Abstract
S-P467L mice expressing dominant negative Peroxisome Proliferator Activated Receptor-γ selectively in vascular smooth muscle, exhibit impaired vasodilation, augmented vasoconstriction, hypertension and tachycardia. We hypothesized that tachycardia in S-P467L mice is due to baroreflex dysfunction. S-P467L mice displayed increased sympathetic traffic to the heart and decreased baroreflex gain and effectiveness. Carotid arteries exhibited inward remodeling but no changes in distensibility or stress/strain. Aortic depressor nerve activity in response to increased arterial pressure was blunted in S-P467L mice. However, the arterial pressure and heart rate responses to aortic depressor nerve stimulation were unaltered in S-P467L mice suggesting the central and efferent limbs of the baroreflex arc remain intact. There was no transgene expression in nodose ganglion, and no change in expression of the acid-sensing ion channel-2 or -3 in nodose ganglion. There was a trend for decreased expression of transient receptor potential vanilloid-1 receptor mRNA in nodose ganglion, but no difference in the immunochemical staining of transient receptor potential vanilloid-1 receptor in the termination area of the left aortic depressor nerve in S-P467L mice. Although there was no difference in the maximal calcium response to capsaicin in cultured nodose neurons from S-P467L mice, there was decreased desensitization of transient receptor potential vanilloid-1 receptor channels. In conclusion, S-P467L mice exhibit baroreflex dysfunction due to a defect in the afferent limb of the baroreflex arc caused by impaired vascular function, altered vascular structure, or compromised neurovascular coupling. These findings implicate vascular smooth muscle Peroxisome Proliferator Activated Receptor-γ as a critical determinant of neurovascular signaling.
(MeSH): Baroreflex, Heart Rate, Blood Pressure, Peroxisome Proliferator-Activated Receptors, Sympathetic Nervous System, Vascular Smooth Muscle
Introduction
Peroxisome proliferator-activated receptor-gamma (PPARγ) is a ligand-activated transcription factor belonging to the nuclear receptor superfamily. Classically, PPARγ plays an important role in adipogenesis and metabolic processes but has recently emerged as a crucial element in cardiovascular diseases such as hypertension. Mutations of PPARγ lead to hypertension in humans and in experimental animal models.1,2 Moreover, thiazolidinediones, high affinity PPARγ agonists, which increase insulin sensitivity, have been shown to be beneficial for lowering arterial pressure (AP) and improving vascular function.3
We have previously shown that mice carrying a dominant-negative form of PPARγ specifically in smooth muscle (S-P467L mice) exhibit hypertension, severe aortic dysfunction and hypertrophy of cerebral arterioles.4 Vascular dysfunction occurs because of impaired expression of the PPARγ target gene Regulator of G-protein signaling 5 (RGS5) leading to enhanced myogenic tone and angiotensin-II-mediated contraction in the mesenteric circulation, and impaired function of the Cullin-3, Cullin Ring Ligase (CRL) pathway leading to increased RhoA and Rho kinase activity in aorta.5,6 Interestingly, these transgenic mice also exhibited robust tachycardia despite increased systemic AP, suggesting that interference with vascular smooth muscle PPARγ may impair the baroreflex.4
The baroreflex is an important beat-to-beat regulator of AP and heart rate (HR). Baroreceptors, located in the adventitia of the aortic arch and carotid sinus, provide a highly sensitive system that detects changes in AP. These receptors send afferent signals to the central nervous system, and the efferent innervation of blood vessels, heart, and kidneys make adjustments to maintain normal AP.7 Clinical and experimental hypertension commonly involves cardiovascular autonomic impairment associated with high sympathetic activity and blunted baroreflex sensitivity with baroreceptor resetting.8 In this study, we hypothesize that the tachycardia observed in S-P467L mice is attributable to cardiovascular autonomic impairment and baroreflex dysfunction. Our data suggest that impairment in PPARγ function in smooth muscle can impair neurovascular coupling which can attenuate afferent signaling to the central nervous system.
Materials and Methods
A detailed description of the transgenic mouse model, baseline cardiovascular measures, assessment of baroreflex sensitivity and efficacy, spectral analysis, mechanical properties of carotid artery, primary cultures of mouse nodose ganglia (NG), neurovascular coupling including calcium imaging as a measure of transient receptor potential vanilloid 1 receptor channels (Trpv1) functional properties, molecular measures, and data and statistical analysis are provided in the online-only data supplement. All studies were approved by the Institutional Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
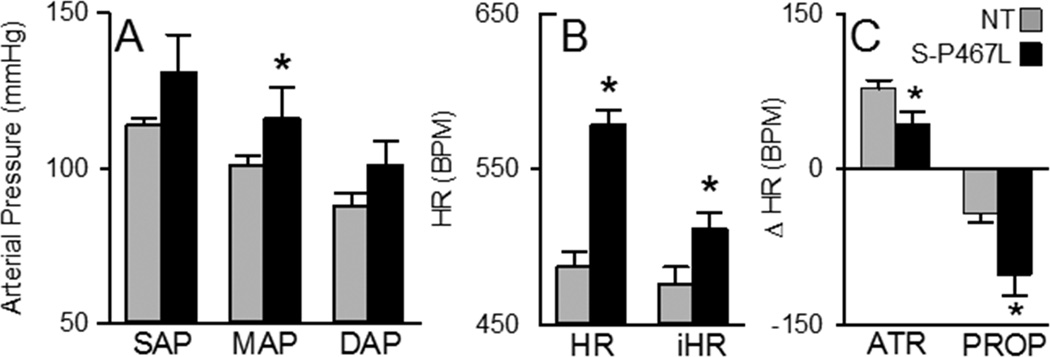
We first measured AP and HR with radiotelemetry. S-P467L mice exhibited elevated AP (Figure 1A) and were markedly tachycardic (Figure 1B). Intrinsic HR, measured after simultaneous sympathetic and parasympathetic blockade, was elevated in S-P467L mice (Figure 1B). Consistent with increased sympathetic outflow to the heart, we observed a larger bradycardic response in S-P467L mice after administering the β-adrenergic antagonist propranolol (Figure 1C). Similarly, the methyl-atropine induced tachycardic response was significantly smaller in S-P467L mice. Power spectral analysis revealed comparable relative low frequency and high frequency components between groups (Figure S1A). The low frequency component of HR (Figure S1B) and AP (Figure S1C) predominated in S-P467L mice. S-P467L mice also exhibited increased systolic AP variability (Figure S1D). Applying the sequence method to examine spontaneous baroreflex fluctuations of AP revealed no changes in the number of sequences (Figure S1E), but S-P467L mice exhibited decreases in both baroreflex gain (Figure S1F) and effectiveness (Figure S1G).
