glutathione concentrations and the shuttling of cystine and cysteine at the membrane supports a redox cycle.52 Oxidative stress is likely to increase further during IDO activation due to the enhanced production of two kynurenine metabolites that are potent generators of radical oxygen species, 3-hydroxykynurenine53 and 3-hydroxyanthranilic acid.54 This should result in further activation of system xc− and increased extracellular release of glutamate. The enhanced release of glutamate in exchange with cystine would then contribute to excitotoxicity especially if the astrocytic excitatory amino acid transporter (EAAT)1 is down-regulated by inflammation as proposed by Takaki et al. based on the results of studies carried out on a mixed culture of astrocytes and microglia.50
Most of the data concerning the involvement of the system xc− antiporter in the neurotoxic properties of glutamate released by activated microglia have been obtained in vitro. The possibility that system xc− is also activated in vivo by LPS has been recently confirmed.51 Non-traumatic injections of low doses of LPS with a micropipette into the spinal cord activated microglia and this was associated with upregulation of xCT specifically in microglia. The resulting neuronal loss was greatly enhanced when cystine was co-injected with LPS.
Based on these findings, it can be proposed that system xc− converts oxidative stress to excitotoxic stress in the context of IDO activation. This would be the main source for the release of microglial glutamate as microglia aggregates are unlikely to form in a non-injured brain. Blockade of system xc− is probably not the suitable target for breaking the chain of events leading to inflammation-induced depression. It runs the risk of increasing IDO expression and activation as reported in human dendritic cells.55 This would result in an enhanced production of kynurenine and ultimately quinolinic acid. An alternative strategy is to favor the efflux of glutamate from the brain to the blood using the blood glutamate scavenging technique.
Is blood glutamate scavenging a viable strategy to alleviate inflammation-induced depression?
Brain extracellular levels of glutamate are normally in the micromolar range but they can increase up to 100 fold in conditions of excitotoxicity.56 Astrocytes express EAAT1 and EAAT2 that enable them to uptake the excess of glutamate and convert it to glutamine via glutamine synthetase. However, when glutamate is released by activated microglia this mechanism is not sufficient to compensate for the increased extracellular levels of glutamate in astrocytes.50 The elevation in astrocytic glutamate levels results in a decreased expression of astrocytic EAAT1 that leads to further elevations in extracellular concentrations of glutamate.50
Another important mechanism for the clearance of brain extracellular glutamate is represented by the efflux of glutamate from the brain interstitial fluid into the blood. The brain-to-blood efflux of glutamate can be evidenced by the increased circulating levels of glutamate that is observed in response to an intracerebral microinjection of glutamate.57 The brain-to-blood efflux of glutamate is dependent on the transport of glutamate from the brain extracellular fluid into brain endothelial cells through EAATs that are expressed on the abluminal or brain side of the endothelium.58 This process is facilitated by the presence of astrocytes at the blood-brain barrier level and their ability to release glutamate in close proximity to endothelial cells.59 Glutamate taken up by endothelial cells is then released into the blood by facilitative glutamate transporters present on the luminal or blood side of the endothelium. The efficacy of this mechanism is limited by the relatively high levels of blood glutamate, about 40 micromoles per liter. An efficient way to substantially enhance the brain-to-blood efflux of glutamate is to accelerate the degradation of glutamate in the blood by administering oxaloacetate that activates glutamic oxaloacetic transaminase (GOT) and transforms glutamate into 2-ketoglutarate and oxaloacetate into aspartate.60 Blood glutamate scavenging can be enhanced further by adding recombinant GOT to oxaloacetate.61,62 The ability of blood glutamate scavenging to decrease brain glutamate levels was demonstrated in vivo in a model of brain ischemia in the rat using magnetic resonance spectroscopy.63 The neuroprotective effect of blood glutamate scavenging has been confirmed in a number of experimental conditions associated with high brain concentrations of glutamate, such as traumatic brain injury, epilepsy, and ischemic stroke.64
Conclusion and perspectives
It would be premature to conclude from the evidence presented in this review that depression can be treated by therapies targeting inflammation. Inflammation is associated with depression but does not always lead to depression. Conversely, depression is not always associated with inflammation.65 The development of depression in inflamed subjects requires the co-occurrence of vulnerability factors including life events such as early physical abuse and genetic factors such as polymorphism in inflammatory genes and in genes regulating sensitivity of the brain to insults. To achieve personalized treatments for depression, it is still necessary to develop suitable biomarkers for identifying those patients who are the most likely to benefit from treatments targeting the chain of events leading from inflammation to depression. Figure 4 shows the key events to be targeted in this chain, which are the transport of kynurenine into the brain, the production and action of kynurenine metabolites in the brain, and probably the increased concentrations of glutamate in the brain interstitial fluids.
Figure 4. The pathophysiology of inflammation-induced depression.