Figure 1. Arterial Pressure and Heart Rate.
Measurement of systolic arterial pressure (SAP), mean arterial pressure (MAP), diastolic arterial pressure (DAP), heart rate (HR) and intrinsic heart rate (iHR) using radiotelemetry. A) Average 1-hour recording of SAP, MAP and DAP. B) Average HR over a 1-hour period calculated from the AP recordings and iHR calculated from the AP recordings after simultaneous blockage of sympathetic and vagal tone to the heart with propranolol and methyl-atropine, respectively. C) HR response to intraperitoneal injection of methyl-atropine (ATR) and propranolol (PROP). S-P467L (black) and non-transgenic (gray) mice. *, P<0.05 vs NT (n=8).
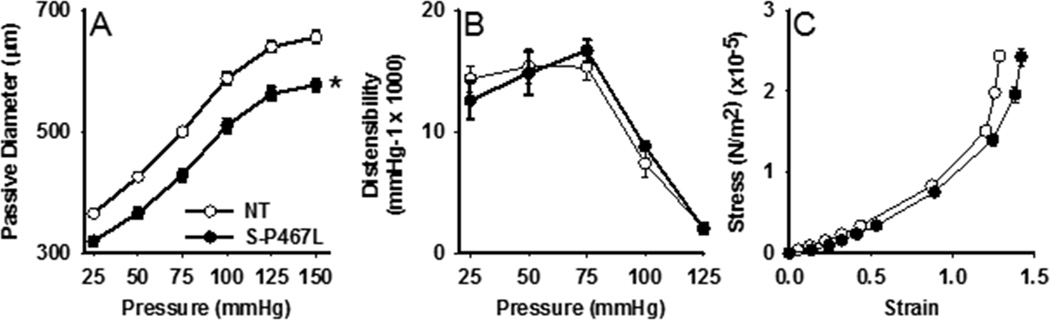
To assess whether the impairment in baroreflex function is due to alterations in vessel structure and/or function, the mechanical properties of carotid arteries were determined in the absence of vascular tone. The lumen diameter was significantly reduced in S-P467L carotid arteries across all pressures tested (Figure 2A). However, there were no differences in vascular distensibility (Figure 2B), and although the stress-strain curve was shifted to the right in S-P467L carotid arteries, the slope was not statistically different between the groups (Figure 2C, NT: 2.61±0.03, S-P467L: 2.38±0.11, p>0.05), suggesting a similar stiffness.
Figure 2. Vascular Mechanics.
A) The passive diameter of the carotid artery was assessed under calcium-free conditions in response to increases in intraluminal pressure. B) Distensibility was calculated from the pressure diameter relationship. S-P467L (N=6); NT (N=5); *P<0.05 vs NT. C) A stress/strain curve was calculated from the internal and external diameter of the vessels that were exposed to a series of pressure steps from 0, 10, 20, 30, 50, 75, 100, 125 to 150 mmHg. S-P467L (N=4); NT (N=3).
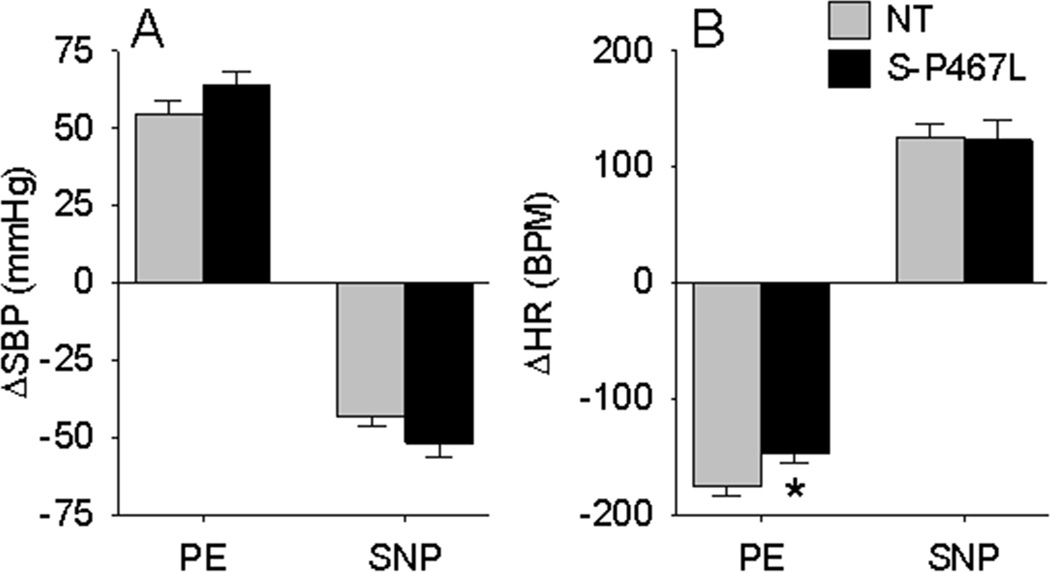
Since baroreflex dysfunction in S-P467L mice could originate from either the afferent or central (efferent) limb, we analyzed AP and HR in conjunction with aortic depressor nerve (ADN) activity at baseline and in response to various stimuli. We first examined the HR response to phenylephrine (PE) and sodium nitroprusside (SNP) in NT and S-P467L mice under conscious conditions. S-P467L mice exhibited tachycardia at baseline (621±18 vs 527±26 bpm, P<0.01), and moderately elevated systolic AP (149.3±10.1 vs 139.5±8.4 mmHg). S-P467L and NT mice exhibited equivalent pressor and depressor responses to PE and SNP, respectively (Figure 3A). Whereas both S-P467L and NT mice exhibited similar tachycardic responses to SNP, the bradycardic response to PE was significantly blunted in S-P467L mice (Figure 3B).
Figure 3. BP and HR Response to PE and SNP.
The change in systolic BP (A) and HR (B) in conscious S-P467L and NT mice is shown in response to slow intravenous infusion of phenylephrine (PE, 5µg) followed by sodium nitroprusside (SNP, 5µg).
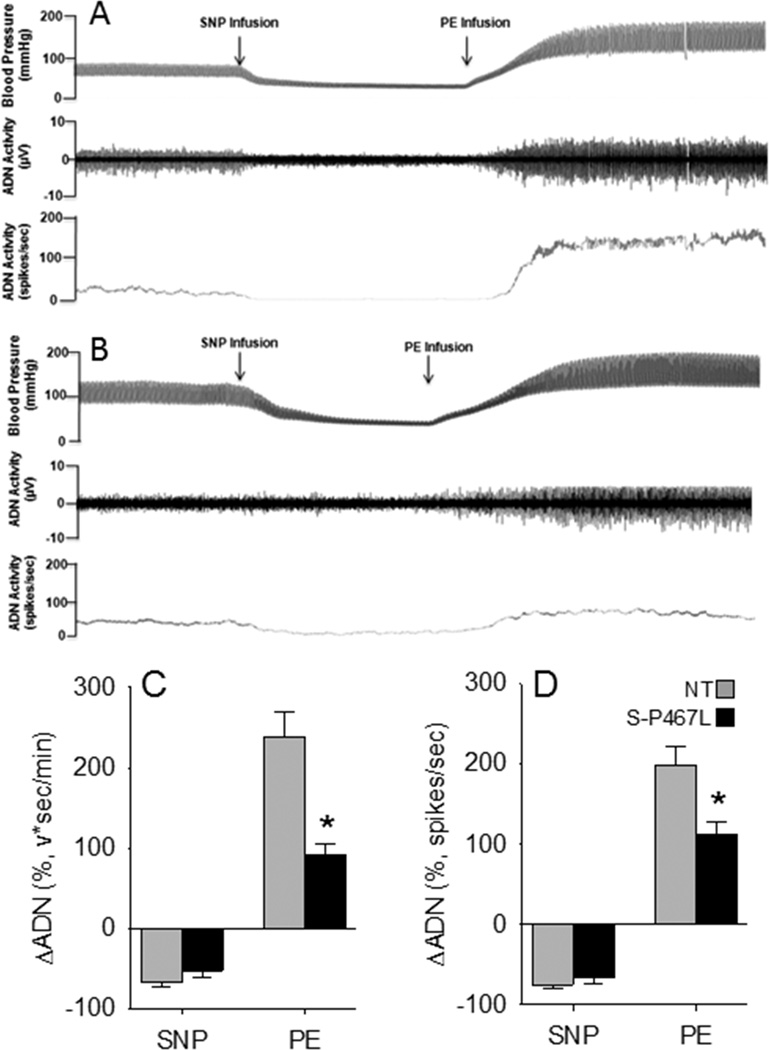
We next measured ADN activity in NT and S-P467L mice under anesthesia. ADN activity occurred in bursts and was synchronous with the arterial pressure pulse in both NT and S-P467L mice (Figure S2, Figure 4A–B).9 There was no difference in baseline ADN activity comparing S-P467L (95±13 spikes/sec) with NT (97±9 spikes/sec) mice. The ADN response to SNP was comparable between NT and S-P467L mice (Figure 4C–D). ADN activity rose abruptly in response to the pressor effects of PE in both groups, but the magnitude of the response was markedly blunted in S-P467L mice (Figure 4C–D).
Figure 4. Relationship Between Arterial Pressure, HR and Aortic Depressor Nerve Activity.
Representative tracings of blood pressure and ADN activity in anesthetized NT (A) and S-P467L (B) mice. The change in ADN activity (C–D) is shown in response to changes in arterial pressure induced by infusion of sodium nitroprusside (SNP) followed by phenylephrine (PE). S-P467L (black) and non-transgenic (gray) mice. NT (N=10); S-P467L (N=8); *, P<0.05 vs NT.