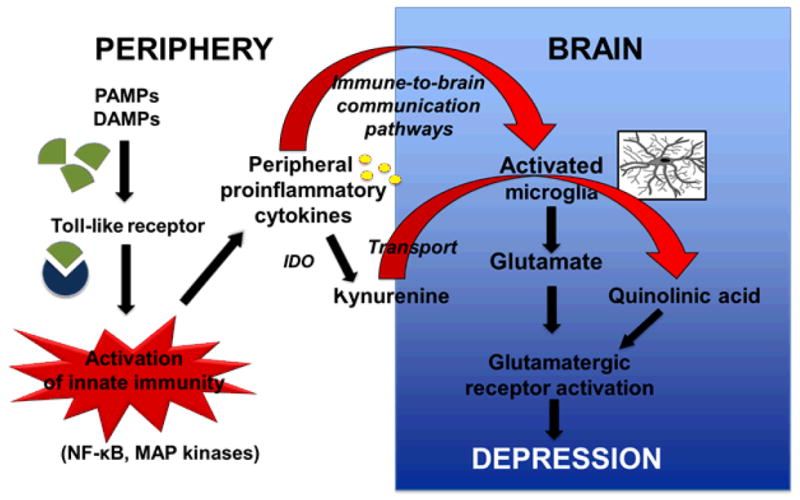
Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) released by dying cells activate pattern recognition receptors including Toll-like receptors in the membrane of innate immune cells. The subsequent activation of nuclear factor kappa-B (NFκB) and mitogen activated protein (MAP) kinase signaling pathways leads to the production of proinflammatory cytokines that activate a number of enzymatic processes including the formation of kynurenine from tryptophan that is catalyzed by indoleamine 2,3 dioxygenase. Cytokine signaling to the brain activates microglia that produces inflammatory mediators and further metabolize kynurenine transported into the brain into neurotoxic compounds including quinolinic acid. Activated microglia also release glutamate. Both glutamate and quinolinic acid enhance glutamatergic neurotransmission that ultimately leads to the development of symptoms of depression.
Acknowledgments
This work was supported by the University of Texas MD Anderson Cancer Center and grants from the National Institute of Neurological Diseases and Stroke of the National Institutes of Health [Grants R01 NS073939; R01 NS074999].
Footnotes
Conflict of Interest
Robert Dantzer works as a consultant for Ironwood Pharma.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Evans DL, et al. Mood disorders in the medically ill: scientific review and recommendations. Biological psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg D. The detection and treatment of depression in the physically ill. World psychiatry : official journal of the World Psychiatric Association. 2010;9:16–20. doi: 10.1002/j.2051-5545.2010.tb00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton T, Staab J, Evans DL. Medical co-morbidity in depressive disorders. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2007;19:289–303. doi: 10.1080/10401230701653542. [DOI] [PubMed] [Google Scholar]
- 4.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, behavior, and immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denicoff KD, et al. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Annals of internal medicine. 1987;107:293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- 7.Renault PF, et al. Psychiatric complications of long-term interferon alfa therapy. Archives of internal medicine. 1987;147:1577–1580. [PubMed] [Google Scholar]
- 8.Capuron L, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 9.Tyring S, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau M, et al. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain, behavior, and immunity. 2008;22:1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capuron L, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor JC, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor JC, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. Journal of immunology. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain, behavior, and immunity. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 18.Gabbay V, Ely BA, Babb J, Liebes L. The possible role of the kynurenine pathway in anhedonia in adolescents. Journal of neural transmission. 2012;119:253–260. doi: 10.1007/s00702-011-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elovainio M, et al. Moderating effect of indoleamine 2, 3-dioxygenase (IDO) activation in the association between depressive symptoms and carotid atherosclerosis: evidence from the Young Finns study. Journal of affective disorders. 2011;133:611–614. doi: 10.1016/j.jad.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Sublette ME, Postolache TT. Neuroinflammation and depression: the role of indoleamine 2,3-dioxygenase (IDO) as a molecular pathway. Psychosomatic medicine. 2012;74:668–672. doi: 10.1097/PSY.0b013e318268de9f. [DOI] [PubMed] [Google Scholar]
- 21.Gold AB, et al. The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. Journal of neuroinflammation. 2011;8:17. doi: 10.1186/1742-2094-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swardfager W, et al. Indoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery disease. Psychoneuroendocrinology. 2009;34:1560–1566. doi: 10.1016/j.psyneuen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Kurz K, Schroecksnadel S, Weiss G, Fuchs D. Association between increased tryptophan degradation and depression in cancer patients. Current opinion in clinical nutrition and metabolic care. 2011;14:49–56. doi: 10.1097/MCO.0b013e328340d849. [DOI] [PubMed] [Google Scholar]
- 24.Raison CL, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Molecular psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker AK, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu CB, et al. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Heesch F, et al. Lipopolysaccharide-induced anhedonia is abolished in male serotonin transporter knockout rats: an intracranial self-stimulation study. Brain, behavior, and immunity. 2013;29:98–103. doi: 10.1016/j.bbi.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Andreetta F, Barnes NM, Wren PB, Carboni L. p38 MAP kinase activation does not stimulate serotonin transport in rat brain: Implications for sickness behaviour mechanisms. Life sciences. 2013;93:30–37. doi: 10.1016/j.lfs.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews. Neuroscience. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner J, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erhardt S, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 33.Larkin GL, Beautrais A. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation. International journal of neuropsychopharmacology. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 34.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: Results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Current psychiatry reports. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- 35.Garcia LS, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Program of the Neuropsychopharmacology Biological Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Nosyreva E, et al. Acute suppression of spontaneous neurotransmissiondrives synaptic potentiation. Journal of neuroscience. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heyes MP, Quearry BJ, Markey SP. Systemic endotoxin increases L-tryptophan, 5-hydroxyindoleacetic acid, 3-hydroxykynurenine and quinolinic acid content of mouse cerebral cortex. Brain research. 1989;491:173–179. doi: 10.1016/0006-8993(89)90101-7. [DOI] [PubMed] [Google Scholar]