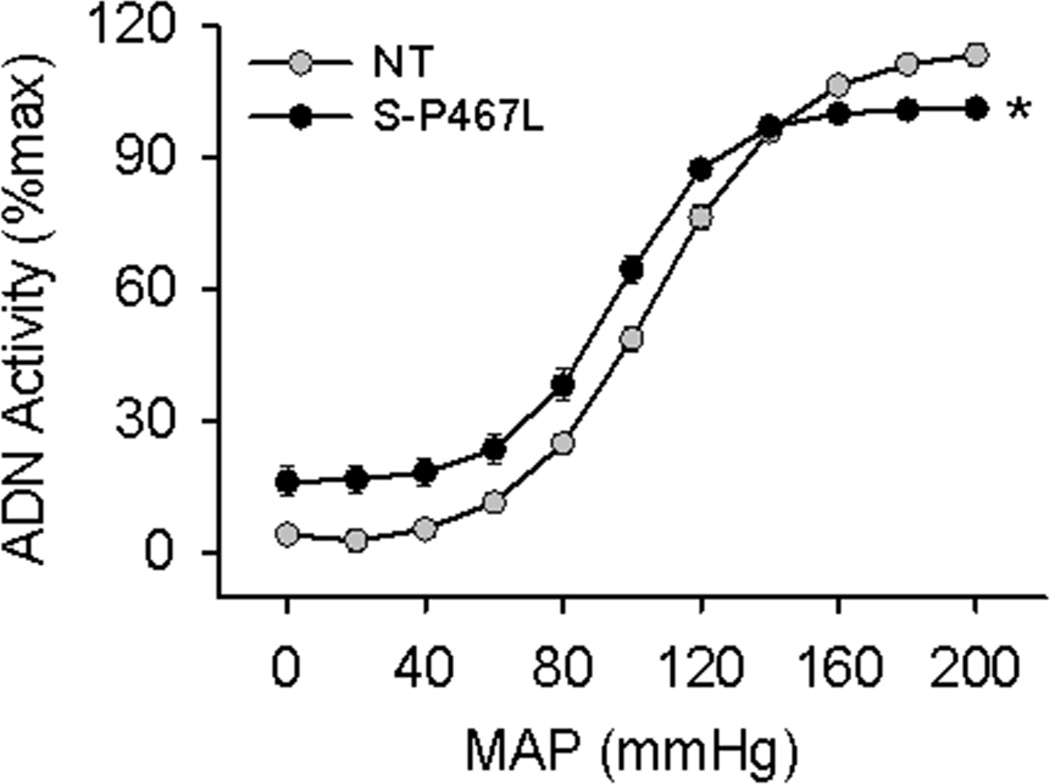
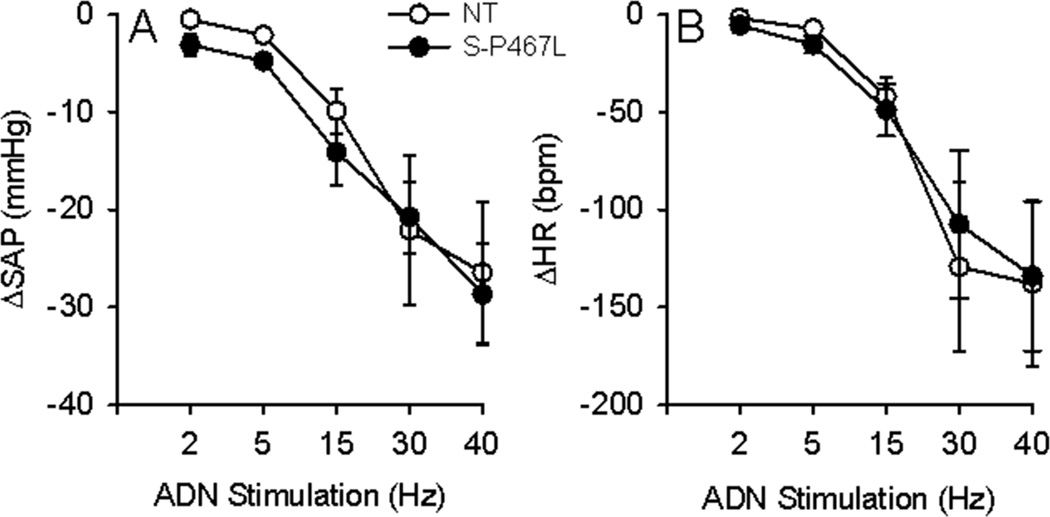
The sigmoidal fitting ADN-AP curve is shown in Figure 5. The baroreflex range, gain, and saturation pressure (MAPsat) were all significantly decreased in S-P467L mice, whereas the MAP at 50% of ADN activity (MAP50) and threshold pressure (MAPth) were not different between groups (Table 1). To examine the central integration of the afferent signal, we directly stimulated the ADN and monitored changes in AP and HR. Unlike the impaired response in S-P467L induced by acute increase in AP, the systolic AP and HR responses to ADN stimulation exhibited no differences between groups (Figure 6).
Figure 5. Baroreflex Curves.
Analysis of baroreceptor afferent function curves derived from responses to intravenous injections of sodium nitroprusside and phenylephrine in S-P467L (filled circles) and NT (open circles) mice. ADN, aortic depressor nerve; MAP, mean arterial pressure.
Table 1.
Baroreflex Parameters
| Parameter | NT | S-P467L |
|---|---|---|
| Range (mmHg) | 114 ± 4 | 85 ± 4* |
| Gain (%max) | 1.69 ± 0.08 | 1.26 ± 0.05* |
| MAP50 (mmHg) | 101 ± 2.4 | 95 ± 2 |
| MAPth (mmHg) | 45 ± 2 | 46 ± 4.9 |
| MAPsat (mmHg) | 172 ± 5.3 | 156 ± 3.8* |
Tabulated data derived from individual curves. NT (N=10); S-P467L (N=8);
, P<0.05 vs NT.
Figure 6. HR and AP Responses to ADN Stimulation.
Reflex decreases in systolic arterial pressure (ΔSAP, A) and heart rate (ΔHR, B) in response to electric stimulation of the aortic depressor nerve (ADN) in anesthetized S-P467L (filled circles) and NT (open circles) mice. N=9 per group.
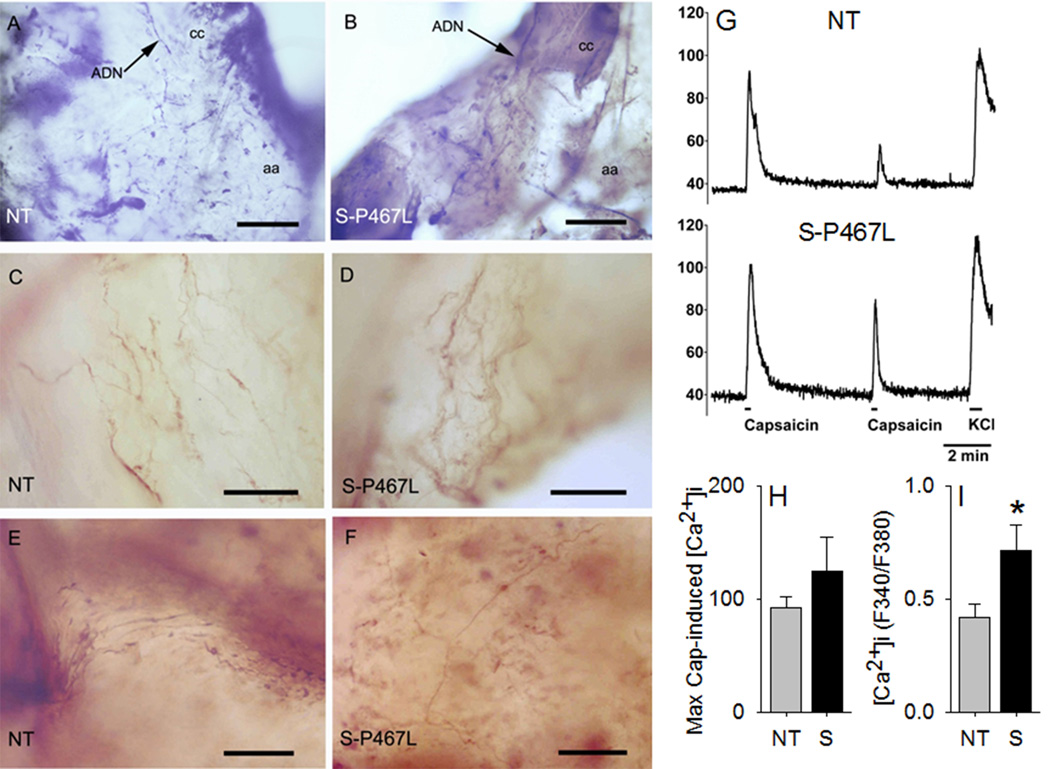
We next determined if defects in the nodose ganglia (NG) contribute to the impaired baroreflex sensitivity in S-P467L mice. Since PPARγ expression was recently identified in nodose neurons, we first assessed whether the smooth muscle promoter-driven transgene was expressed in nodose ganglia (NG) in S-P467L mice.10 No signal was detected in either aorta or NG of NT mice. Although a signal (Ct value ~28) was detected in NG from S-P467L mice, it was expressed approximately 500-fold less than in aorta (Figure S3A). There was no significant change in expression of the PPARγ target genes CD36 or aP2 in NG from P467L compared with NT (data not shown). Previous studies reported that the acid-sensing ion channel 2 (ASIC2) is expressed in aortic baroreceptor neurons and that Asic2-deficient mice exhibit baroreflex dysfunction.11 However, there was no difference in the level of expression of either Asic2 (Figure S3B) or Asic3 (Figure S3C) mRNA in the NG of S-P467L mice. Transient receptor potential vanilloid-1 (Trpv1) receptor channels have been implicated in baroreflex function,12 but there was no detectable expression of Trpv1 mRNA in the aorta. Initial investigations suggested a decrease in mRNA encoding Trpv1 in NG from S-P467L mice, but ultimately, this trend was not significant (Figure S3D). Based on our early finding, we assessed if there was histological evidence to support a defect in neural vascular coupling at the aorta arch. No obvious differences were observed between NT and S-P467L mice in the immunochemical staining pattern of Trpv1 in the termination area of the left ADN (Figure 7A–F). In all specimens, both thick and thin fibers could be seen arising from the ADN in the aortic wall and bouton-like swellings and heavily branched terminal arrays similar to those reported elsewhere.13 The mean numbers of grid intersections were 18.9±15.5 and 18.6±10.7 (NT vs S-P467L, t=0.237, p=1.0) for large fibers and 6.57±7.9 and 9.2±7.81 (NT vs S-P467L, t=0.851, p=0.4) indicating there were no difference between the mean number of large fiber intersections and only a modest difference for fine fiber intersections.
Figure 7. Neurovascular Coupling and Function of Trpv1 Channels in Nodose Ganglia Neurons.
Fibers and terminals of the aortic depressor nerve (ADN) on the aortic arch (aa) immunochemically stained with an antibody against Trpv1 in non-transgenic (NT) and S-P467 transgenic mice. A) Low power micrograph from a whole mount preparation from a NT animal showing the ADN innervating the region of the aortic arch at the junction with the left common carotid artery (cc). B) Similar preparation as in A from a S-P467L mouse. Note the similar appearance of the ADN and its arborizations in both mice. C–D) Complex endings of Trpv1 positive ADN axons in the vicinity of the aortic arch-left carotid junction. E–F) Arborizations of thin Trpv1 positive axons on the anterior wall of the aortic arch. Scale bars in A and B=250μm; scale bars in C–F=25μm. G) Representative traces of Ca2+ imaging in response to two successive capsaicin applications (100 nM, 15 sec), followed by KCl application (50 mM, 30 sec) in NG neurons. H) Maximal capsaicin-induced [Ca2+]i responses normalized to KCl. I) Magnitude of Trpv1 desensitization, expressed as the ratio of 2nd vs 1st capsaicin-induced peak [Ca2+]i in the same neuron. N=38 for NT and N=58 for S-P467L. *, P<0.05 vs NT.
Finally, we examined if there was any change in the number of functional Trpv1 channels in the NG neurons by monitoring Trpv1-mediated increase in intracellular Ca2+ ([Ca2+]i) responses (Figure 7G).14 No significant change was observed in the magnitude of capsaicin-induced [Ca2+]i rise, normalized to the KCl-induced [Ca2+]i rise in NG neurons of either genotype (Figure 7H), indicating no change in the levels of functional Trpv1 channel in these neurons. Interestingly, there was a significant decrease in the magnitude of Trpv1 channel desensitization in the NG neurons from S-P467L mice (Figure 7I).