- 38.Saito K, Markey SP, Heyes MP. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51:25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- 39.Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. Journal of neurochemistry. 2002;82:258–268. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 40.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. Journal of neurochemistry. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 41.Omidi Y, Barar J, Ahmadian S, Heidari HR, Gumbleton M. Characterization and astrocytic modulation of system L transporters in brain microvasculature endothelial cells. Cell biochemistry and function. 2008;26:381–391. doi: 10.1002/cbf.1455. [DOI] [PubMed] [Google Scholar]
- 42.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu X, et al. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. Journal of neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawson MA, et al. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2, 3-dioxygenase-dependent depression-like behaviors. Journal of neuroinflammation. 2013;10:87. doi: 10.1186/1742-2094-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obrenovitch TP, Urenjak J. Accumulation of quinolinic acid with neuroinflammation: does it mean excitotoxicity? Advances in experimental medicine and biology. 2003;527:147–154. doi: 10.1007/978-1-4615-0135-0_17. [DOI] [PubMed] [Google Scholar]
- 46.Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. European journal of immunology. 1992;22:2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi H, et al. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. The Journal of biological chemistry. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 48.Yawata I, et al. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life sciences. 2008;82:1111–1116. doi: 10.1016/j.lfs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Eugenin EA, et al. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takaki J, et al. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. Journal of neuroinflammation. 2012;9:275. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kigerl KA, et al. System x(c)(−) regulates microglia and macrophage glutamate excitotoxicity in vivo. Experimental neurology. 2012;233:333–341. doi: 10.1016/j.expneurol.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−) : cystine supplier and beyond. Amino acids. 2012;42:231–246. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- 53.Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain research. 1989;495:225–231. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 54.Smith AJ, Smith RA, Stone TW. 5-Hydroxyanthranilic acid, a tryptophan metabolite, generates oxidative stress and neuronal death via p38 activation in cultured cerebellar granule neurones. Neurotoxicity research. 2009;15:303–310. doi: 10.1007/s12640-009-9034-0. [DOI] [PubMed] [Google Scholar]
- 55.Mattox ML, D’Angelo JA, Grimes ZM, Fiebiger E, Dickinson BL. The cystine/glutamate antiporter regulates indoleamine 2,3-dioxygenase protein levels and enzymatic activity in human dendritic cells. American journal of clinical and experimental immunology. 2012;1:113–123. [PMC free article] [PubMed] [Google Scholar]
- 56.Gras G, et al. EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids. 2012;42:221–229. doi: 10.1007/s00726-011-0866-6. [DOI] [PubMed] [Google Scholar]
- 57.Hosoya K, Sugawara M, Asaba H, Terasaki T. Blood-brain barrier produces significant efflux of L-aspartic acid but not D-aspartic acid: in vivo evidence using the brain efflux index method. Journal of neurochemistry. 1999;73:1206–1211. doi: 10.1046/j.1471-4159.1999.0731206.x. [DOI] [PubMed] [Google Scholar]
- 58.Helms HC, Madelung R, Waagepetersen HS, Nielsen CU, Brodin B. In vitro evidence for the brain glutamate efflux hypothesis: brain endothelial cells cocultured with astrocytes display a polarized brain-to-blood transport of glutamate. Glia. 2012;60:882–893. doi: 10.1002/glia.22321. [DOI] [PubMed] [Google Scholar]
- 59.Cohen-Kashi-Malina K, Cooper I, Teichberg VI. Mechanisms of glutamate efflux at the blood-brain barrier: involvement of glial cells. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:177–189. doi: 10.1038/jcbfm.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience. 2009;158:301–308. doi: 10.1016/j.neuroscience.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 61.Nagy D, et al. Effects of blood glutamate scavenging on cortical evoked potentials. Cellular and molecular neurobiology. 2010;30:1101–1106. doi: 10.1007/s10571-010-9542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruban A, Mohar B, Jona G, Teichberg VI. Blood glutamate scavenging as a novel neuroprotective treatment for paraoxon intoxication. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013 doi: 10.1038/jcbfm.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campos F, et al. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1378–1386. doi: 10.1038/jcbfm.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leibowitz A, Boyko M, Shapira Y, Zlotnik A. Blood glutamate scavenging: insight into neuroprotection. International journal of molecular sciences. 2012;13:10041–10066. doi: 10.3390/ijms130810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raison CL, Miller AH. Do cytokines really sing the blues? Cerebrum : the Dana forum on brain science. 2013;2013:10. [PMC free article] [PubMed] [Google Scholar]