Discussion
There is emerging evidence that the ligand activated transcription factor PPARγ, best known for its regulation of adipogenesis, lipid metabolism and insulin sensitivity, plays autocrine roles in tissues outside of its classical sites of action (e.g. liver, skeletal muscle and adipose tissue). Endothelial PPARγ-deficiency (through gene ablation) or PPARγ-interference (employing a dominant negative mutant) results in impaired NO-mediated vasodilatation and increased AP in response to a high fat diet.15–17 Smooth muscle PPAR-γdeficiency blunts the protective effects of thiazolidinediones on atherosclerosis and augments angiotensin II-induced oxidative stress, inflammation and vascular remodeling.18,19 Selective vascular smooth muscle interference with PPARγ causes a loss of NO-responsiveness, an increase in vasoconstriction and hypertension.4
Despite mild hypertension, S-P467L mice exhibit robust tachycardia suggesting impaired autonomic function.4 S-P467L mice exhibited a blunted tachycardic response to vagal blockade and a markedly augmented bradycardic response to sympathetic blockade. The direct measurements of HR in response to pharmacological blockade suggest there is increased sympathetic activity to the heart. This conclusion was further supported by spectral analysis, although it should be noted that the application and usefulness of spectral analysis remains controversial.
HR can be controlled by baroreceptors and chemoreceptors on free nerve endings in the aortic arch and carotid sinus, which transmit afferent signals to the brain in response to the local stretch of the vessel or changes in oxygen or pH (reviewed in20,21). Theoretically, a lesion in any portion of the baroreflex arc can lead to baroreflex impairment. That the ADN response to an increase in AP was severely impaired in S-P467L mice suggests impairment in the afferent limb of the baroreflex arc. Because the HR response to direct ADN stimulation was normal, suggests that the central and efferent limbs of the baroreflex are functioning normally. This stands in contrast with other models of hypertension including the spontaneously hypertensive rat (SHR), DOCA-salt-treated rat and obese Zucker rat, where the depressor response elicited by direct stimulation of ADN was attenuated suggesting a defect in the central component of the baroreflex arc.22–24 Unlike the SHR, which exhibits marked hypertension, the S-P467L mouse model displays only a small increase in arterial blood pressure but profound baroreflex dysfunction. Therefore, it is unlikely that the baroreflex dysfunction observed in S-P467L mice is due to increased arterial pressure.
The mechanical properties of the vascular wall and substances released by vascular cells have major influence on baroreceptor activity. For example, impaired release of prostacyclin, increased generation of reactive oxygen species and platelet aggregation have been shown to reduce baroreceptor activity during hypertension and atherosclerosis.25 ApoE-deficient mice were reported to exhibit both hypertension and tachycardia suggesting that hypercholesterolemia, atherosclerosis and/or vascular structure and function may affect baroreflex regulation.26 Vascular hypertrophy has been previously associated with the degree of baroreflex impairment.27 We showed here that there was a decrease in the diameter (without a change in wall thickness) of the carotid artery, and our previous observations indicate that cerebral arterioles, but not mesenteric arteries in S-P467L mice undergo hypertrophy, increased distensibility and remodeling.4,5 Thus, we must consider the possibility that the baroreceptor afferents in S-P467L mice may be unresponsive to increases in AP due to structural changes in the vessel. Alternatively, functional defects in the vessel may be playing an important role. Previous ex vivo studies revealed that the aorta from S-P467L mice exhibits markedly impaired responses to both endogenous and exogenous nitric oxide.4,6,28 Interestingly, mesenteric vessels from S-P467L mice display BKCa channel dysfunction,5 and polymorphisms in the KCNMB1 gene encoding the 1 subunit is associated with HR variability and baroreflex function in humans.29
PPARγ is expressed in primary cultured microglia and cortical neurons where its protective effects may be due to its anti-oxidant and anti-inflammatory actions.30,31 PPARγ is also expressed in the arcuate nucleus of the hypothalamus where it regulates the activity of a subset of neurons to control food intake.32,33 Nevertheless, it remains unclear if PPARγ is expressed in the afferent nerves communicating baroreflex signals from the peripheral vasculature to the brain. Recently, PPARγ expression in the vagal nerve was reported to play a role in high fat diet-induced thermogenesis.10 We found no evidence for the expression of the transgene encoding dominant negative PPARγ, nor a change in expression of two PPARγ target genes in peripheral NG nerves. Thus, PPARγ in nerves derived from the aortic arch and carotid sinus baroreceptors was unlikely to be affected. Asic2, Asic3 and Trpv1 have been mechanistically implicated to modulate the baroreflex.11,12 There was no change in Asic channel expression, but initial studies suggested that expression of Trpv1 might be decreased. This was attractive because Trpv1 channels are localized on the nerve fibers and terminals in the aortic arch and in NG neurons, and because ablation or inhibition of Trpv1 containing neurons blunts the baroreflex.12 Although there were no detectable differences in the number of Trpv1-expressing fibers at the termination of the left ADN, and no change in the maximal response to the Trpv1 ligand capsaicin, there was a significant decrease in the extent of channel desensitization in cultured NG neurons from S-P467L mice. This decrease in the magnitude of Trpv1 desensitization could lead to prolonged channel opening duration, which could promote action potential firing, that is, increased excitation of these neurons upon Trpv1 activation. Physiologically, the Trpv1 channel could be activated by acidic pH (≤6.0) or noxious temperatures (≥43°C).34 However, pathological conditions (e.g. inflammation) could lead to the activation of Trpv1 at normal body temperature, as well as by relatively weak acidic pH (~6.8 to 6.4).14,35 Under such circumstances, if the Trpv1 channel desensitization is decreased, it could lead to prolonged excitation of NG neurons. Of course, we recognize that this would stand in apparent contradiction to data showing that loss of Trpv1 causes baroreceptor dysfunction.12 It is also possible that the decrease in channel desensitization constitutes a compensatory effect elicited by the decrease in Trpv1 expression, which could ultimately lead to a normalization of channel function.
Perspectives
The most important observations from this study are that mice with impaired PPARγ activity in vascular smooth muscle exhibit tachycardia mechanistically caused by any combination of: a) increased cardiac sympathetic nervous system activity, b) impaired baroreflex, and c) a blunted ADN response to increased AP localized to the afferent limb of the baroreflex arc. Therefore, there may be some degree of baroreflex failure in S-P467L mice, which drives the increased HR.
Alteration in baroreflex function is a common problem in diabetes. Baroreflex sensitivity is decreased in patients with impaired glucose tolerance,36 and is blunted in diabetic patients.37 Likewise, animal models of diabetes display baroreflex alterations in a similar fashion to human diabetes.38,39 Depressed baroreflex sensitivity was reported to be predictive of other cardiovascular events in diabetes.40 Of greatest relevance to the current study, pioglitazone, a high affinity synthetic PPARγ agonist increased baroreflex sensitivity in type II diabetic patients.41 Similarly, rosiglitazone, another member of the thiazolidinedione family of PPARγ agonists improves baroreflex gain in rats with diet-induced obesity.42 Given that diabetes is often associated with PPARγ impairment, these findings combined with our current study raises the possibility that diabetes-associated baroreflex alteration is due to defective vascular PPARγ. Indeed our findings implicate vascular smooth muscle PPARγ as a critical determinant of neurovascular signaling. Thus, S-P467L mice may be a useful model to understand the decreased baroreflex sensitivity in diabetes.
Supplementary Material
Novelty and Significance.
What is New?
Mice expressing dominant negative PPARγ specifically in vascular smooth muscle (S-P467L) exhibit increased sympathetic activity to the heart and decreased effectiveness of the baroreflex.
S-P467L mice exhibit decreased aortic depressor nerve (ADN) activity in response to changes in arterial pressure, whereas arterial pressure and heart rate responses to direct ADN stimulation were similar in S-P467L and control mice
These data suggest that the afferent limb of the baroreflex arc is impaired whereas the central and efferent limbs of the baroreflex arc remain intact.
What is Relevant?
PPARγ is a ligand-activated transcription factor classically playing an important role in adipogenesis and metabolic processes but more recently cardiovascular diseases such as hypertension.
The baroreflex is an important beat-to-beat regulator of AP and heart rate (HR), controlled by baroreceptors, located in the adventitia of the aortic arch and carotid sinus.
Summary
The most important observations from this study relate to the finding that mice with impaired PPARγ activity in vascular smooth muscle exhibit tachycardia mechanistically caused by any combination of a) increased cardiac sympathetic nervous system activity, b) impaired baroreflex regulation, c) a blunted ADN response to increased AP localized to the afferent limb of the baroreflex arc. Therefore there may be some degree of baroreflex failure in S-P467L mice, which drives the increased HR. These findings implicate vascular smooth muscle PPARγ as a critical determinant of neurovascular signaling.
Acknowledgements
The authors would like to thank Deborah R. Davis for assistance with mice. We also thank Norma Sinclair, JoAnne Schwarting, and Patricia Yarolem for genotyping mice. Transgenic mice were generated at the University of Iowa Transgenic Animal Facility supported in part by grants from the NIH and from the Roy J. and Lucille A. Carver College of Medicine.
Sources of Funding: This work was supported through research grants from the NIH to CDS and KR (HL084207), to CDS (HL048058, HL061446, HL062984), to ADM (CA171927), to DPM (NS069898), and from the American Heart Association to PK (11POST5720021) and KR (14EIA18860041). The authors also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Footnotes
Disclosures: None.
References
- 1.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 2.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, deLange WJ, Keen HL, Tsai Y-S, Maeda N, Sigmund CD, Faraci FM. Interference with PPARγ Signaling Causes Cerebral Vascular Dysfunction, Hypertrophy, and Remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 4.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketsawatsomkron P, Lorca RA, Keen HL, Weatherford ET, Liu X, Pelham CJ, Grobe JL, Faraci FM, England SK, Sigmund CD. PPARgamma Regulates Resistance Vessel Tone Through a Mechanism Involving RGS5-Mediated Control of PKC and BKCa Channel Activity. Circ Res. 2012;111:1446–1458. doi: 10.1161/CIRCRESAHA.112.271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 Regulates Vascular Smooth Muscle Function and Arterial Blood Pressure via PPARgamma and RhoA/Rho-Kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 9.Ma X, Abboud FM, Chapleau MW. Analysis of afferent, central, and efferent components of the baroreceptor reflex in mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1033–R1040. doi: 10.1152/ajpregu.00768.2001. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Bookout AL, Lee S, Sun K, Jia L, Lee C, Udit S, Deng Y, Scherer PE, Mangelsdorf DJ, Gautron L, Elmquist JK. PPARgamma in Vagal Neurons Regulates High-Fat Diet Induced Thermogenesis. Cell Metab. 2014;19:722–730. doi: 10.1016/j.cmet.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Li DP, Chen SR, Hittelman WN, Pan HL. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J Pharmacol Exp Ther. 2009;331:851–859. doi: 10.1124/jpet.109.160473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Huang C, Ai J, Yan B, Gu H, Ma Z, Li AY, Xinyan S, Harden SW, Hatcher JT, Wurster RD, Cheng ZJ. Structural remodeling of vagal afferent innervation of aortic arch and nucleus ambiguus (NA) projections to cardiac ganglia in a transgenic mouse model of type 1 diabetes (OVE26) J Comp Neurol. 2010;518:2771–2793. doi: 10.1002/cne.22363. [DOI] [PubMed] [Google Scholar]
- 14.Loo L, Shepherd AJ, Mickle AD, Lorca RA, Shutov LP, Usachev YM, Mohapatra DP. The C-type natriuretic peptide induces thermal hyperalgesia through a noncanonical Gbetagamma-dependent modulation of TRPV1 channel. J Neurosci. 2012;32:11942–11955. doi: 10.1523/JNEUROSCI.1330-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL, Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor gamma (PPAR{gamma}) reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol. 2009;297:H1647–H1654. doi: 10.1152/ajpheart.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian V, Golledge J, Ijaz T, Bruemmer D, Daugherty A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPAR{gamma} Circ Res. 2010;107:953–958. doi: 10.1161/CIRCRESAHA.110.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesi C, Rehman A, Rautureau Y, Kasal DA, Briet M, Leibowitz A, Simeone SM, Ebrahimian T, Neves MF, Offermanns S, Gonzalez FJ, Paradis P, Schiffrin EL. Protective role of vascular smooth muscle cell PPARgamma in angiotensin II-induced vascular disease. Cardiovasc Res. 2013;97:562–570. doi: 10.1093/cvr/cvs362. [DOI] [PubMed] [Google Scholar]
- 20.Chapleau MW, Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev. 2011;16:109–127. doi: 10.1007/s10741-010-9174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohmeier TE, Iliescu R. Chronic activation of the baroreflex and the promise for hypertension therapy. Handb Clin Neurol. 2013;117:395–406. doi: 10.1016/B978-0-444-53491-0.00032-8. [DOI] [PubMed] [Google Scholar]
- 22.Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol. 2010;588:1515–1525. doi: 10.1113/jphysiol.2009.186387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura Y, Takeda K, Nakata T, Hayashi J, Kawasaki S, Lee LC, Sasaki S, Nakagawa M, Ijichi H. Central attenuation of aortic baroreceptor reflex in prehypertensive DOCA-salt-loaded rats. Hypertension. 1988;12:259–266. doi: 10.1161/01.hyp.12.3.259. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez ER, Krieger AJ, Sapru HN. Central resetting of baroreflex in the spontaneously hypertensive rat. Hypertension. 1983;5:346–352. doi: 10.1161/01.hyp.5.3.346. [DOI] [PubMed] [Google Scholar]
- 25.Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995;26:341–347. doi: 10.1161/01.hyp.26.2.341. [DOI] [PubMed] [Google Scholar]
- 26.Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 27.Sapru HN, Wang SC. Modification of aortic barorecptor resetting in the spontaneously hypertensive rat. Am J Physiol. 1976;230:664–674. doi: 10.1152/ajplegacy.1976.230.3.664. [DOI] [PubMed] [Google Scholar]
- 28.Pelham CJ, Keen HL, Lentz SR, Sigmund CD. Dominant negative PPARgamma promotes atherosclerosis, vascular dysfunction, and hypertension through distinct effects in endothelium and vascular muscle. Am J Physiol Regul Integr Comp Physiol. 2013;304:R690–R701. doi: 10.1152/ajpregu.00607.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gollasch M, Tank J, Luft FC, Jordan J, Maass P, Krasko C, Sharma AM, Busjahn A, Bahring S. The BK channel beta1 subunit gene is associated with human baroreflex and blood pressure regulation. J Hypertens. 2002;20:927–933. doi: 10.1097/00004872-200205000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Bernardo A, Ajmone-Cat MA, Gasparini L, Ongini E, Minghetti L. Nuclear receptor peroxisome proliferator-activated receptor-gamma is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J Neurochem. 2005;92:895–903. doi: 10.1111/j.1471-4159.2004.02932.x. [DOI] [PubMed] [Google Scholar]
- 31.Cimini A, Benedetti E, Cristiano L, Sebastiani P, D'Amico MA, D'Angelo B, Di LS. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130:325–337. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 32.Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW, Olefsky JM. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 35.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JS, Lu FH, Yang YC, Chang SH, Huang YH, Chen JJ, Chang CJ. Impaired baroreflex sensitivity in subjects with impaired glucose tolerance, but not isolated impaired fasting glucose. Acta Diabetol. 2014 doi: 10.1007/s00592-013-0548-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Frattola A, Parati G, Gamba P, Paleari F, Mauri G, di RM, Castiglioni P, Mancia G. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in diabetes mellitus. Diabetologia. 1997;40:1470–1475. doi: 10.1007/s001250050851. [DOI] [PubMed] [Google Scholar]
- 38.Maeda CY, Fernandes TG, Timm HB, Irigoyen MC. Autonomic dysfunction in short-term experimental diabetes. Hypertension. 1995;26:1100–1104. doi: 10.1161/01.hyp.26.6.1100. [DOI] [PubMed] [Google Scholar]
- 39.Lin M, Harden SW, Li L, Wurster RD, Cheng ZJ. Impairment of baroreflex control of heart rate in conscious transgenic mice of type 1 diabetes (OVE26) Auton Neurosci. 2010;152:67–74. doi: 10.1016/j.autneu.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J. 2010;74:1379–1383. doi: 10.1253/circj.cj-09-0960. [DOI] [PubMed] [Google Scholar]
- 41.Yokoe H, Yuasa F, Yuyama R, Murakawa K, Miyasaka Y, Yoshida S, Tsujimoto S, Sugiura T, Iwasaka T. Effect of pioglitazone on arterial baroreflex sensitivity and sympathetic nerve activity in patients with acute myocardial infarction and type 2 diabetes mellitus. J Cardiovasc Pharmacol. 2012;59:563–569. doi: 10.1097/FJC.0b013e31824f91a7. [DOI] [PubMed] [Google Scholar]
- 42.Zhao D, McCully BH, Brooks VL. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. J Pharmacol Exp Ther. 2012;343:206–213. doi: 10.1124/jpet.112.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.